Fabrication of a 3D Corneal Model Using Collagen Bioink and Human Corneal Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Information
2.2. Bioprinting Setup
2.3. Gelatin Dome
2.4. Acellular Collagen Constructs
2.4.1. Water Retention
2.4.2. Reflection Confocal Microscopy
2.5. Cell Culture
2.6. Cell-Embedded Collagen Construct Model
2.6.1. Collagen Solution Formulation
2.6.2. RNA Extraction
2.6.3. qRT-PCR
2.7. Statistical Analysis
3. Results
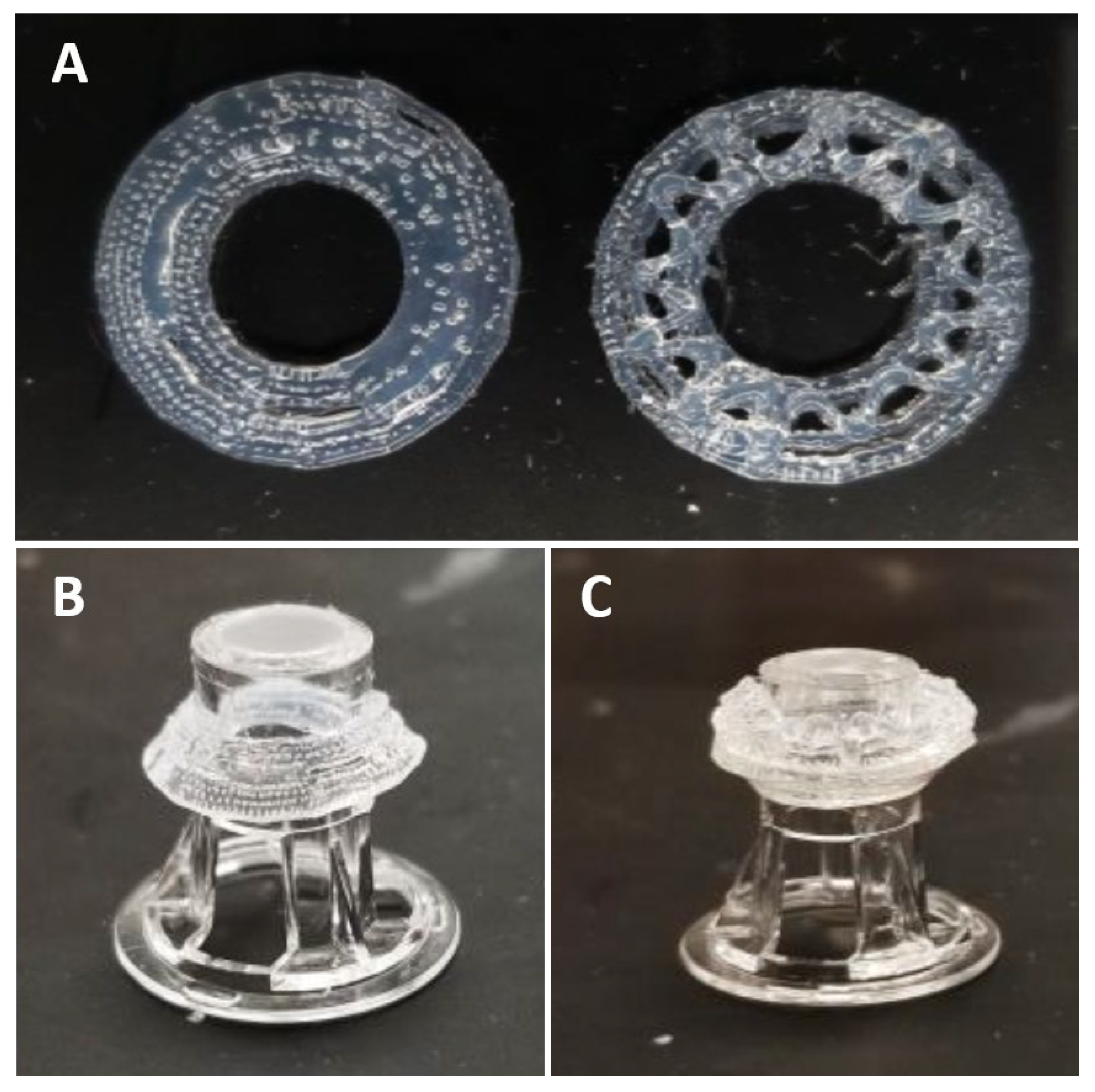
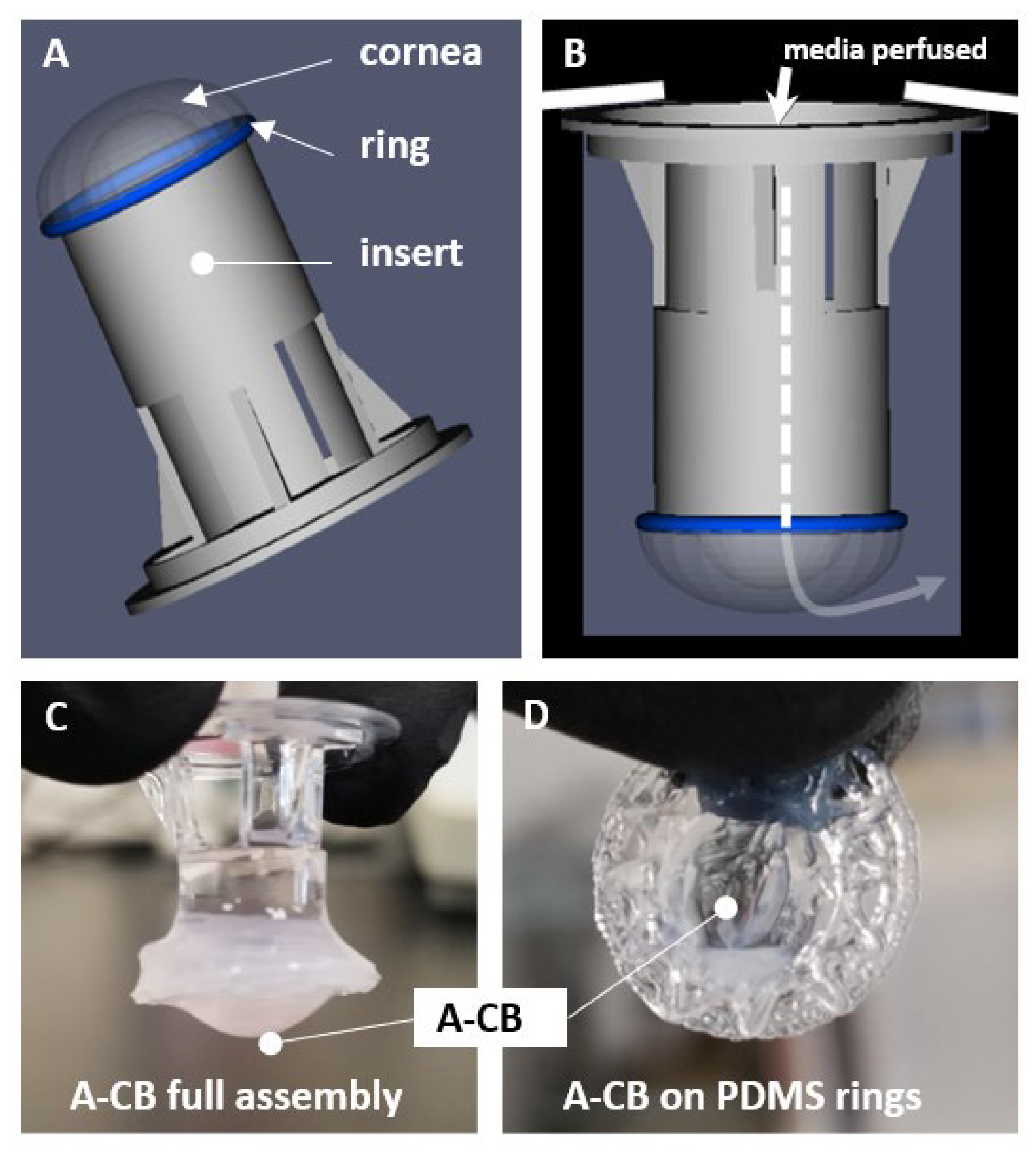
3.1. 3D Bioprinted Human Corneal Model
3.2. Acellular Model Results (A-CB)
Observation of Water Retention
3.3. Cellular Model Results (3D-hCB)
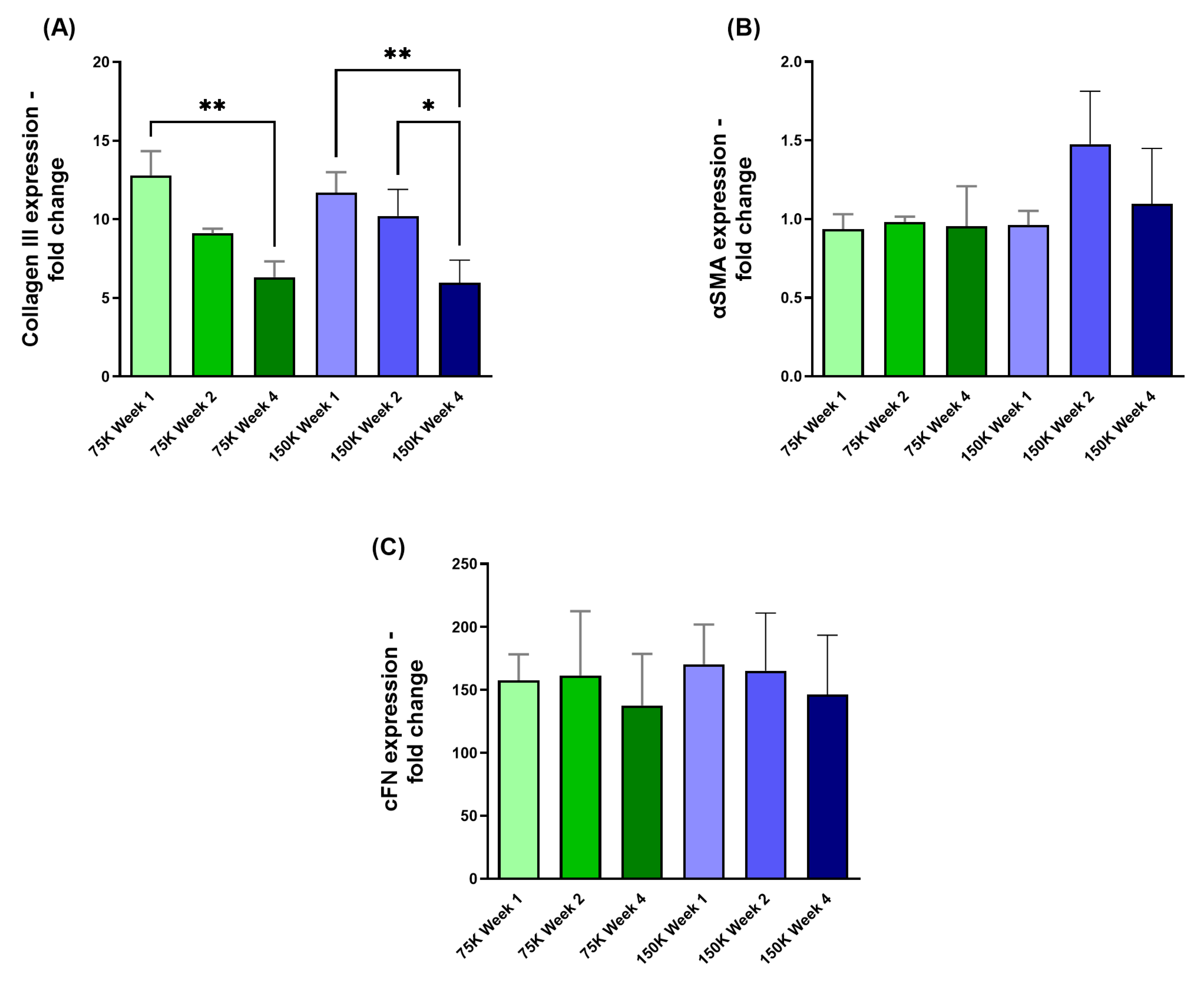
3.3.1. Collagen III (COL III), α-Smooth Muscle Actin (αSMA), and Cellular Fibronectin (cFN)
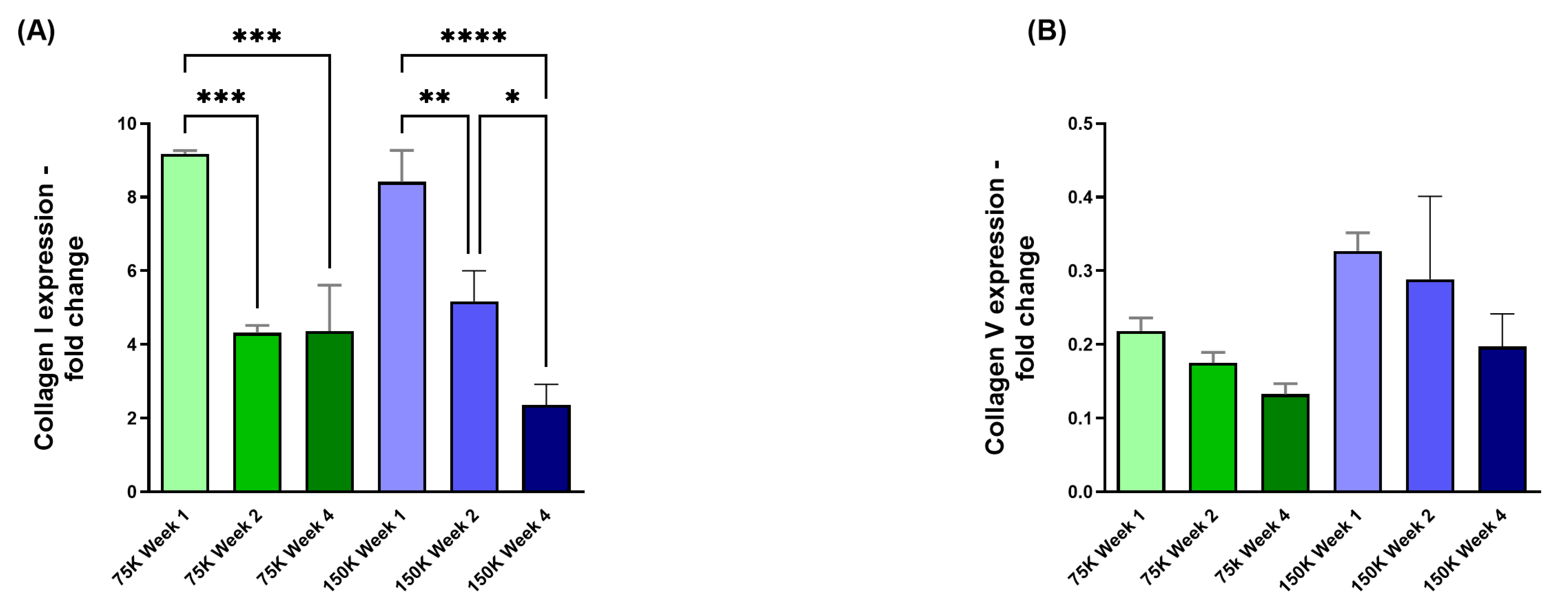
3.3.2. Collagen I (COL I) and Collagen V (COL V)
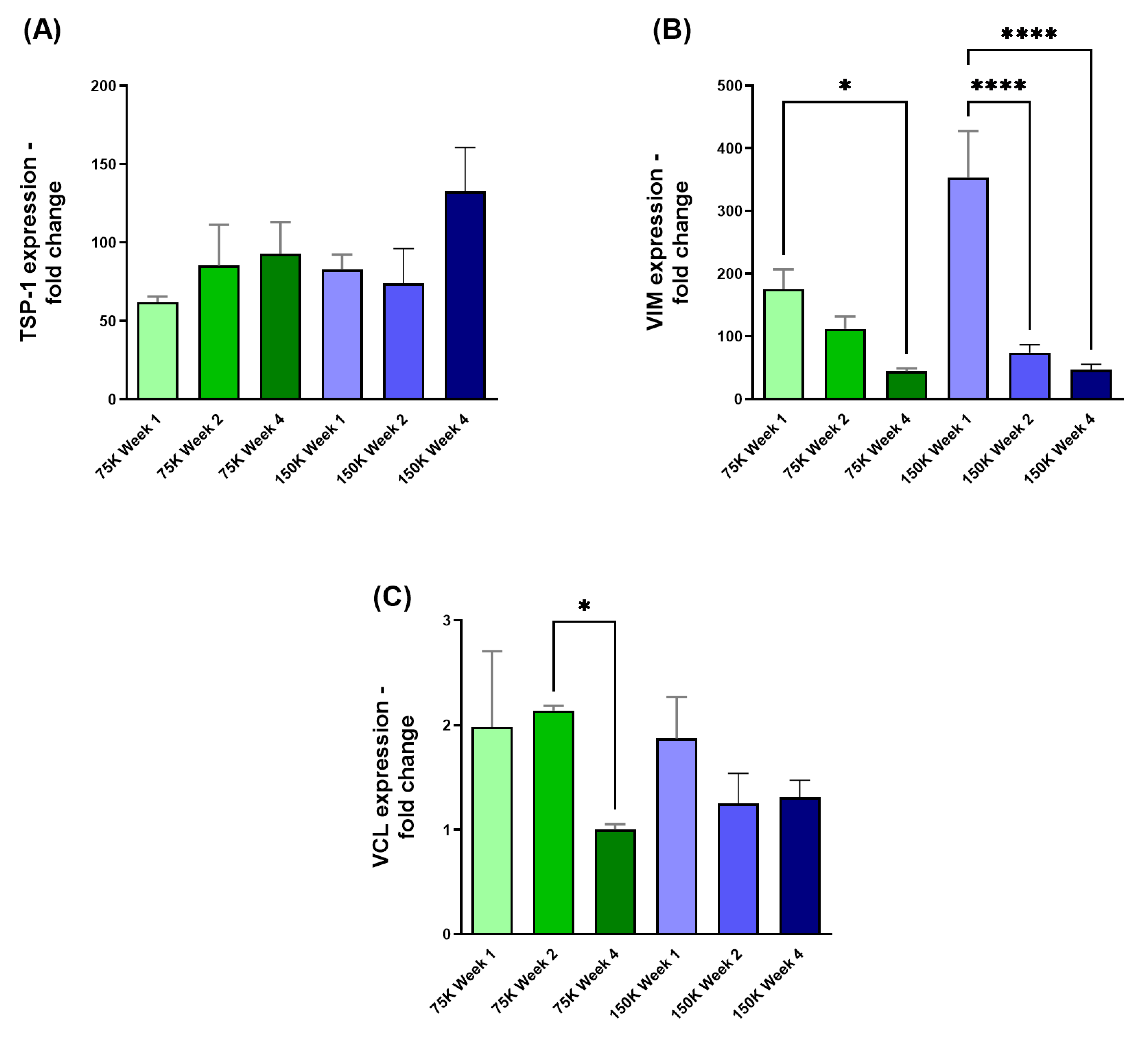
3.3.3. Thrombospondin-1 (TSP-1), Vimentin (VIM), and Vinculin (VCL)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDMS | Polydimethylsiloxane |
| BAB200 | BioassemblyBot®200 |
| TSIM | Tissue Structure Information Modeling |
| A-CBs | Acellular collagen bioprinted constructs |
| 3D-hCBs | HCF-embedded corneal bioprinted constructs |
| RCM | Reflection confocal microscopy |
| qRT-PCR | Quantitative Real-Time PCR |
| ECM | Extracellular Matrix |
| dECM | Decellularized Extracellular Matrix |
References
- Jung, Y.H.; Park, B.; Kim, J.U.; Kim, T.I. Bioinspired Electronics for Artificial Sensory Systems. Adv. Mater. 2019, 31, e1803637. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; Wood, J. Vision and falls. Clin. Exp. Optom. 2005, 88, 212–222. [Google Scholar] [PubMed]
- Shakarchi, A.F.; Mihailovic, A.; West, S.K.; Friedman, D.S.; Ramulu, P.Y. Vision Parameters Most Important to Functionality in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4556–4563. [Google Scholar]
- Clarke, A.; Tyler, L.K. Understanding What We See: How We Derive Meaning From Vision. Trends Cogn. Sci. 2015, 19, 677–687. [Google Scholar]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar]
- Villoslada, P.; Martinez-Lapiscina, E.H. Time is vision: The importance of the early discovery and diagnosis of optic neuritis. Mult. Scler. 2017, 23, 1806–1807. [Google Scholar]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef]
