From Autologous Bone Tissue to Bioengineered Material Solutions in Post-Traumatic Orbital Wall Reconstruction: An Overview
Abstract
1. Introduction
2. Types of Materials Used for Orbital Reconstruction
2.1. Biological Materials
2.1.1. Autologous Bone
2.1.2. Autologous Cartilage
2.1.3. Allografts
2.1.4. Xenografts
2.2. Alloplastic Materials
2.2.1. Titanium
2.2.2. Biological Ceramics
2.2.3. Polymers
Non-Absorbable Permanent Polymer Implants
Absorbable Polymer Implants
2.2.4. Composite Materials
2.2.5. Analytical Summary—Materials Versus Clinical Outcomes
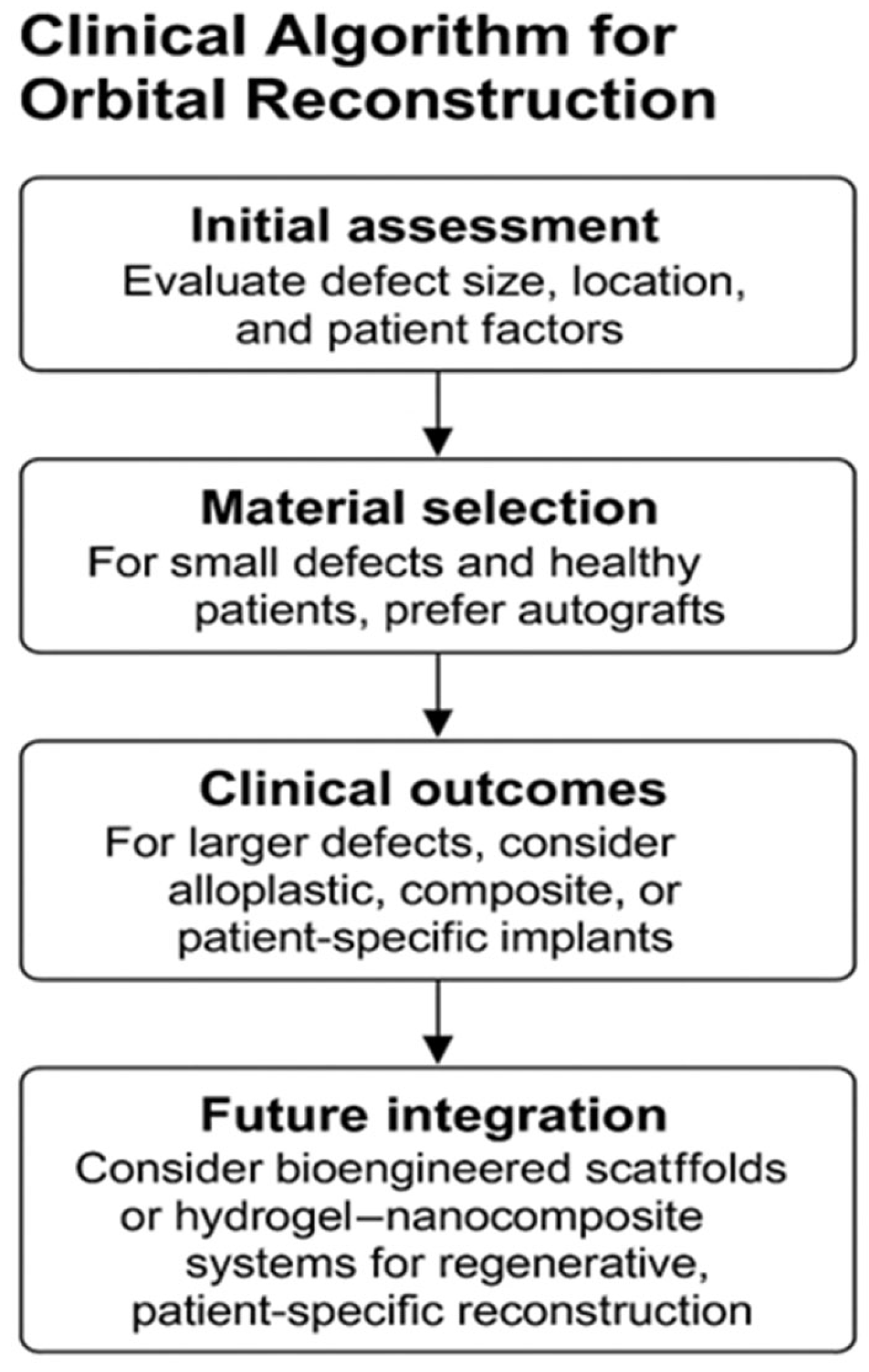
2.3. Patient-Specific Implants
3. Recent Discoveries
4. Surface Modifications of Metallic Biomaterials Using Composite Substances
5. Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, N.V.; Uppuluri, A.; Bhagat, N.; Langer, P.D. Epidemiology of motor vehicle accident-associated ocular trauma. Int. Ophthalmol. 2024, 44, 433. [Google Scholar] [CrossRef] [PubMed]
- Devandan, M.; Daniel, L.; Sanjay, A.B. Prevalence of Ocular Injuries Following Road Traffic Accidents Among Two-Wheeler Riders/Pillions and the Factors Influencing Them Reported in a Tertiary Care Center in Chengalpattu in Tamil Nadu, India. Cureus 2024, 16, e72470. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Kwon, D.Y.; Oleru, O.; Seyidova, N.; Shamamian, P.E.; Montalmant, K.E.; Sarosi, A.; Taub, P.J. The Burden of Road Traffic Accidents on Facial Fractures: National Trends, Injury Patterns, and Disparities in 154,185 Patients. Craniomaxillofac. Trauma Reconstr. 2024, 17, NP182–NP191. [Google Scholar] [CrossRef] [PubMed]
- Homer, N.; Huggins, A. Contemporary management of orbital blowout fractures. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 310–316. [Google Scholar] [CrossRef]
- Vasile, V.A.; Istrate, S.; Iancu, R.C.; Piticescu, R.M.; Cursaru, L.M.; Schmetterer, L.; Garhöfer, G.; Cherecheanu, A.P. Biocompatible Materials for Orbital Wall Reconstruction—An Overview. Materials 2022, 15, 2183. [Google Scholar] [CrossRef]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.J.; Bos, R.R.M.; Becking, A.G. Controversies in orbital reconstruction—III. Biomaterials for orbital reconstruction: A review with clinical recommendations. Int. J. Oral Maxillofac. Surg. 2016, 45, 41–50. [Google Scholar] [CrossRef]
- Kauke-Navarro, M.; Knoedler, L.; Baecher, H.; Sherwani, K.; Knoedler, S.; Allam, O.; Diatta, F.; Alperovich, M.; Safi, A.-F. A systematic review of implant materials for facial reconstructive and aesthetic surgery. Front. Surg. 2025, 12, 1548597. [Google Scholar] [CrossRef]
- Tessier, P.; Woillez, M.; Lekieffre, M.; Assemanr, R. Posttraumatic diplopia and osseous grafts. Observations. Bull. Mem. Soc. Fr. Ophtalmol. 1960, 73, 271–291. [Google Scholar]
- Chowdhury, K.; Krause, G.E. Selection of Materials for Orbital Floor Reconstruction. Arch. Otolaryngol.-Head Neck Surg. 1998, 124, 1398–1401. [Google Scholar] [CrossRef]
