Outcomes Following Iliac Vein Stenting for Non-Thrombotic Iliac Vein Lesions—A Narrative Review Based on Large Sample Studies
Abstract
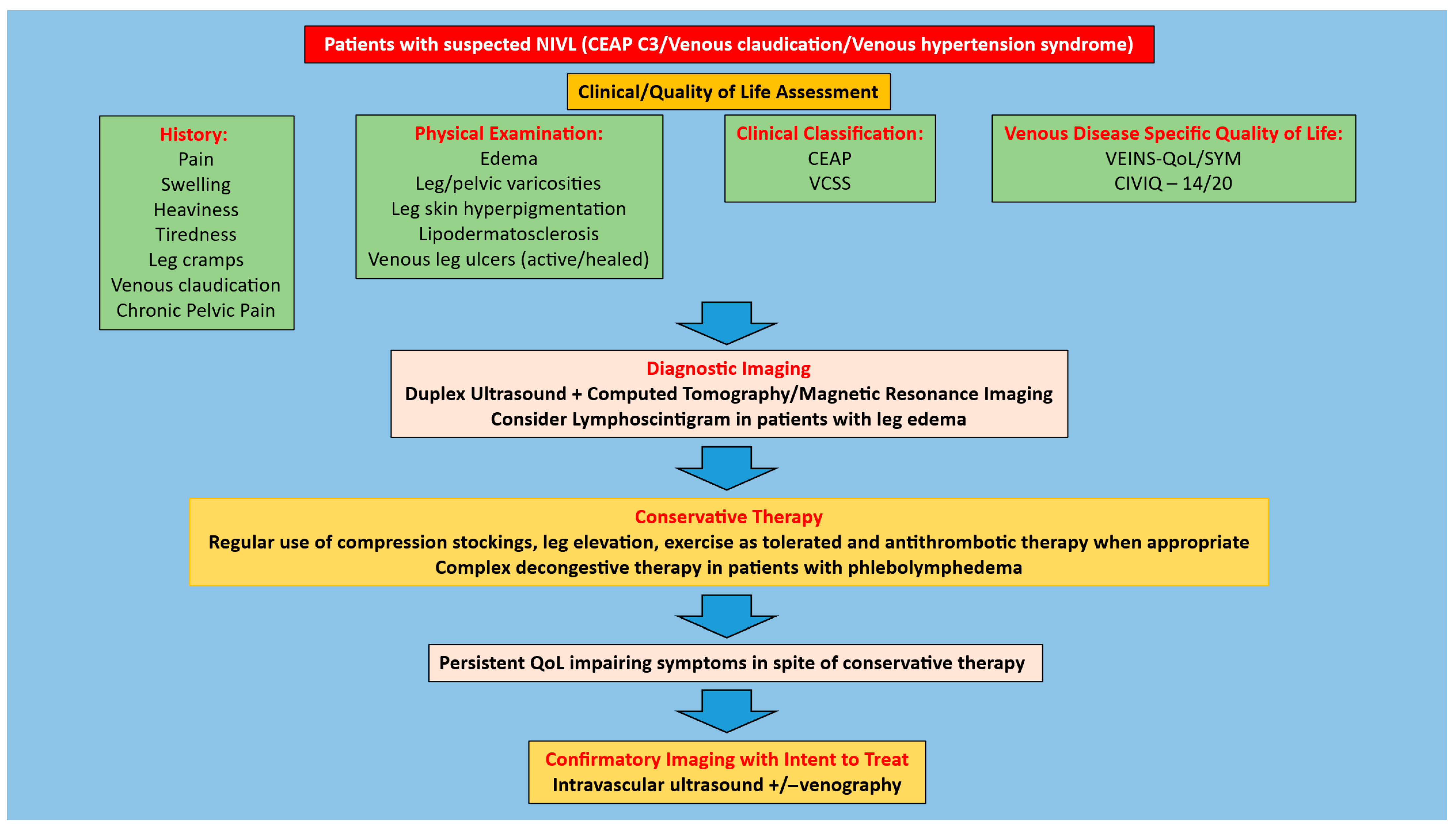
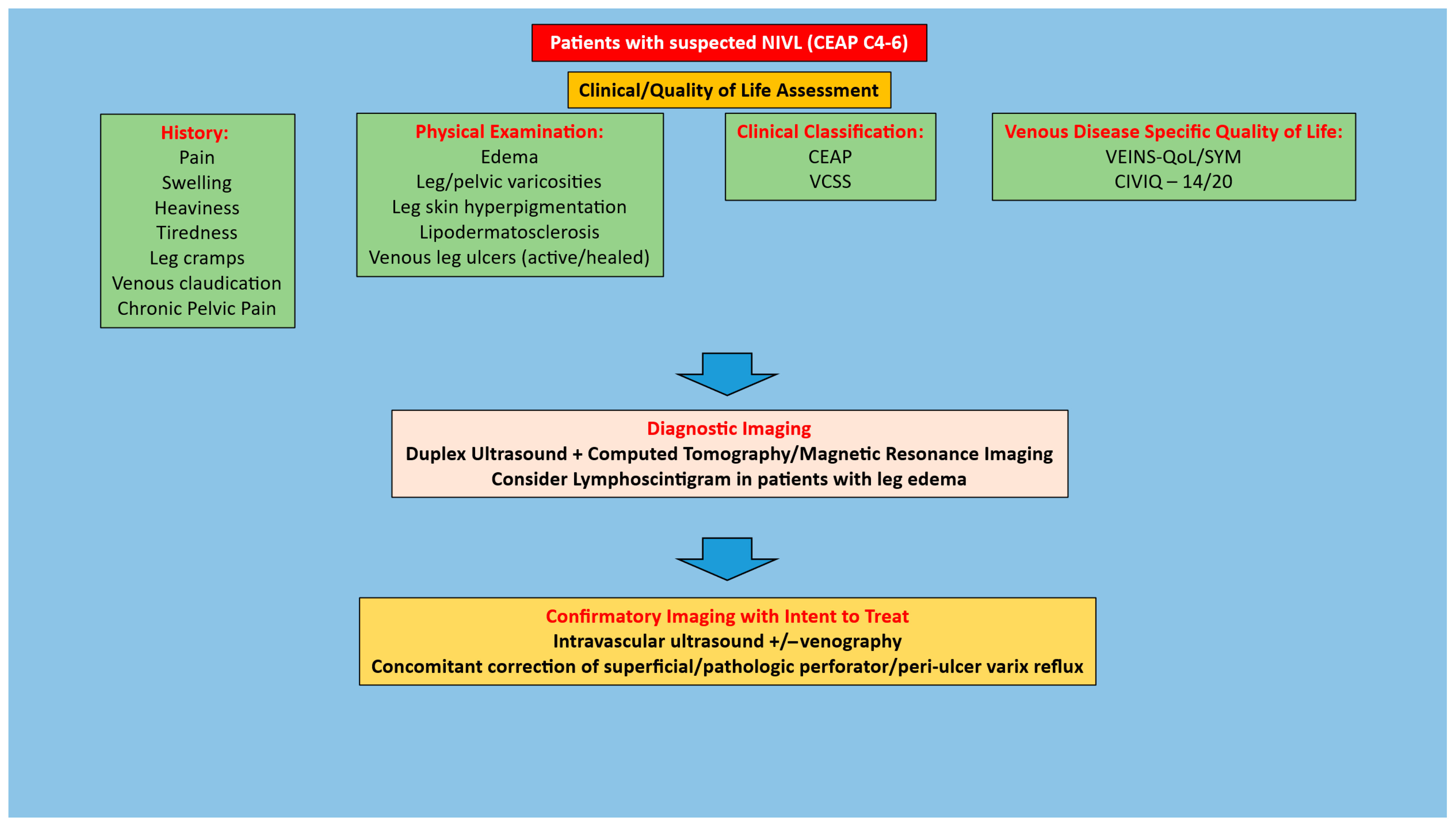
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
3. Results
3.1. Clinical Outcomes
3.1.1. Grade of Swelling
3.1.2. Visual Analog Scale Pain Score
3.1.3. Combined Pain and Swelling
3.1.4. Ulcer Healing
3.1.5. Venous Clinical Severity Score (VCSS)
3.2. Quality-of-Life Outcomes
3.3. Stent-Related Outcomes
3.4. Complications
4. Discussion
4.1. Patient Selection for Iliofemoral Venous Stenting
4.2. Stenting for NIVL
4.3. Discharge and Follow-Up Post-Stenting for NIVL
4.4. Clinical Improvement Following Stenting
4.5. Quality-of-Life Improvement Post-Stenting
4.6. Stent-Related Outcomes
4.7. Study Heterogeneity
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartung, O.; Loundou, A.D.; Barthelemy, P.; Arnoux, D.; Boufi, M.; Alimi, Y.S. Endovascular management of chronic disabling ilio-caval obstructive lesions: Long-term results. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 118–124. [Google Scholar] [CrossRef]
- Gutzeit, A.; Zollikofer Ch, L.; Dettling-Pizzolato, M.; Graf, N.; Largiader, J.; Binkert, C.A. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: Long-term results 1987–2009. Cardiovasc. Interv. Radiol. 2011, 34, 542–549. [Google Scholar] [CrossRef]
