Comparative Evaluation of Hyaluronic Acid (hyaDENT BG® Gel) and Enamel Matrix Proteins (Emdogain®) in the Regenerative Treatment of Angular Bone Defects Using Xenograft (Bio-Oss Collagen®)—A Clinical Trial
Abstract
1. Introduction
1.1. Bone Substitutes and Bone Filling
1.2. Barrier Membranes
1.3. Biomolecules
2. Materials and Methods
2.1. Study Population
- Adults over the age of 18 diagnosed with periodontal disease—Stage III or IV periodontitis;
- Sites with bone defects and PPD ≥ 6 mm, accompanied by bleeding on probing (BoP) at re-evaluation, conducted six weeks after non-surgical periodontal therapy;
- Full Mouth Plaque Score (FMPS) < 20% and Full Mouth Bleeding Score (FMBS) < 15% prior to surgical treatment;
- Good general health with no systemic conditions and no known allergies to materials or medications used in the study.
- Proximal angular bone defects;
- Two-wall or three-wall bone defects;
- At least one defect with PPD ≥ 6 mm, CAL ≥ 5 mm, and an infraosseous component of the defect measuring ≥ 3 mm;
- Vital teeth or teeth with adequately performed endodontic treatment.
- Medical conditions contraindicating surgical intervention;
- Pregnancy or lactation;
- Heavy smokers;
- Untreated periodontal disease;
- Poor oral hygiene;
- Acute infectious lesions in the area of intervention;
- Teeth with increased mobility (Grade II or III);
- Restorations or carious lesions on root surfaces associated with the bone defect.
2.2. Clinical Measurements
2.3. Surgical Procedure
2.4. Radiographic Measurements
2.5. Statistical Analysis
- Descriptive Analysis: This analysis included estimates of central tendency (such as mean, median) and dispersion (including standard deviation, range) to provide a comprehensive overview of the data characteristics.
- Distribution Normality Assessment: The Shapiro–Wilk non-parametric test was applied to evaluate the distribution type of the data. Given the small sample size, a normal distribution was not expected.
- Comparative Analysis for Independent Samples: The Mann–Whitney non-parametric test was applied to test for statistically significant differences between the two treatment groups. This test was specifically chosen due to the non-normal distribution of the data, as identified by the Shapiro–Wilk test.
- Comparative Analysis for Related Samples: The Wilcoxon signed-rank test was used to comparison of clinical parameters within the groups of patients with the same therapy, comparing their status prior to intervention and following the treatment. This test was specifically chosen due to the non-normal distribution of the data, as identified by the Shapiro–Wilk test.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMD | enamel matrix derivatives |
| PGA | propylene glycol alginate |
| HA | hyaluronic acid |
| PPD | probing pocket depth |
| BoP | bleeding on probing |
| FMPS | Full Mouth Plaque Score |
| FMBS | Full Mouth Bleeding Score |
| CAL | clinical attachment loss |
| F | furcation involvement |
| M | tooth mobility |
| GR | gingival recession |
| R | radiographic analysis |
| BF | bone filling |
| CBCT | cone- beam computed tomography |
| OFD | open- flap debridement |
References
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Darveau RP, Curtis MA, editors. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Jin, L.; Lamster, I.; Greenspan, J.; Pitts, N.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal Regeneration—Intrabony Defects: A Consensus Report From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Biologics-based regenerative technologies for periodontal soft tissue engineering. J. Periodontol. 2020, 91, 147–154. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Mortellaro, C.; Fabbro, M.D. Tissue Engineering: Use of Growth Factors in Bone Regeneration. In Biomaterials in Regenerative Medicine; Dobrzański, L.A., Ed.; InTech: Suginami City, Tokyo, 2018; Available online: http://www.intechopen.com/books/biomaterials-in-regenerative-medicine/tissue-engineering-use-of-growth-factors-in-bone-regeneration (accessed on 27 October 2025).