- Ilankovan, V.; Jackson, I.T. Experience in the use of calvarial bone grafts in orbital reconstruction. Br. J. Oral Maxillofac. Surg. 1992, 30, 92–96. [Google Scholar] [CrossRef]
- Tessier, P.; Kawamoto, H.; Posnick, J.; Raulo, Y.; Tulasne, J.F.; Wolfe, S.A. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo--tools and techniques: V. A 9650-case experience in craniofacial and maxillofacial surgery. Plast. Reconstr. Surg. 2005, 116 (Suppl. S5), 54S–71S. [Google Scholar] [CrossRef]
- Kontio, R.K.; Laine, P.; Salo, A.; Paukku, P.; Lindqvist, C.; Suuronen, R. Reconstruction of internal orbital wall fracture with iliac crest free bone graft: Clinical, computed tomography, and magnetic resonance imaging follow-up study. Plast. Reconstr. Surg. 2006, 118, 1365–1374. [Google Scholar] [CrossRef]
- Girdler, N.M.; Hosseini, M. Orbital floor reconstruction with autogenous bone harvested from the mandibular lingual cortex. Br. J. Oral Maxillofac. Surg. 1992, 30, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Matsuzawa, Y.; Mori, H.; Matsunaga, K.; Kamiishi, H. Orbital wall reconstruction with bone grafts from the outer cortex of the mandible. J. Cranio-Maxillofac. Surg. 2004, 32, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Reiss, S.; Kulker, D.; Laure, B.; Paré, A. Reconstruction of the orbitozygomatic framework: State of the art and perspectives. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101788. [Google Scholar] [CrossRef]
- Tuan, R.S.; Chen, A.F.; Klatt, B.A. Cartilage regeneration. J. Am. Acad. Orthop. Surg. 2013, 21, 303–311. [Google Scholar] [CrossRef]
- Harish, S. Autogenous Grafts for Orbital Floor Reconstruction: A review. Int. J. Oral Craniofac. Sci. 2017, 3, 046–052. [Google Scholar] [CrossRef]
- Abukhder, M.; Onions, E.; Flaherty, E.; Tarassoli, S.; Hassan, M.R.; Whelan, R. A systematic literature review and narrative synthesis on the use of autologous cartilage in the repair of orbital fractures. Ann. Med. Surg. 2024, 86, 968–974. [Google Scholar] [CrossRef]
- Boyette, J.R.; Pemberton, J.D.; Bonilla-Velez, J. Management of orbital fractures: Challenges and solutions. Clin. Ophthalmol. 2015, 9, 2127–2137. [Google Scholar] [CrossRef]
- Bratton, E.M.; Durairaj, V.D. Orbital implants for fracture repair. Curr. Opin. Ophthalmol. 2011, 22, 400–406. [Google Scholar] [CrossRef]
- Shih, S.; Askinas, C.; Caughey, S.; Vernice, N.; Berri, N.; Dong, X.; Spector, J.A. Sourcing and development of tissue for transplantation in reconstructive surgery: A narrative review. J. Plast. Reconstr. Aesthetic Surg. 2023, 83, 266–275. [Google Scholar] [CrossRef]
- Hammel, J.H.; Zatorski, J.M.; Cook, S.R.; Pompano, R.R.; Munson, J.M. Engineering in vitro immune-competent tissue models for testing and evaluation of therapeutics. Adv. Drug Deliv. Rev. 2022, 182, 114111. [Google Scholar] [CrossRef]
- Swenson, R.W.; Koopmann, C.F. Grafts and implants. Otolaryngol. Clin. N. Am. 1984, 17, 413–428. [Google Scholar] [CrossRef]