- Soleimani, M.; Ebrahimi, Z.; Ebrahimi, K.S.; Farhadian, N.; Shahlaei, M.; Cheraqpour, K.; Ghasemi, H.; Moradi, S.; Chang, A.Y.; Sharifi, S.; et al. Application of biomaterials and nanotechnology in corneal tissue engineering. J. Int. Med. Res. 2023, 51, 3000605231190473. [Google Scholar] [CrossRef]
- Chua, A.; Chua, M.J.; Kam, P. Recent advances and anaesthetic considerations in corneal transplantation. Anaesth. Intensive Care 2018, 46, 162–170. [Google Scholar]
- Del Barrio, J.L.A.; Bhogal, M.; Ang, M.; Ziaei, M.; Robbie, S.; Montesel, A.; Gore, D.M.; Mehta, J.S.; Alió, J.L. Corneal transplantation after failed grafts: Options and outcomes. Surv. Ophthalmol. 2021, 66, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Moramarco, A.; Gardini, L.; Iannetta, D.; Versura, P.; Fontana, L. Post Penetrating Keratoplasty Ectasia: Incidence, Risk Factors, Clinical Features, and Treatment Options. J. Clin. Med. 2022, 11, 2678. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Zeppieri, M.; Enaholo, E.S.; Chukwuyem, E.; Salati, C. An Overview of Corneal Transplantation in the Past Decade. Clin. Pract. 2023, 13, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Kirkness, C.M.; Ficker, L.A.; Steele, A.D.; Rice, N.S. The success of penetrating keratoplasty for keratoconus. Eye 1990, 4 Pt 5, 673–688. [Google Scholar] [CrossRef]
- Pramanik, S.; Musch, D.C.; Sutphin, J.E.; Farjo, A.A. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology 2006, 113, 1633–1638. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Choi, A.J.; Hefley, B.S.; Nicholas, S.E.; Cunningham, R.L.; Karamichos, D. Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma. Int. J. Mol. Sci. 2023, 24, 13635. [Google Scholar] [CrossRef]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal myofibroblasts and fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [PubMed]
- Zhou, C.; Robert, M.-C.; Kapoulea, V.; Lei, F.; Stagner, A.M.; Jakobiec, F.A.; Dohlman, C.H.; Paschalis, E.I. Sustained Subconjunctival Delivery of Infliximab Protects the Cornea and Retina Following Alkali Burn to the Eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bains, K.K.; Fukuoka, H.; Hammond, G.M.; Sotozono, C.; Quantock, A.J. Recovering vision in corneal epithelial stem cell deficient eyes. Cont. Lens Anterior Eye 2019, 42, 350–358. [Google Scholar]