- Lin, Z.; Rios, H.F.; Cochran, D.L. Emerging Regenerative Approaches for Periodontal Reconstruction: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S134–S152. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Nemcovsky, C.E.; Beitlitum, I. Combination Therapy for Reconstructive Periodontal Treatment in the Lower Anterior Area: Clinical Evaluation of a Case Series. Dent. J. 2018, 6, 50. [Google Scholar] [CrossRef]
- Ausenda, F.; Rasperini, G.; Acunzo, R.; Gorbunkova, A.; Pagni, G. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials 2019, 12, 2197. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Hjørting-Hansen, E.; Buser, D. The fate of the autogenous bone graft. Forum Implant. 2009, 5, 4–11. [Google Scholar]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Mancini, L.; Fratini, A.; Marchetti, E. Periodontal Regeneration. Encyclopedia 2021, 1, 87–98. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qu, T.; Zhang, X.; Yu, Q.; Chen, F. Designing biomaterials for in situ periodontal tissue regeneration. Biotechnol. Prog. 2012, 28, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Yao, Y.; Giannobile, W.V.; Wang, H.L. Current and future trends in periodontal tissue engineering and bone regeneration. Plast. Aesthet. Res. 2021, 8, 3. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Somerman, M.J. Growth and Amelogenin-Like Factors in Periodontal Wound Healing. A Systematic Review. Ann. Periodontol. 2003, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel Matrix Derivative: A Review of Cellular Effects In Vitro and a Model of Molecular Arrangement and Functioning. Tissue Eng. Part B Rev. 2012, 18, 181–202. [Google Scholar] [CrossRef]