- Neigel, J.M.; Ruzicka, P.O. Use of demineralized bone implants in orbital and craniofacial reconstruction and a review of the literature. Ophthalmic Plast. Reconstr. Surg. 1996, 12, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsieh, D.J.; Wu, Y.C.; Yen, K.C.; Srinivasan, P.; Lee, H.C.; Chen, Y.-C.; Lee, S.-S. Reconstruction of the orbital floor using supercritical CO2 decellularized porcine bone graft. Int. J. Med. Sci. 2021, 18, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Biomaterials and implants for orbital floor repair. Acta Biomater. 2011, 7, 3248–3266. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.X.; Sun, X.M.; Zhang, Y.G.; Zhang, Y. Materials to facilitate orbital reconstruction and soft tissue filling in posttraumatic orbital deformaties. Plast. Aesthetic Res. 2016, 3, 86. [Google Scholar] [CrossRef]
- Neumann, A. Biomaterials for craniofacial reconstruction. Laryngorhinootologie 2009, 88 (Suppl. S1), S48–S63. [Google Scholar] [CrossRef]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2021, 5, 371–395. [Google Scholar] [CrossRef]
- Hanawa, T. Biocompatibility of titanium from the viewpoint of its surface. Sci. Technol. Adv. Mater. 2022, 23, 457–472. [Google Scholar] [CrossRef]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L.; Kolarova, H. Treatments for enhancing the biocompatibility of titanium implants. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2020, 164, 23–33. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Kämmerer, P.; Ortolano, L.C.; Sagheb, K.; Seiler, M. Customised products for orbital wall reconstruction: A systematic review. Br. J. Oral Maxillofac. Surg. 2022, 60, e702–e711. [Google Scholar] [CrossRef] [PubMed]
- Canzi, G.; Corradi, F.; Novelli, G.; Bozzetti, A.; Sozzi, D. “6 Anatomical Landmarks” Technique for Satisfactory Free-Hand Orbital Reconstruction With Standard Preformed Titanium Mesh. Craniomaxillofac. Trauma Reconstr. 2022, 15, 51–57. [Google Scholar] [CrossRef]
- Du, R.; Su, Y.X.; Yan, Y.; Choi, W.S.; Yang, W.F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic Approach for Making 3D-Printed Patient-Specific Implants for Craniomaxillofacial Reconstruction. Engineering 2020, 6, 1291–1301. [Google Scholar] [CrossRef]
- Martola, M.; Lindqvist, C.; Hänninen, H.; Al-Sukhun, J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 345–352. [Google Scholar] [CrossRef]
- Katakura, A.; Shibahara, T.; Noma, H.; Yoshinari, M. Material analysis of AO plate fracture cases. J. Oral Maxillofac. Surg. 2004, 62, 348–352. [Google Scholar] [CrossRef]
- Vasile, V.A.; Pirvulescu, R.A.; Iancu, R.C.; Garhöfer, G.; Schmetterer, L.; Ghita, A.M.; Ionescu, D.; Istrate, S.; Piticescu, R.M.; Cursaru, L.M.; et al. Titanium Implants Coated with Hydroxyapatite Used in Orbital Wall Reconstruction—A Literature Review. Materials 2024, 17, 1676. [Google Scholar] [CrossRef]
- Tarsitano, A.; Badiali, G.; Pizzigallo, A.; Marchetti, C. Orbital Reconstruction: Patient-Specific Orbital Floor Reconstruction Using a Mirroring Technique and a Customized Titanium Mesh. J. Craniofac. Surg. 2016, 27, 1822–1825. [Google Scholar] [CrossRef]