- Barrientez, B.; Nicholas, S.E.; Whelchel, A.; Sharif, R.; Hjortdal, J.; Karamichos, D. Corneal injury: Clinical and molecular aspects. Exp. Eye Res. 2019, 186, 107709. [Google Scholar]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar]
- Dohlman, T.H.; Yin, J.; Dana, R. Methods for Assessing Corneal Opacity. Semin. Ophthalmol. 2019, 34, 205–210. [Google Scholar]
- Priyadarsini, S.; McKay, T.B.; Sarker-Nag, A.; Allegood, J.; Chalfant, C.; Ma, J.-X.; Karamichos, D. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp. Eye Res. 2016, 153, 90–100. [Google Scholar]
- Chen, Z.; Liu, X.; You, J.; Tomaskovic-Crook, E.; Yue, Z.; Talaei, A.; Sutton, G.; Crook, J.; Wallace, G. Electro-compacted collagen for corneal epithelial tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 1151–1160. [Google Scholar] [PubMed]
- Karamichos, D.; Nicholas, S.E.; Khan, A.; Riaz, K.M. Collagen Crosslinking for Keratoconus: Cellular Signaling Mechanisms. Biomolecules 2023, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Ronnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [PubMed]
- Karsdal, M.A.; Genovese, F.; Madsen, E.A.; Manon-Jensen, T.; Schuppan, D. Collagen and tissue turnover as a function of age: Implications for fibrosis. J. Hepatol. 2016, 64, 103–109. [Google Scholar]
- Jia, S.; Bu, Y.; Lau, D.-S.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.-H.J. Advances in 3D bioprinting technology for functional corneal reconstruction and regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1065460. [Google Scholar]
- Kutlehria, S.; Dinh, T.C.; Bagde, A.; Patel, N.; Gebeyehu, A.; Singh, M. High-throughput 3D bioprinting of corneal stromal equivalents. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2981–2994. [Google Scholar]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. A 2019, 107, 1945–1953. [Google Scholar]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969. [Google Scholar]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar]