- Darby, I.B.; Morris, K.H. A Systematic Review of the Use of Growth Factors in Human Periodontal Regeneration. J. Periodontol. 2013, 84, 465–476. [Google Scholar] [CrossRef]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 320–351. [Google Scholar] [CrossRef]
- Fernandes, F.; Peixoto, D.; Correia, C.; Silva, M.; Paiva, M.C.; Alves, N.M. Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration. Nanomaterials 2025, 15, 276. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohora, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 52, 308–316. [Google Scholar] [PubMed]
- Asparuhova, M.B.; Chappuis, V.; Stähli, A.; Buser, D.; Sculean, A. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin. Oral Investig. 2020, 24, 3923–3937. [Google Scholar] [CrossRef]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar]
- De Brito Bezerra, B.; Mendes Brazão, M.A.; De Campos, M.L.G.; Casati, M.Z.; Sallum, E.A.; Sallum, A.W. Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects: Hyaluronic acid effects on bone healing. Clin. Oral Implant. Res. 2012, 23, 938–942. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.; Chang, J.; Kho, H. Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral Dis. 2011, 17, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Leanza, Y.; Belmonte, A.; Grippaudo, C.; Leonardi, R.; Isola, G. Impact of Hyaluronic Acid and Other Re-Epithelializing Agents in Periodontal Regeneration: A Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 12347. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef]
