Squid Skin Decellularised Dermal Matrix for Enhancing Repair of Acute Cranial Injuries in Rabbit Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
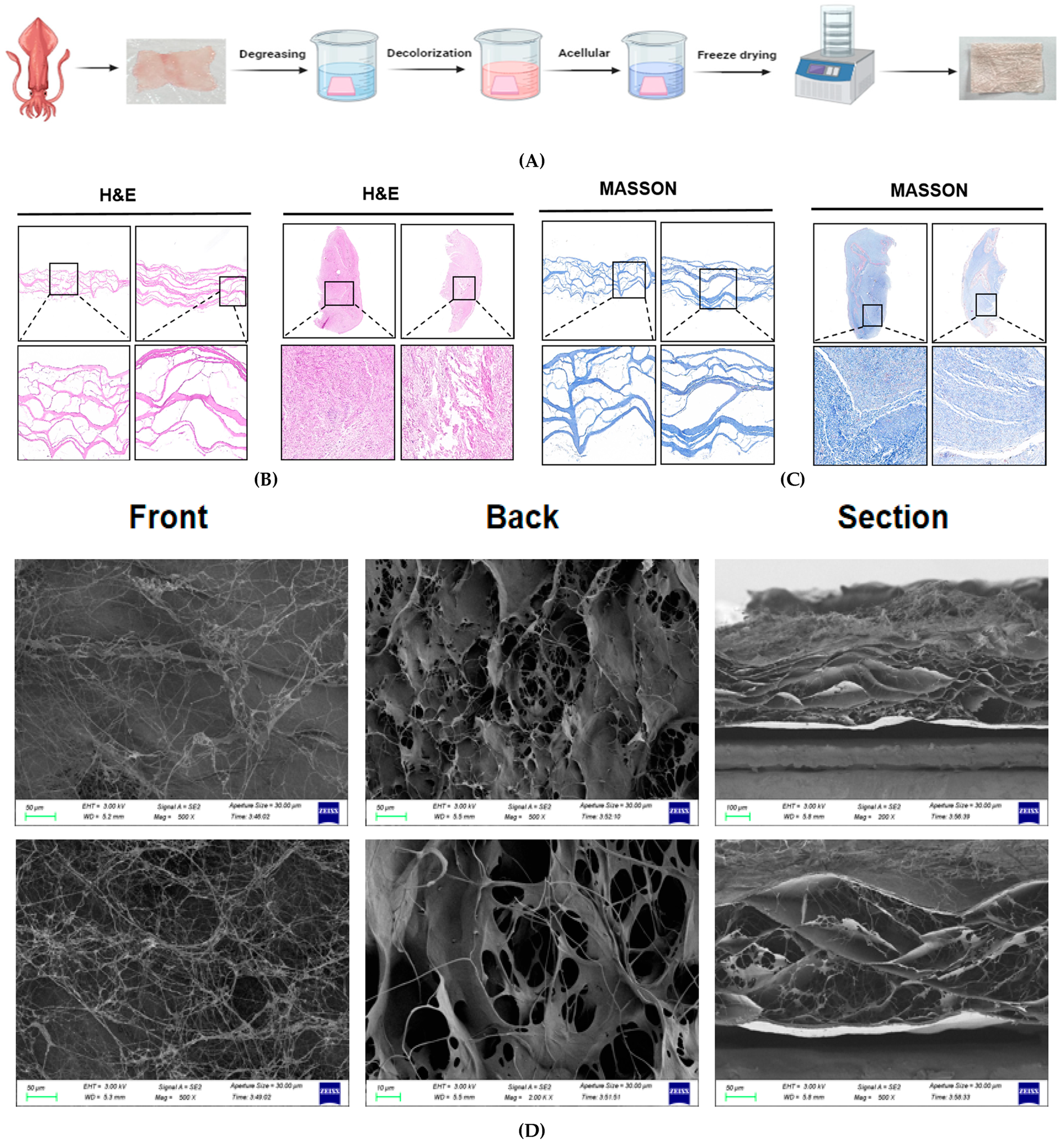
2.2. Squid Decellularised Dermal Matrix Preparation (SADM)
2.3. Physicochemical Properties of SADM
2.3.1. HE Staining
2.3.2. Massion Staining
2.3.3. Microstructure Observation
2.3.4. Porosity
2.3.5. Dissolution Properties
2.3.6. Fourier Infrared Spectroscopy
2.3.7. Determination of Mechanical Properties
2.4. Determination of In Vitro Degradation Rate
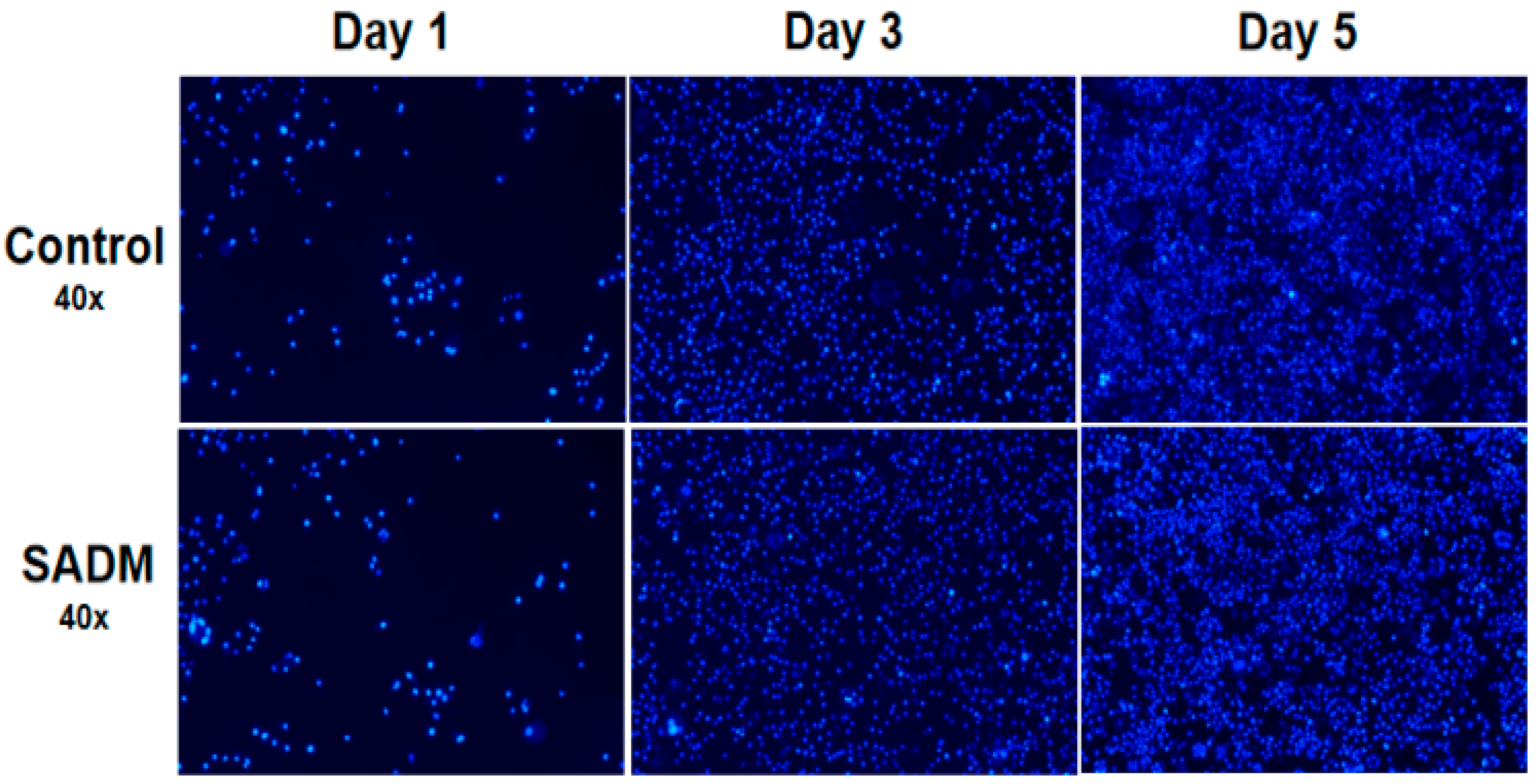
2.5. Cell Culture
Cytotoxicity and Proliferation Rate
2.6. Rabbit Skull Injury Model
2.6.1. Establishment and Surgical Repair of Cranial Parietal Hole-Type Bone Defects in the Rabbit
2.6.2. Observation Indicators
2.6.3. Statistical Analysis
3. Results and Discussion
3.1. Histology
3.2. Scanning Electron Microscopy
3.3. Porosity Analysis
3.4. Evaluation of Swelling Performance
3.5. Fourier Infrared Spectral Analysis
3.6. Measurement of Mechanical Properties
3.7. In Vitro Degradation Rate Analysis
3.8. Cell Proliferation and Cytotoxicity
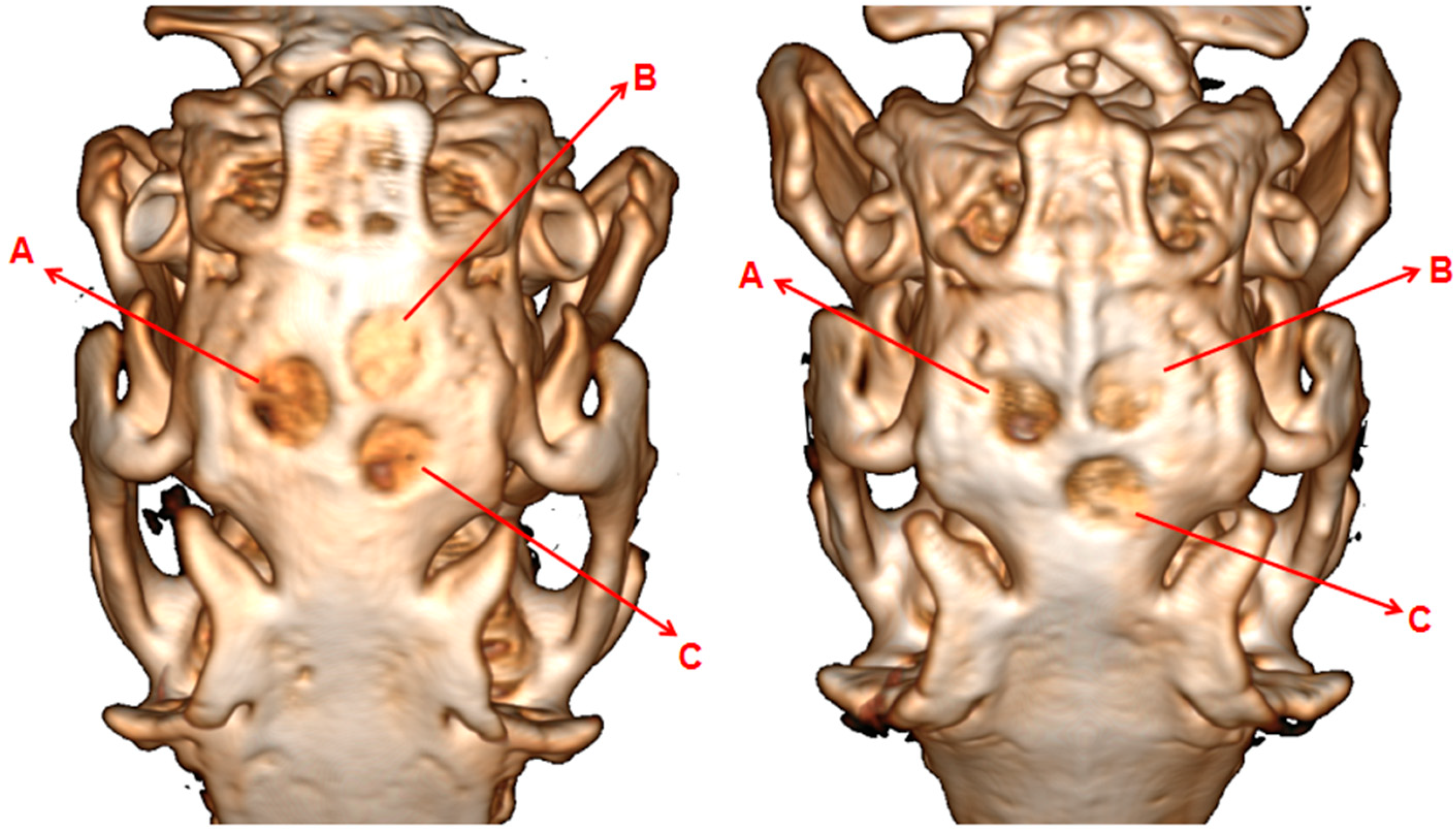
3.9. Characteristics of SADM Repair of Bone Cavity Defects in the Rabbit Cranial Parietal Bone
3.9.1. Behavioural Characteristics of Rabbits with Cranial Cave-In Bone Defects
3.9.2. Effect of SADM on New Osteogenesis in Rabbit Skulls
3.9.3. Effects of SADM on New Osteogenesis in Rabbit Cranium Observed in Saffron Solid Green Sections
3.9.4. Histometric Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.T.; Ge, B.F.; Xu, Y.K. Practical Orthopaedics, 3rd ed.; People’s Army Medical Press: Beijing, China, 2005. [Google Scholar]
- Park, S.A.; Shin, J.W.; Yang, Y.I.; Kim, Y.K.; Park, K.D.; Lee, J.W.; Jo, I.H.; Kim, Y.J. In vitro study of osteogenic differentiation of bone marrow stromal cells on heat-treated porcine trabecular bone blocks. Biomaterials 2004, 25, 527–535. [Google Scholar] [CrossRef]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 22. [Google Scholar] [CrossRef]
- Brotto, M.; Johnson, M.L. Endocrine Crosstalk Between Muscle and Bone. Curr. Osteoporos. Rep. 2014, 12, 135–141. [Google Scholar] [CrossRef]