- Wu, Y.; Qin, M.; Yang, X. Organ bioprinting: Progress, challenges and outlook. J. Mater. Chem. B 2023, 11, 10263–10287. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [PubMed]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cerezo, L.; Jesus, M.R.; Macias-Garcia, A.; Marcos-Romero, A.C.; Diaz-Parralejo, A. Evolution of bioprinting and current applications. Int. J. Bioprint. 2023, 9, 742. [Google Scholar]
- Balters, L.; Reichl, S. 3D bioprinting of corneal models: A review of the current state and future outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar]
- Bhattacharjee, P.; Ahearne, M. Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering. Pharmaceutics 2021, 13, 319. [Google Scholar] [CrossRef]
- Frankowski, J.; Kurzatkowska, M.; Sobczak, M.; Piotrowska, U. Utilization of 3D bioprinting technology in creating human tissue and organoid models for preclinical drug research—State-of-the-art. Int. J. Pharm. 2023, 644, 123313. [Google Scholar]
- Tavafoghi, M.; Khademhosseini, A.; Ahadian, S. Advances and challenges in bioprinting of biological tissues and organs. Artif. Organs 2021, 45, 1441–1445. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar]
- Xu, X.; Zhao, J.; Wang, M.; Wang, L.; Yang, J. 3D Printed Polyvinyl Alcohol Tablets with Multiple Release Profiles. Sci. Rep. 2019, 9, 12487. [Google Scholar]
- Baik, J.-M.; Yu, Y.-S.; Kim, J.H.; Ahn, C.B.; Son, K.H.; Choi, E.S.; Lee, J.W. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci. Rep. 2020, 10, 7554. [Google Scholar]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Gingras, A.A.; Jansen, P.A.; Smith, C.; Zhang, X.; Niu, Y.; Zhao, Y.; Roberts, C.J.; Herderick, E.D.; Swindle-Reilly, K.E. 3D Bioprinting of Acellular Corneal Stromal Scaffolds with a Low Cost Modified 3D Printer: A Feasibility Study. Curr. Eye Res. 2023, 48, 1112–1121. [Google Scholar] [CrossRef]
- Grönroos, P.; Mörö, A.; Puistola, P.; Hopia, K.; Huuskonen, M.; Viheriälä, T.; Ilmarinen, T.; Skottman, H. Bioprinting of human pluripotent stem cell derived corneal endothelial cells with hydrazone crosslinked hyaluronic acid bioink. Stem Cell Res. Ther. 2024, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, M.N.; Kim, J.; Jang, J.; Kim, H.K.; Cho, D.W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 2041731418823382. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, e1800398. [Google Scholar]
- Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-Del-Burgo, L.; Pedraz, J.L. Current Insights Into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. [Google Scholar] [CrossRef]
- Puistola, P.; Miettinen, S.; Skottman, H.; Moro, A. Novel strategy for multi-material 3D bioprinting of human stem cell based corneal stroma with heterogenous design. Mater. Today Bio 2024, 24, 100924. [Google Scholar]
- Nicholas, S.E.; Choi, A.J.; Lam, T.N.; Basu, S.K.; Mandal, N.; Karamichos, D. Potentiation of Sphingolipids and TGF-beta in the human corneal stroma reveals intricate signaling pathway crosstalks. Exp. Eye Res. 2023, 231, 109487. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Sarker-Nag, A.; Rowsey, T.G.; Ma, J.X.; Karamichos, D. Establishment of a 3D In Vitro Model to Accelerate the Development of Human Therapies against Corneal Diabetes. PLoS ONE 2016, 11, e0168845. [Google Scholar]
- Camburu, G.; Zemba, M.; Tataru, C.P.; Purcarea, V.L. The measurement of Central Corneal Thickness. Rom. J. Ophthalmol. 2023, 67, 168–174. [Google Scholar] [PubMed]
- Henriksson, J.T.; McDermott, A.M.; Bergmanson, J.P. Dimensions and morphology of the cornea in three strains of mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3648–3654. [Google Scholar]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar]
- Wang, Y.; Wang, J.; Ji, Z.; Yan, W.; Zhao, H.; Huang, W.; Liu, H. Application of Bioprinting in Ophthalmology. Int. J. Bioprint. 2022, 8, 552. [Google Scholar]
- Lam, E.H.Y.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Sabzevari, A.; Rayat Pisheh, H.; Ansari, M.; Salati, A. Progress in bioprinting technology for tissue regeneration. J. Artif. Organs 2023, 26, 255–274. [Google Scholar]
- Hong, N.; Yang, G.H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 444–459. [Google Scholar]
- Memic, A.; Navaei, A.; Mirani, B.; Cordova, J.A.V.; Aldhahri, M.; Dolatshahi-Pirouz, A.; Akbari, M.; Nikkhah, M. Bioprinting technologies for disease modeling. Biotechnol. Lett. 2017, 39, 1279–1290. [Google Scholar]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [PubMed]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [PubMed]
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar]