- Dvoracek, L.A.; Lee, J.Y.; Unadkat, J.V.; Lee, Y.H.; Thakrar, D.; Losee, J.E.; Goldstein, J.A. Low-Cost, Three-Dimensionally–Printed, Anatomical Models for Optimization of Orbital Wall Reconstruction. Plast. Reconstr. Surg. 2020, 147, 162–166. [Google Scholar] [CrossRef]
- Purnell, C.A.; Vaca, E.E.; Ellis, M.F. Orbital Fracture Reconstruction Using Prebent, Anatomic Titanium Plates: Technical Tips to Avoid Complications. J. Craniofac. Surg. 2018, 29, e515–e517. [Google Scholar] [CrossRef] [PubMed]
- Toivari, M.; Suominen, A.L.; Apajalahti, S.; Lindqvist, C.; Snäll, J.; Thorén, H. Isolated Orbital Fractures Are Severe Among Geriatric Patients. J. Oral Maxillofac. Surg. 2018, 76, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Bettschen, D.; Tsichlaki, D.; Chatzimichail, E.; Klukowska-Rötzler, J.; Müller, M.; Sauter, T.C.; Exadaktylos, A.K.; Ziaka, M.; Doulberis, M.; Burkhard, J.-P. Epidemiology of maxillofacial trauma in elderly patients receiving oral anticoagulant or antithrombotic medication; a Swiss retrospective study. BMC Emerg. Med. 2024, 24, 121. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.K.; Tatum, S.A.; Kellman, R.M. Carbonated Apatite and Hydroxyapatite in Craniofacial Reconstruction. Arch. Fac. Plast. Surg. 2003, 5, 379–383. [Google Scholar] [CrossRef]
- Popa Cherecheanu, A.; Istrate, S.; Iancu, R.; Popescu, M.; Bastian, A.; Ciuluvica, R. Nanostructured hydroxyapatite used as an augmenting material to expand the orbit. Acta Ophthalmol. 2017, 95, 01192. [Google Scholar] [CrossRef]
- Nam, S.B.; Bae, Y.C.; Moon, J.S.; Kang, Y.S. Analysis of the postoperative outcome in 405 cases of orbital fracture using 2 synthetic orbital implants. Ann. Plast. Surg. 2006, 56, 263–267. [Google Scholar] [CrossRef]
- Gradinaru, S.; Popescu, L.M.; Piticescu, R.M.; Zurac, S.; Ciuluvica, R.; Burlacu, A.; Tutuianu, R.; Valsan, S.-N.; Motoc, A.M.; Voinea, L.M. Repair of the orbital wall fractures in rabbit animal model using nanostructured hydroxyapatite-based implant. Nanomaterials 2016, 6, 11. [Google Scholar] [CrossRef]
- Tang, S.; Shen, Y.; Jiang, L.; Zhang, Y. Surface Modification of Nano-Hydroxyapatite/Polymer Composite for Bone Tissue Repair Applications: A Review. Polymers 2024, 16, 1263. [Google Scholar] [CrossRef]
- Schumann, P.; Lindhorst, D.; Wagner, M.E.H.; Schramm, A.; Gellrich, N.C.; Rücker, M. Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology 2013, 80, 211–217. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Dougherty, W.R.; Wellisz, T. The natural history of alloplastic implants in orbital floor reconstruction: An animal model. J. Craniofac. Surg. 1994, 5, 26–32, discussion 33. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.K.; Smith, J.F.; Rozzelle, A.A. Cranio-orbital reconstruction: Safety and image quality of metallic implants on CT and MRI scanning. Plast. Reconstr. Surg. 1994, 94, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Cenzi, R.; Farina, A.; Zuccarino, L.; Carinci, F. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J. Craniofac. Surg. 2005, 16, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.D.; Sanderson, R.C.; Moos, K.F. The use of silastic as an orbital implant for reconstruction of orbital wall defects: Review of 311 cases treated over 20 years. J. Oral Maxillofac. Surg. 1995, 53, 412–417. [Google Scholar] [CrossRef]