- Seager, M.J.; Busuttil, A.; Dharmarajah, B.; Davies, A.H. Editor’s Choice—A Systematic Review of Endovenous Stenting in Chronic Venous Disease Secondary to Iliac Vein Obstruction. Eur. J. Vasc. Endovasc. 2016, 51, 100–120. [Google Scholar] [CrossRef]
- Gagne, P.J.; Gagne, N.; Kucher, T.; Thompson, M.; Bentley, D. Long-term clinical outcomes and technical factors with the Wallstent for treatment of chronic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 45–55. [Google Scholar] [CrossRef]
- Lichtenberg, M.K.W.; Stahlhoff, W.F.; Stahlhoff, S.; Ozkapi, A.; Breuckmann, F.; de Graaf, R. Venovo venous stent for treatment of non-thrombotic or post-thrombotic iliac vein lesions—Long-term efficacy and safety results from the Arnsberg venous registry. VASA Z. Gefasskrankh. 2021, 50, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.K.; Nie, M.L.; Fu, J.; Liu, F.Y.; Chen, Y.K.; Sun, J.M.; Wang, H.Y. Comparing the efficacy of endovascular treatment for iliac vein compression syndrome with or without acute deep venous thrombosis: A single-center retrospective study. Vascular 2022, 30, 341–348. [Google Scholar] [CrossRef]
- Espitia, O.; Douane, F.; Hersant, J.; Abbadie, F.; Sobocinski, J.; Heautot, J.F.; Miossec, A.; Lapebie, F.X.; Hartung, O.; the Venous Stent Network Investigators. Predictive Factors of Stent Patency in Iliofemoral