- Rodríguez-A, M.; Montiel-Company, J.M.; Alpiste-Illueca, F.; Rodríguez-A, L.; Paredes-Gallardo, V.; López-Roldán, A. Comparision of crosslinked hyaluronic acid vs. enamel matrix derivative for periodontal regeneration: An 18-month follow-up randomized clinical trial. Clin. Oral Investig. 2025, 29, 197. [Google Scholar] [CrossRef]
- Covani, U.; Giammarinaro, E.; Panetta, D.; Salvadori, P.A.; Cosola, S.; Marconcini, S. Alveolar Bone Remodeling with or without Collagen Filling of the Extraction Socket: A High-Resolution X-ray Tomography Animal Study. J. Clin. Med. 2022, 11, 2493. [Google Scholar] [CrossRef]
- Nocini, P.F.; Menchini Fabris, G.B.; Gelpi, F.; Lotti, J.; Favero, V.; Zanotti, G.; Jurlaro, A.; Rosskopf, I.; Lotti, T.; Barone, A.; et al. Treatment of skin defects with growth factors and biodegradable collagen carrier: Histological evaluation in animal model. J. Biol. Regul. Homeost. Agents. 2017, 31 (Suppl. 2), 1–13. [Google Scholar]
- Miron, R.J.; Shirakata, Y.; Ahmad, P.; Romandini, M.; Estrin, N.E.; Farshidfar, N.; Bosshardt, D.D.; Sculean, A. 30 years of enamel matrix derivative: Mimicking tooth development as a clinical concept. Periodontology 2000 2025, 1–19. [Google Scholar] [CrossRef]
- Pilloni, A.; Shirakata, Y.; Marini, L.; Božić, D.; Miron, R.J.; Rotundo, R.; Stavropoulos, A.; Sculean, A. Hyaluronic acid: A novel approach in regenerative/reconstructive periodontal therapy? Periodontology 2000 2025, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ostos-Aguilar, B.I.; Pinheiro Furquim, C.; Muniz, F.W.M.G.; Faveri, M.; Meza-Mauricio, J. Clinical efficacy of hyaluronic acid in the treatment of periodontal intrabony defect: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Mamajiwala, A.S.; Sethi, K.S.; Raut, C.P.; Karde, P.A.; Mamajiwala, B.S. Clinical and radiographic evaluation of 0.8% hyaluronic acid as an adjunct to open flap debridement in the treatment of periodontal intrabony defects: Randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5257–5271. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D.; Laugisch, O.; Katsaros, C.; Buser, D.; Sculean, A. Enamel Matrix Protein Adsorption to Root Surfaces in the Presence or Absence of Human Blood. J. Periodontol. 2012, 83, 885–892. [Google Scholar] [CrossRef]
- Vela, O.C.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Ramaglia, L.; Boia, S.; Radulescu, V.; Ilyes, I.; Stratul, S.-I. Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina 2024, 60, 829. [Google Scholar] [CrossRef] [PubMed]
- Abdel Nasser Atia, G.; Shalaby, H.K.; Zehravi, M.; Ghobashy, M.M.; Ahmad, Z.; Khan, F.S.; Dey, A.; Rahman, H.; Joo, S.W.; Barai, H.R.; et al. Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review. Polymers 2022, 14, 2964. [Google Scholar] [CrossRef] [PubMed]
| Parameter (mm) | hyaDENT BG | Emdogain | p-Value |
|---|---|---|---|
| Average (n = 13) | Average (n = 13) | ||
| PPD1 | 7.23 (95%CI [6.62; 7.84]) | 7.23 (95%CI [6.48; 7.98]) | 0.920 |
| CAL1 | 7.69 (95%CI [7.18; 8.21]) | 7.15 (95%CI [5.90; 8.41]) | 0.362 |
| GR1 | 0.46 (95%CI [0.15; 0.78]) | 0.38 (95%CI [0.00; 0.91]) | 0.418 |
| R1 | 5.08 (95%CI [4.56; 5.60]) | 4.62 (95%CI [3.67; 5.56]) | 0.264 |
| Parameter | hyaDENT BG | Emdogain | p-Value |
|---|---|---|---|
| Average (n = 13) | Average (n = 13) | ||
| Operative depth (mm) | 4.54 (95%CI [4.01; 5.07]) | 4.31 (95%CI [3.55; 5.06]) | 0.479 |
| Defect angle (°) | 36.00 (95%CI [32.53; 39.47]) | 37.15 (95%CI [33.28; 41.03]) | 0.801 |