- Wang, P.; Li, X.; Zhu, W.; Zhong, Z.; Moran, A.; Wang, W.; Zhang, K.; Chen, S. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting 2018, 12, e00029. [Google Scholar]
- Lorber, B.; Hsiao, W.K.; Hutchings, I.M.; Martin, K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2014, 6, 015001. [Google Scholar]
- Lorber, B.; Hsiao, W.K.; Martin, K.R. Three-dimensional printing of the retina. Curr. Opin. Ophthalmol. 2016, 27, 262–267. [Google Scholar]
- Fuest, M.; Yam, G.H.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. [Google Scholar] [CrossRef]
- Gibney, R.; Patterson, J.; Ferraris, E. High-Resolution Bioprinting of Recombinant Human Collagen Type III. Polymers 2021, 13, 2973. [Google Scholar] [CrossRef]
- Mörö, A.; Samanta, S.; Honkamäki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation. Biofabrication 2022, 15, 015020. [Google Scholar]
- Park, J.; Lee, K.; Kim, H.; Park, S.; Wijesinghe, R.E.; Lee, J.; Han, S.; Lee, S.; Kim, P.; Cho, D.; et al. Biocompatibility evaluation of bioprinted decellularized collagen sheet implanted in vivo cornea using swept-source optical coherence tomography. J. Biophotonics 2019, 12, e201900098. [Google Scholar] [CrossRef] [PubMed]
- Isidan, A.; Liu, S.; Li, P.; Lashmet, M.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation 2019, 26, e12564. [Google Scholar]
- Zhang, M.; Yang, F.; Han, D.; Zhang, S.-Y.; Dong, Y.; Li, X.; Ling, L.; Deng, Z.; Cao, X.; Tian, J.; et al. 3D bioprinting of corneal decellularized extracellular matrix: GelMA composite hydrogel for corneal stroma engineering. Int. J. Bioprint. 2023, 9, 774. [Google Scholar] [CrossRef]
- Wang, X.; Elbahrawi, R.T.; Abdukadir, A.M.; Ali, Z.M.; Chan, V.; Corridon, P.R. A proposed model of xeno-keratoplasty using 3D printing and decellularization. Front. Pharmacol. 2023, 14, 1193606. [Google Scholar]
- Noro, J.; Vilaca-Faria, H.; Reis, R.L.; Pirraco, R.P. Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar]