- Frost, H.M. From Wolff’s law to the Utah paradigm: Insights about bone physiology and its clinical applications. Anat. Rec. 2010, 262, 398–419. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. Int. J. Oral Maxillofac. Implant. 1989, 4, 19–25. [Google Scholar]
- Rakers, S.; Gebert, M.; Uppalapati, S.; Meyer, W.; Maderson, P.; Sell, A.F.; Kruse, C.; Paus, R. ‘Fish matters’: The relevance of fish skin biology to investigative dermatology. Exp. Dermatol. 2010, 19, 313–324. [Google Scholar] [CrossRef]
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; De Val, J.E.M.S.; Wu, W. Collagen Peptide Upregulates Osteoblastogenesis from Bone Marrow Mesenchymal Stem Cells through MAPK-Runx2. Cells 2019, 8, 446. [Google Scholar] [CrossRef]
- Banerjee, P.; Mehta, A.; Shanthi, C. Investigation into the cyto-protective and wound healing properties of cryptic peptides from bovine Achilles tendon collagen. Chem. Interact. 2014, 211, 1–10. [Google Scholar] [CrossRef]
- Wong, R.; Rabie, A. Effect of Bio-Oss Collagen and Collagen matrix on bone formation. Open Biomed. Eng. J. 2010, 4, 71–76. [Google Scholar] [CrossRef]
- Alam, K.; Jeffery, S.L.A. Acellular Fish Skin Grafts for Management of Split Thickness Donor Sites and Partial Thickness Burns: A Case Series. Mil. Med. 2019, 184 (Suppl. S1), 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Sericin-chitosan-glycosaminoglycans Hydrogels Incorporated with Growth Factors for In Vitro and In Vivo Skin Repair. Carbohydr. Polym. 2021, 258, 117717. [Google Scholar] [CrossRef]
- Zhou, H.; Shang, L.; Li, X.; Zhang, X.; Gao, G.; Guo, C.; Chen, B.; Liu, Q.; Gong, Y.; Shao, C. Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp. Cell Res. 2009, 315, 2953–2962. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 2007, 14, 806–814. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Kjartansson, H.; Olafsson, I.H.; Karason, S.; Thorisson, H.; Baldursson, B.T.; Gunnarsson, E.; Jorundsson, E.; Sigurjonsson, G.F. Use of Acellular Fish Skin for Dura Repair in an Ovine Model: A Pilot Study. Open J. Mod. Neurosurg. 2015, 05, 124–136. [Google Scholar] [CrossRef]