| hyaDENT BG Parameter (mm) | Before | After | p-Value |
|---|---|---|---|
| Average (n = 14) | Average (n = 14) | ||
| PPD | 7.29 (95% CI [6.71; 7.86]) | 3.07 (95% CI [2.59; 3.55]) | 0.001 ** |
| CAL | 7.50 (95% CI [6.87; 8.13]) | 3.14 (95% CI [2.70; 3.59]) | 0.001 ** |
| R | 5.00 (95% CI [4.49; 5.51]) | 0.57 (95% CI [0.03; 1.11]) | 0.001 ** |
| Emdogain Parameter (mm) | Before | After | p-Value |
|---|---|---|---|
| Average (n = 14) | Average (n = 14) | ||
| PPD | 7.43 (95% CI [6.62; 8.24]) | 2.50 (95% CI [2.01; 2.99]) | 0.001 ** |
| CAL | 7.36 (95% CI [6.12; 8.59]) | 2.79 (95% CI [2.22; 23.35]) | 0.001 ** |
| R | 4.71 (95% CI [3.82; 5.60]) | 0.14 (95% CI [0.00; 0.35]) | 0.001 ** |
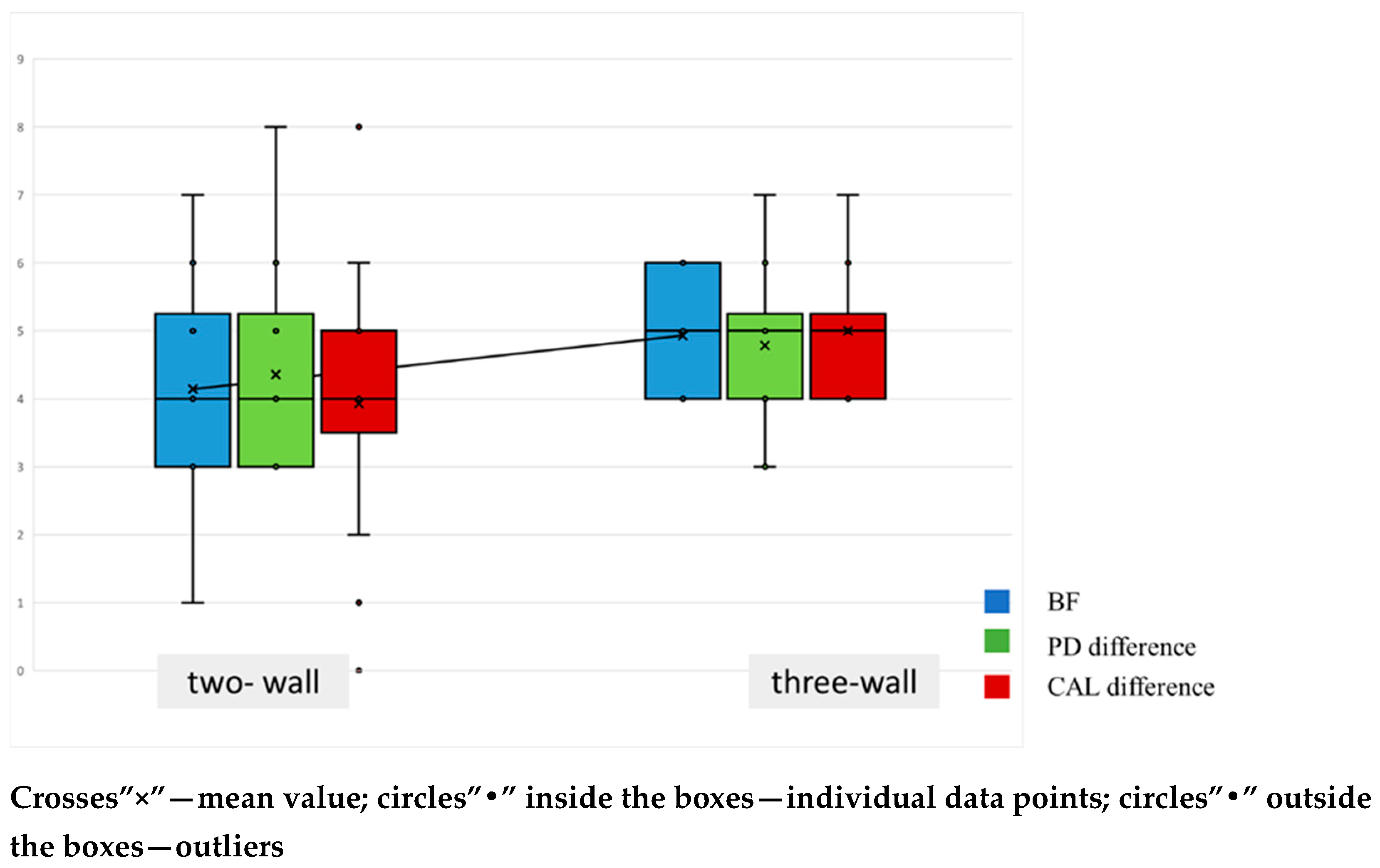
| Parameter (mm) | hyaDENT BG | Emdogain | p-Value |
|---|---|---|---|
| Average (n = 13) | Average (n = 13) | ||
| PPD2 | 2.92 (95% CI [2.54; 3.31]) | 2.54 (95% CI [2.01; 3.07]) | 0.243 |
| CAL2 | 3.00 (95% CI [2.65; 3.35]) | 2.85 (95% CI [2.25; 3.44]) | 0.762 |
| GR2 | 0.31 (95% CI [0.02; 0.60]) | 0.31 (95% CI [0.02; 0.60]) | - |
| R2 | 0.38 (95% CI [0.00; 0.78]) | 0.15 (95% CI [0.00; 0.38]) | 0.479 |
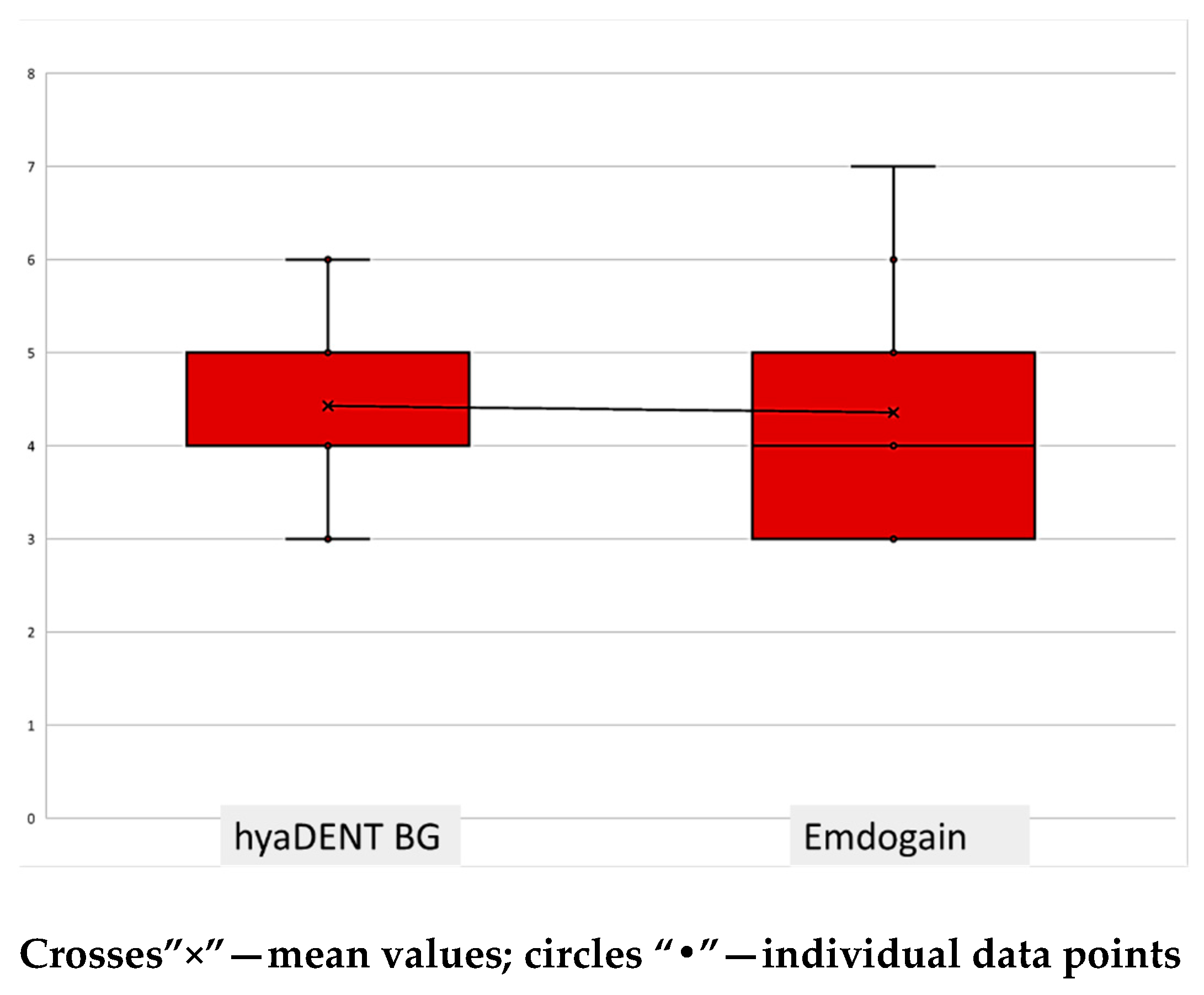
| BF | 4.69 (95% CI [4.24; 5.15]) | 4.54 (95% CI [3.70; 5.38]) | 0.650 |
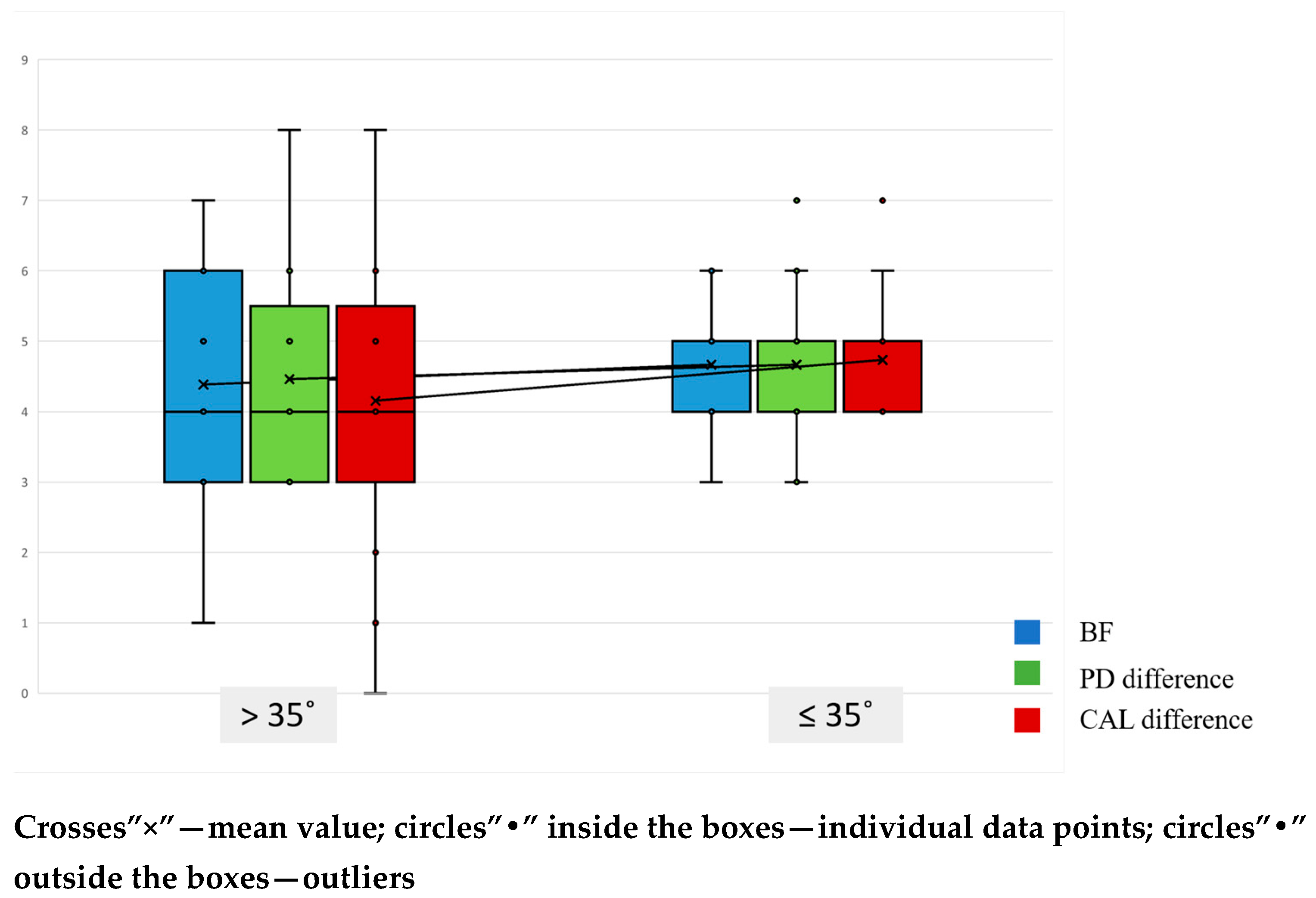
| Parameter | ≤35° | >35° | p-Value |
|---|---|---|---|
| Average (n = 14) | Average (n = 14) | ||
| PPD2 (mm) | 2.53 (95% CI [2.07; 3.00]) | 3.08 (95% CI [2.56; 3.60]) | 0.142 |
| CAL2 (mm) | 2.80 (95% CI [2.28; 3.32]) | 3.15 (95% CI [2.67; 3.64]) | 0.413 |
| BF (mm) | 4.67 (95% CI [4.17; 5.16]) | 4.38 (95% CI [3.38; 5.39]) | 0.683 |