- Laxenaire, A.; Lévy, J.; Blanchard, P.; Lerondeau, J.C.; Tesnier, F.; Scheffer, P. Complications of silastic implants used in orbital repair. Rev. Stomatol. Chir. Maxillo-Fac. 1997, 98 (Suppl. S1), 96–99. [Google Scholar]
- Takabayashi, K.; Maeda, Y.; Kataoka, N. Modified procedure for reconstructing the inferomedial orbital wall: Silicone sheet implantation without surgical removal. Eur. Arch. Otorhinolaryngol. 2024, 281, 515–521. [Google Scholar] [CrossRef]
- Velnar, T.; Bunc, G.; Klobučar, R.; Gradisnik, L. Biomaterials and host versus graft response: A short review. Bosn. J. Basic Med. Sci. 2016, 16, 82–90. [Google Scholar] [CrossRef]
- Enislidis, G. Treatment of orbital fractures: The case for treatment with resorbable materials. J. Oral Maxillofac. Surg. 2004, 62, 869–872. [Google Scholar] [CrossRef]
- Eppley, B.L.; Sadove, A.M. Resorbable PLLA–PGA fixation in pediatric craniofacial surgery: Clinical experience in 188 consecutive cases. Plast. Reconstr. Surg. 1997, 100, 1–7. [Google Scholar] [CrossRef]
- Martins, J.A.; Lach, A.A.; Morris, H.L.; Carr, A.J.; Mouthuy, P.A. Polydioxanone implants: A systematic review on safety and performance in patients. J. Biomater. Appl. 2019, 34, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Gosau, M.; Schöneich, M.; Draenert, F.G.; Ettl, T.; Driemel, O.; Reichert, T.E. Retrospective analysis of orbital floor fractures--complications, outcome, and review of literature. Clin. Oral Investig. 2011, 15, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.; Fan, K.; Brokmeier, J.; Thieringer, F.M.; Berg, B.I. Orbital Floor Fractures: Treatment and Diagnostics—A Survey Among Swiss, German and Austrian Maxillofacial Units. Craniomaxillofac. Trauma Reconstr. 2024, 14, NP60–NP67. [Google Scholar] [CrossRef] [PubMed]
- Bourry, M.; Hardouin, J.B.; Fauvel, F.; Corre, P.; Lebranchu, P.; Bertin, H. Clinical evaluation of the efficacy of materials used for primary reconstruction of orbital floor defects: Meta-analysis. Head Neck 2021, 43, 679–690. [Google Scholar] [CrossRef]
- Zhang, L.; Fay, A. Composite implants in oculoplastic surgery. Semin. Ophthalmol. 2010, 25, 303–308. [Google Scholar] [CrossRef]
- Kim, J.; Kang, D. Absorbable Versus Non-absorbable Implants for Orbital Fracture Repair: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. 2025; Epub ahead of print. [Google Scholar]
- Maher, D.I.; Hall, A.J.; Gwini, S.; Ben Artsi, E. Patient-specific Implants for Orbital Fractures: A Systematic Review. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 417–424. [Google Scholar] [CrossRef]
- Taxis, J.; Ungerboeck, L.; Motel, C.; Eckert, A.W.; Platz Batista da Silva, N.; Nieberle, F.; Ludwig, N.; Meier, J.K.; Ettl, T.; Reichert, T.E.; et al. Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes. Tomography 2023, 9, 1515–1525. [Google Scholar] [CrossRef]