- Dorweiler, B.; Trinh, T.T.; Dünschede, F.; Vahl, C.F.; Debus, E.S.; Storck, M.; Diener, H. Die marine Omega-3-Wundmatrix zur Behandlung komplizierter Wunden. Gefasschirurgie 2017, 22, 558–567. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Regenstein, J.M.; Lin, L. Comparison of collagen and gelatin extracted from the skins of Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus). Food Biosci. 2016, 13, 41–48. [Google Scholar] [CrossRef]
- Cho, J.-K.; Jin, Y.-G.; Rha, S.-J.; Kim, S.-J.; Hwang, J.-H. Biochemical characteristics of four marine fish skins in Korea. Food Chem. 2014, 159, 200–207. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Loss, M.; Wedler, V.; Künzi, W.; Meuli-Simmen, C.; Meyer, V.E. Artificial Skin, Split-Thickness Autograft and Cultured Autologous Keratinocytes Combined to Treat a Severe Burn Injury of 93% of TBSA. Burns 2000, 26, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.R.; Drew, P.; Dickson, W.A. The Use of a Bilaminate Artificial Skin Substitute (IntegraTM) in Acute Resurfacing of burns: An Early Experience. Br. J. Plast. Surg. 2001, 54, 208. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularisation Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix Molecules and Skin Biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S.; et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 127, 112202. [Google Scholar] [CrossRef]
- Fosnot, J.; Kovach, S.J., 3rd; Serletti, J.M. Acellular Dermal Matrix:General Principles for the Plastic Surgeon. Aesthetic Surg. J. 2011, 31, 5S–12S. [Google Scholar] [CrossRef]
- Wang, L.-P.; Wang, H.-J.; Hou, X.-S.; Raza, A.; Koyama, Y.; Ito, T.; Wang, J.-Y. Preparation of stretchable composite film and its application in skin burn repair. J. Mech. Behav. Biomed. Mater. 2021, 113, 104114. [Google Scholar] [CrossRef]
- Stone, R., 2nd; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collaen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus). Characterisation and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A.; Gruber, R. Collagen membranes adsorb the transforming growth factor-β receptor I kinase-dependent activity of enamel matrix derivative. J. Periodontol. 2016, 87, 583–590. [Google Scholar] [CrossRef]