| R2 (mm) | 0.27 (95% CI [0.00; 0.60]) | 0.46 (95% CI [0.00; 0.99]) | 0.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosseva-Panova, V.; Maynalovska, H.; Mlachkova, A.; Tosheva, E.; Ivanov, I.; Pashova-Tasseva, Z. Comparative Evaluation of Hyaluronic Acid (hyaDENT BG® Gel) and Enamel Matrix Proteins (Emdogain®) in the Regenerative Treatment of Angular Bone Defects Using Xenograft (Bio-Oss Collagen®)—A Clinical Trial. J. Funct. Biomater. 2025, 16, 431. https://doi.org/10.3390/jfb16120431
Dosseva-Panova V, Maynalovska H, Mlachkova A, Tosheva E, Ivanov I, Pashova-Tasseva Z. Comparative Evaluation of Hyaluronic Acid (hyaDENT BG® Gel) and Enamel Matrix Proteins (Emdogain®) in the Regenerative Treatment of Angular Bone Defects Using Xenograft (Bio-Oss Collagen®)—A Clinical Trial. Journal of Functional Biomaterials. 2025; 16(12):431. https://doi.org/10.3390/jfb16120431
Chicago/Turabian StyleDosseva-Panova, Velitchka, Hristina Maynalovska, Antoaneta Mlachkova, Ekaterina Tosheva, Ivan Ivanov, and Zdravka Pashova-Tasseva. 2025. "Comparative Evaluation of Hyaluronic Acid (hyaDENT BG® Gel) and Enamel Matrix Proteins (Emdogain®) in the Regenerative Treatment of Angular Bone Defects Using Xenograft (Bio-Oss Collagen®)—A Clinical Trial" Journal of Functional Biomaterials 16, no. 12: 431. https://doi.org/10.3390/jfb16120431
APA StyleDosseva-Panova, V., Maynalovska, H., Mlachkova, A., Tosheva, E., Ivanov, I., & Pashova-Tasseva, Z. (2025). Comparative Evaluation of Hyaluronic Acid (hyaDENT BG® Gel) and Enamel Matrix Proteins (Emdogain®) in the Regenerative Treatment of Angular Bone Defects Using Xenograft (Bio-Oss Collagen®)—A Clinical Trial. Journal of Functional Biomaterials, 16(12), 431. https://doi.org/10.3390/jfb16120431