- Zhang, B.; Xue, Q.; Hu, H.-Y.; Yu, M.-F.; Gao, L.; Luo, Y.-C.; Li, Y.; Li, J.-T.; Ma, L.; Yao, Y.-F.; et al. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J. Zhejiang Univ. Sci. B 2019, 20, 945–959. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, J.; Tian, J.; Deng, X.; Balayan, A.; Sun, Y.; Xiang, Y.; Guan, J.; Schimelman, J.; Hwang, H.; et al. Rapid 3D bioprinting of a multicellular model recapitulating pterygium microenvironment. Biomaterials 2022, 282, 121391. [Google Scholar]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 770780. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Cao, Y.; Wu, J.; Zhang, W.S. Role of corneal collagen fibrils in corneal disorders and related pathological conditions. Int. J. Ophthalmol. 2017, 10, 803–811. [Google Scholar]
- Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Ceresa, B.P.; Calkins, D.J. Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration. Front. Pharmacol. 2021, 12, 705623. [Google Scholar] [CrossRef]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hashizume, H.; Hitomi, J.; Shigeno, M.; Sawaguchi, S.; Abe, H.; Ushiki, T. The subfibrillar arrangement of corneal and scleral collagen fibrils as revealed by scanning electron and atomic force microscopy. Arch. Histol. Cytol. 2000, 63, 127–135. [Google Scholar] [CrossRef]
- Liang, W.; Ma, J.X.; Van, L.; Vasini, B.; Karamichos, D. Prolactin-Induced Protein facilitates corneal wound healing. Exp. Eye Res. 2022, 225, 109300. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef]
- Foulsham, W.; Dohlman, T.H.; Mittal, S.K.; Taketani, Y.; Singh, R.B.; Masli, S.; Dana, R. Thrombospondin-1 in ocular surface health and disease. Ocul. Surf. 2019, 17, 374–383. [Google Scholar] [CrossRef]
- Soong, H.K. Vinculin in focal cell-to-substrate attachments of spreading corneal epithelial cells. Arch. Ophthalmol. 1987, 105, 1129–1132. [Google Scholar] [PubMed]
- Ritchey, E.R.; Code, K.; Zelinka, C.P.; Scott, M.A.; Fischer, A.J. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol. Vis. 2011, 17, 2440–2454. [Google Scholar] [PubMed]
- Das, S.K.; Gupta, I.; Cho, Y.K.; Zhang, X.; Uehara, H.; Muddana, S.K.; Bernhisel, A.A.; Archer, B.; Ambati, B.K. Vimentin knockdown decreases corneal opacity. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4030–4040. [Google Scholar]
- Miron-Mendoza, M.; Poole, K.; DiCesare, S.; Nakahara, E.; Bhatt, M.P.; Hulleman, J.D.; Petroll, W.M. The Role of Vimentin in Human Corneal Fibroblast Spreading and Myofibroblast Transformation. Cells 2024, 13, 1094. [Google Scholar] [CrossRef]