- Sarban, S.; Senkoylu, A.; Isikan, U.E.; Korkusuz, P.; Korkusuz, F. Can rhBMP-2 containing collagen sponges enhance bone repair in ovariectomised rats?: A preliminary study. Clin. Orthop. Relat. Res. 2009, 467, 3113–3120. [Google Scholar] [CrossRef]
- Yan, W.; Chen, S. Analysis of squid production and import/export trade in China. China Fish. Econ. 2013, 31, 74–79. [Google Scholar]
- Nam, K.A.; You, S.G.; Kim, S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008, 73, C249–C255. [Google Scholar] [CrossRef]
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016, 46, 123–130. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Genta, I.; Modena, T.; Conti, B. Effect of porogen on the physico-chemical properties and degradation performance of PLGA scaffolds. Polym. Degrad. Stab. 2010, 95, 694–701. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, J.; Gu, Y.; Shao, J.; Zhou, S.; Zhuang, J.; Song, L.; Wang, X. Cryopreserved skin epithelial cell sheet combined with acellular amniotic membrane as an off-the-shelf scaffold for urethral regeneration. Mater. Sci. Eng. C 2021, 122, 111926. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, L.; Wang, H.; Wei, L.; Gao, X.; Zeng, Z.; Liu, S.; Fan, Y.; Liu, T.; Chen, J. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mater. Sci. Eng. C 2021, 121, 111749. [Google Scholar] [CrossRef]
- Wang, S.; He, S.; Zhang, X.; Sun, J.; Huang, Q.; Liu, J.; Han, C.; Yin, Z.; Ding, B.; Yin, J. Acellular bovine pericardium matrix in immediate breast reconstruction compared with conventional implant-based breast reconstruction. JPRAS Open 2021, 29, 1–9. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. Methods Mol. Biol. 2021, 2347, 17–25. [Google Scholar]
- Sivaraman, K.; Muthukumar, K.; Shanthi, C. Adhesion and proliferation properties of type I collagen-derived peptide for possible use in skin tissue engineering application. Cell Biol. Int. 2022, 46, 391–402. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299. [Google Scholar] [CrossRef]
- Norton, M.R.; Odell, E.W.; Thompson, I.D.; Cook, R.J. Efficacy of bovine bone mineral for alveolar augmentation: A human histologic study. Clin. Oral Implant. Res. 2003, 14, 775–783. [Google Scholar] [CrossRef]
- Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Biochemical and Microstructural Properties of Lizardfish (Saurida tumbil) Scale Collagen Extracted with Various Organic Acids. Gels 2022, 8, 266. [Google Scholar] [CrossRef]
- Beard, H.K.; Ueda, M.; Faulk, W.P.; Glynn, L.E. Cell-mediated and humoral immunity to chick type II collagen and its cyanogen bromide peptides in guinea-pigs. Immunology 1978, 34, 323–335. [Google Scholar] [PubMed]
- Duan, Z. Research on the Properties of Humanoid Collagen Haemostatic Sponge. Ph.D. Thesis, Northwest University, Kirkland, WA, USA, 2008. [Google Scholar]
- Hutmacher, D.; Hürzeler, M.B.; Schliephake, H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int. J. Oral Maxillofac. Implant. 1996, 11, 667–678. [Google Scholar]
- Pati, F.; Datta, P.; Adhikari, B.; Dhara, S.; Ghosh, K.; Das Mohapatra, P.K. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. J. Biomed. Mater. Res. Part A 2012, 100A, 1068–1079. [Google Scholar] [CrossRef]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared Spectroscopy of Collagen and Collagen-like Polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef]
- Abe, Y.; Krimm, S. Normal Vibrations of Crystalline Polyglycine I. Biopolymers 1972, 11, 1817–1839. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier Transform Ir Spectroscopy of Collagen and Gelatin Solutions: Deconvolution of the Amide I Band for Conformational Studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Wabnitz, G.H.; Goursot, C.; Jahraus, B.; Kirchgessner, H.; Hellwig, A.; Klemke, M.; Konstandin, M.H.; Samstag, Y. Mitochondrial Translocation of Oxidized Cofilin Induces Caspase-Independent Necrotic-like Programmed Cell Death of T Cells. Cell Death Dis. 2010, 1, e58. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, S.; Sun, Y.; Ma, S.; Li, X.; Zhang, T.; Zhou, Y. Preparation of poly(butylene succinate)/poly(propylene carbonate) biofilm and evaluation of related properties. China Tissue Eng. Res. 2015, 19, 3355–3360. [Google Scholar]