- Dubron, K.; Verbist, M.; Jacobs, R.; Olszewski, R.; Shaheen, E.; Willaert, R. Augmented and Virtual Reality for Preoperative Trauma Planning, Focusing on Orbital Reconstructions: A Systematic Review. J. Clin. Med. 2023, 12, 5203. [Google Scholar] [CrossRef]
- Raisian, S.; Fallahi, H.R.; Khiabani, K.S.; Heidarizadeh, M.; Azdoo, S. Customized Titanium Mesh Based on the 3D Printed Model vs. Manual Intraoperative Bending of Titanium Mesh for Reconstructing of Orbital Bone Fracture: A Randomized Clinical Trial. Rev. Recent Clin. Trials 2017, 12, 154–158. [Google Scholar] [CrossRef]
- Strong, E.B.; Fuller, S.C.; Wiley, D.F.; Zumbansen, J.; Wilson, M.D.; Metzger, M.C. Preformed vs intraoperative bending of titanium mesh for orbital reconstruction. Otolaryngol. Head Neck Surg. 2013, 149, 60–66. [Google Scholar] [CrossRef]
- Singh, A.K.; Khanal, N.; Chaulagain, R.; Sharma, N.; Thieringer, F.M. Is the Pre-Shaping of an Orbital Implant on a Patient-Specific 3D-Printed Model Advantageous Compared to Conventional Free-Hand Shaping? A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3426. [Google Scholar] [CrossRef]
- Kotecha, S.; Ferro, A.; Harrison, P.; Fan, K. Orbital reconstruction: A systematic review and meta-analysis evaluating the role of patient-specific implants. Oral Maxillofac. Surg. 2022, 27, 213–226. [Google Scholar] [CrossRef]
- Sharaf, B.; Leon, D.E.; Wagner, L.; Morris, J.M.; Salinas, C.A. Virtual Planning and 3D Printing in the Management of Acute Orbital Fractures and Post-Traumatic Deformities. Semin. Plast. Surg. 2022, 36, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current biomaterial-based bone tissue engineering and translational medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dai, W.; Gupta, A.; Zhang, B.; Wu, Z.; Zhang, Y.; Pan, L.; Wang, L. Frontiers of Hydroxyapatite Composites in Bionic Bone Tissue Engineering. Materials 2022, 15, 8475. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, L.; Wu, T. Recent Advances in Bioengineering Bone Revascularization Based on Composite Materials Comprising Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 12492. [Google Scholar] [CrossRef]
- Zhang, F.F.; Hao, Y.; Zhang, K.X.; Yang, J.J.; Zhao, Z.Q.; Liu, H.J.; Li, J.-T. Interplay between mesenchymal stem cells and macrophages: Promoting bone tissue repair. World J. Stem Cells 2024, 16, 375–388. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomaterials Science and Engineering. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Haider, H.; Kurtz, S.; Marcolongo, M.; Urish, K. New materials for hip and knee joint replacement: What’s hip and what’s in kneed? J. Orthop. Res. 2020, 38, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Cai, Z.; Du, P.; Li, K.; Chen, L.; Xie, G. A Review of the Development of Titanium-Based and Magnesium-Based Metallic Glasses in the Field of Biomedical Materials. Materials 2024, 17, 4587. [Google Scholar] [CrossRef]