- Coelho-Rato, L.S.; Parvanian, S.; Modi, M.K.; Eriksson, J.E. Vimentin at the core of wound healing. Trends Cell Biol. 2024, 34, 239–254. [Google Scholar]
- Uno, K.; Hayashi, H.; Kuroki, M.; Uchida, H.; Yamauchi, Y.; Kuroki, M.; Oshima, K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem. Biophys. Res. Commun. 2004, 315, 928–934. [Google Scholar]
- Radstake, W.E.; Gautam, K.; Miranda, S.; Van Rompay, C.; Vermeesen, R.; Tabury, K.; Verslegers, M.; Dowson, A.; Gorissen, J.; van Loon, J.J.W.A.; et al. Gravitational effects on fibroblasts’ function in relation to wound healing. NPJ Microgravity 2023, 9, 48. [Google Scholar]
- Torricelli, A.A.; Wilson, S.E. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp. Eye Res. 2014, 129, 151–160. [Google Scholar]
- McKay, T.B.; Hutcheon , A.E.; Guo, X.; Zieske, J.D.; Karamichos, D. Modeling the cornea in 3-dimensions: Current and future perspectives. Exp. Eye Res. 2020, 197, 108127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, A.J.; Hefley, B.S.; Strobel, H.A.; Moss, S.M.; Hoying, J.B.; Nicholas, S.E.; Moshayedi, S.; Kim, J.; Karamichos, D. Fabrication of a 3D Corneal Model Using Collagen Bioink and Human Corneal Stromal Cells. J. Funct. Biomater. 2025, 16, 118. https://doi.org/10.3390/jfb16040118
Choi AJ, Hefley BS, Strobel HA, Moss SM, Hoying JB, Nicholas SE, Moshayedi S, Kim J, Karamichos D. Fabrication of a 3D Corneal Model Using Collagen Bioink and Human Corneal Stromal Cells. Journal of Functional Biomaterials. 2025; 16(4):118. https://doi.org/10.3390/jfb16040118
Chicago/Turabian StyleChoi, Alexander J., Brenna S. Hefley, Hannah A. Strobel, Sarah M. Moss, James B. Hoying, Sarah E. Nicholas, Shadi Moshayedi, Jayoung Kim, and Dimitrios Karamichos. 2025. "Fabrication of a 3D Corneal Model Using Collagen Bioink and Human Corneal Stromal Cells" Journal of Functional Biomaterials 16, no. 4: 118. https://doi.org/10.3390/jfb16040118
APA StyleChoi, A. J., Hefley, B. S., Strobel, H. A., Moss, S. M., Hoying, J. B., Nicholas, S. E., Moshayedi, S., Kim, J., & Karamichos, D. (2025). Fabrication of a 3D Corneal Model Using Collagen Bioink and Human Corneal Stromal Cells. Journal of Functional Biomaterials, 16(4), 118. https://doi.org/10.3390/jfb16040118