- Park, S.; Choi, S.; Shimpi, A.A.; Estroff, L.A.; Fischbach, C.; Paszek, M.J. Collagen Mineralization Decreases NK Cell-Mediated Cytotoxicity of Breast Cancer Cells via Increased Glycocalyx Thickness. Adv. Mater. 2024, 36, e2311505. [Google Scholar] [CrossRef]
- Ellis, M.W.; Riaz, M.; Huang, Y.; Qyang, Y.B. Epigallocatechin Gallate Facilitates Extracellular Elastin Fiber Formation in Induced Pluripotent Stem Cell Derived Vascular Smooth Muscle Cells for Tissue Engineering. J. Mol. Cell. Cardiol. 2022, 163, 167–174. [Google Scholar] [CrossRef]
- Eyrebrook, A.L. The periosteum: Its function reassessed. Clin. Orthop. Relat. Res. 1984, 189, 300. [Google Scholar]



| Peaks | Absorption [cm−1] | Observations |
|---|---|---|
| Amide A | 3254 | O-H N-H Tension |
| Amide B | 2927–2861 | CH2 asymmetric stretching |
| Amide I | 1640 | C=O stretch Hydrogen bonding and COO-coupling |
| Amide II | 1541 | N-H bending coupled to CN stretching |
| Amide III |
| Groups | Mean | SD | p-Value | |
|---|---|---|---|---|
| New bone area (%) | Control | 7.73 | 2.71 | 0.017 * |
| SADM | 32.76 | 5.11 | ||
| HEAL-ALL | 28.12 | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Pang, Y.; Yang, H.; Zhou, Q.; Hou, J.; Wu, W.; Elango, J. Squid Skin Decellularised Dermal Matrix for Enhancing Repair of Acute Cranial Injuries in Rabbit Model. J. Funct. Biomater. 2025, 16, 159. https://doi.org/10.3390/jfb16050159
Liu L, Pang Y, Yang H, Zhou Q, Hou J, Wu W, Elango J. Squid Skin Decellularised Dermal Matrix for Enhancing Repair of Acute Cranial Injuries in Rabbit Model. Journal of Functional Biomaterials. 2025; 16(5):159. https://doi.org/10.3390/jfb16050159
Chicago/Turabian StyleLiu, Lixin, Yida Pang, Haoze Yang, Qiyi Zhou, JinHua Hou, Wenhui Wu, and Jeevithan Elango. 2025. "Squid Skin Decellularised Dermal Matrix for Enhancing Repair of Acute Cranial Injuries in Rabbit Model" Journal of Functional Biomaterials 16, no. 5: 159. https://doi.org/10.3390/jfb16050159
APA StyleLiu, L., Pang, Y., Yang, H., Zhou, Q., Hou, J., Wu, W., & Elango, J. (2025). Squid Skin Decellularised Dermal Matrix for Enhancing Repair of Acute Cranial Injuries in Rabbit Model. Journal of Functional Biomaterials, 16(5), 159. https://doi.org/10.3390/jfb16050159