- Long, S.; Zhu, J.; Jing, Y.; He, S.; Cheng, L.; Shi, Z. A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants. Coatings 2023, 13, 1917. [Google Scholar] [CrossRef]
- Vasile, V.A.; Istrate, S.; Cursaru, L.M.; Piticescu, R.M.; Ghita, A.M.; Popescu, D.M.; Garhöfer, G.; Catrina, A.M.; Spandole-Dinu, S.; Haidoiu, C.; et al. A New Approach for Orbital Wall Reconstruction in a Rabbit Animal Model Using a Hybrid Hydroxyapatite–Collagen-Based Implant. Int. J. Mol. Sci. 2024, 25, 12712. [Google Scholar] [CrossRef]
- Hossain, I.; Chattopadhyay, P.K.; Perumal, S.J. Abbreviated Key Title: Saudi Journal Oral Dental Research ISSN. Saudi J. Oral Dent. Res. 2021, 6, 581–591. [Google Scholar]
- Xia, Y.; Chen, Z.; Zheng, Z.; Chen, H.; Chen, Y. Nanomaterial-integrated injectable hydrogels for craniofacial bone reconstruction. J. Nanobiotechnol. 2024, 22, 525. [Google Scholar] [CrossRef]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 24, 529. [Google Scholar] [CrossRef]
- Kumar, M.S.; Varma, P.; Kandasubramanian, B. From lab to life: Advances in in-situ bioprinting and bioink technology. Biomed. Mater. 2024, 20, 012004. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Fernandes, M.; Vieira, M.; Peixoto, D.; Alves, N.M. Nature-Based Hydrogels Combined with Nanoparticles for Bone Regeneration. J. Funct. Biomater. 2025, 16, 317. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.-J.; Kotula, A.P.; Takagi, S.; Chow, L.; Alimperti, S. Engineering 3D Printed Scaffolds with Tunable Hydroxyapatite. J. Funct. Biomater. 2022, 13, 34. [Google Scholar] [CrossRef]
- Samandari, M.; Mostafavi, A.; Quint, J.; Memić, A.; Tamayol, A. In situ bioprinting: Intraoperative implementation of regenerative medicine. Trends Biotechnol. 2022, 40, 1229–1247. [Google Scholar] [CrossRef]
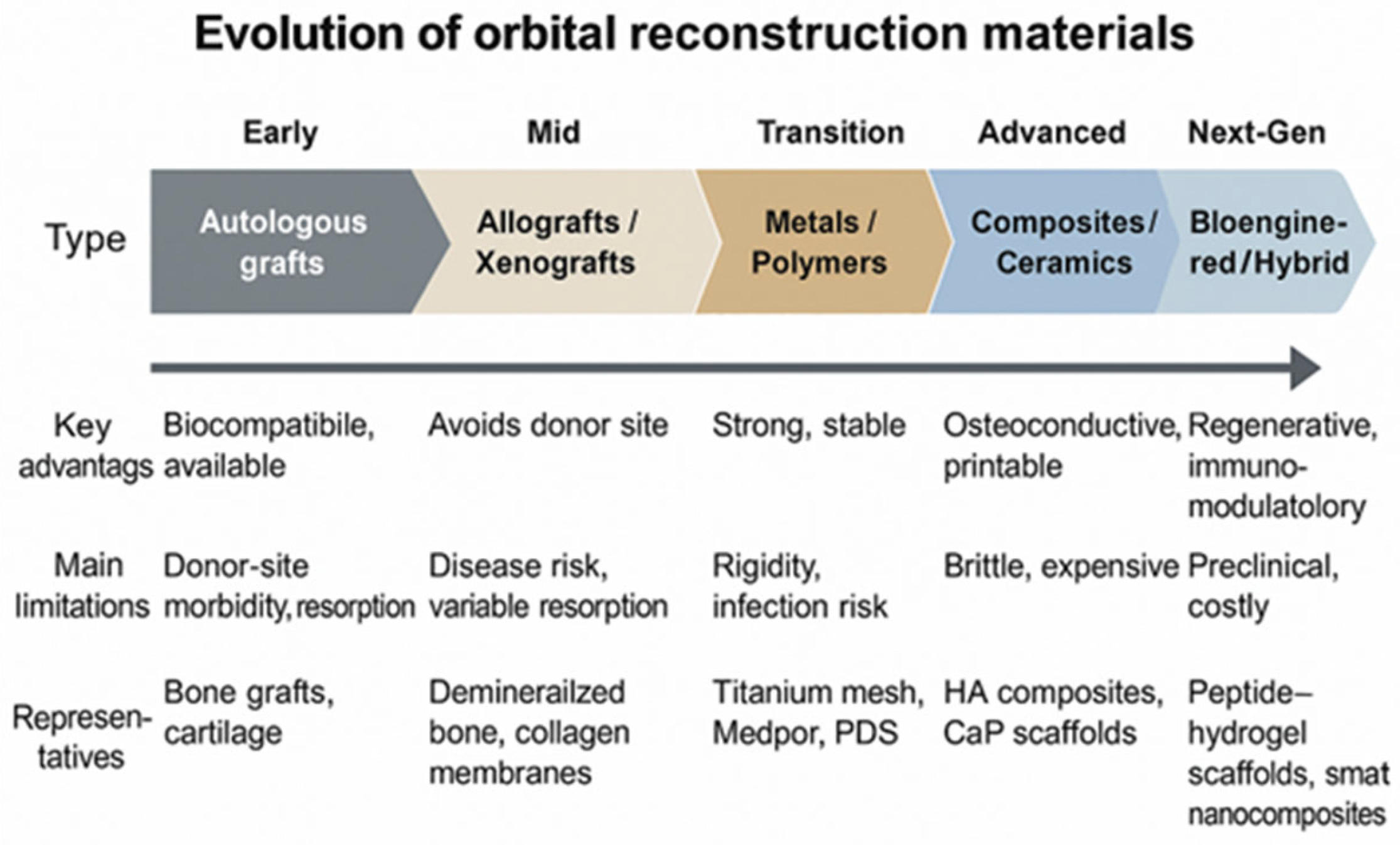
| Material Category | Representative Examples | Main Advantages | Principal Limitations | Clinical Remarks |
|---|---|---|---|---|
| Autologous tissues | Calvarial bone, iliac crest, rib, nasal cartilage | Excellent biocompatibility; osteoconductive; low infection rate; readily available | Donor-site morbidity; limited quantity; unpredictable resorption | Best suited for small to medium defects; declining use with advent of alloplasts |
| Allografts/Xenografts | Lyophilized dura, demineralized bone matrix, porcine collagen | Avoids donor site surgery; biological integration possible | Risk of disease transmission; immune reaction; variable resorption | Use restricted; limited long-term data |
| Metals | Titanium mesh, titanium plates | High mechanical strength; stable volume; radiopaque; 3D-printing feasible | Rigid; possible cold conduction; expensive; may require removal | Widely used for large or complex defects; custom meshes available |
| Polymers (non-resorbable) | Porous polyethylene (Medpor), silicone, PTFE | Easily shaped; promotes fibrovascular ingrowth; good stability | Risk of late infection or extrusion; non-resorbable | Common for medium defects; careful asepsis required |
| Polymers (resorbable) | PLLA, PDS, PGA, copolymers | Eliminates second surgery; avoids growth restriction in children | Limited strength; unpredictable degradation; local inflammation possible | Preferred in pediatric or small orbital defects |
| Composites/Bioactive ceramics | HA-polymer composites, calcium phosphate ceramics | Osteoconductive; good stability; customizable; radiopaque | Brittle; expensive; limited clinical validation | Promising in hybrid 3D-printed and scaffold-based reconstructions |
| Bioactive Peptide | Function |
|---|---|
| PepGen P-15 | Promoted: extracellular matrix production; proliferation and osteogenic differentiation; cell attachment, migration, and survival |
| Arginine-glycine-aspartic acid (RGD) | Promoted: proliferation, mineralization, and osteogenic differentiation; cell attachment and survival |
| Ser-Val—Val-Tyr-Gly-Leu-Arg (SVVYGLR) | Promoted: proliferation and neovascularization; angiogenesis and osteogenesis; adhesion; migration; tube formation of endothelial cells |
| Gly-Phel-Hydroxy-proline-arginine (GFGOER) | Promoted: differentiation, bone regeneration, osseointegration |
| Collagen binding motif (CBM) | Promoted: bone-related cell adhesion and growth; osteogenic differentiation |
| Fibronectin-derived peptides (FN-derived peptides) | Promoted: bone-related cell spreading; adhesion and mineralization |
| P17-BMP-2 | Promoted: bone repair; osteoblast differentiation and bone regeneration |
| P20-BMP-2 and P 24- BMP-2 | Promoted: osteogenesis and differentiation of MSCs into osteoblasts |
| BMP-7 derived BFP-1 | Enhanced: Ca2+ content in cells, ALP activity, bone regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, O.; Garhoefer, G.; Ionescu, D.; Ionescu, T.; Istrate, S.; Popa-Cherecheanu, A.; Mincă, D.G. From Autologous Bone Tissue to Bioengineered Material Solutions in Post-Traumatic Orbital Wall Reconstruction: An Overview. J. Funct. Biomater. 2025, 16, 430. https://doi.org/10.3390/jfb16120430
Lazăr O, Garhoefer G, Ionescu D, Ionescu T, Istrate S, Popa-Cherecheanu A, Mincă DG. From Autologous Bone Tissue to Bioengineered Material Solutions in Post-Traumatic Orbital Wall Reconstruction: An Overview. Journal of Functional Biomaterials. 2025; 16(12):430. https://doi.org/10.3390/jfb16120430
Chicago/Turabian StyleLazăr, Ovidiu, Gerhard Garhoefer, Diana Ionescu, Tudor Ionescu, Sînziana Istrate, Alina Popa-Cherecheanu, and Dana Galieta Mincă. 2025. "From Autologous Bone Tissue to Bioengineered Material Solutions in Post-Traumatic Orbital Wall Reconstruction: An Overview" Journal of Functional Biomaterials 16, no. 12: 430. https://doi.org/10.3390/jfb16120430
APA StyleLazăr, O., Garhoefer, G., Ionescu, D., Ionescu, T., Istrate, S., Popa-Cherecheanu, A., & Mincă, D. G. (2025). From Autologous Bone Tissue to Bioengineered Material Solutions in Post-Traumatic Orbital Wall Reconstruction: An Overview. Journal of Functional Biomaterials, 16(12), 430. https://doi.org/10.3390/jfb16120430