Venous Diseases in a Multicentre Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Badesha, A.S.; Black, S.A.; Khan, G.; Harper, A.J.; Thulasidasan, N.; Doyle, A.; Khan, T. A meta-analysis of the medium- to long-term outcomes in patients with chronic deep venous disease treated with dedicated venous stents. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101722. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Kibrik, P.; Shaydakov, M.E.; Singh, M.; Ting, W. Indications, technical aspects, and outcomes of stent placement in chronic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101904. [Google Scholar] [CrossRef]
- Williams, Z.F.; Dillavou, E.D. A systematic review of venous stents for iliac and venacaval occlusive disease. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 145–153. [Google Scholar] [CrossRef]
- Dake, M.D.; O’Sullivan, G.; Shammas, N.W.; Lichtenberg, M.; Mwipatayi, B.P.; Settlage, R.A.; for the VERNACULAR Trial Investigators. Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction. Cardiovasc Interv. Radiol. 2021, 44, 1918–1929. [Google Scholar] [CrossRef]
- Comerota, A.J.; Gagne, P.; Brown, J.A.; Segbefia, E.; Hofmann, L.V.; VIVO Clinical Study Investigators. Final 3-Year Study Outcomes from the Evaluation of the Zilver Vena Venous Stent for the Treatment of Symptomatic Iliofemoral Venous Outflow Obstruction (The VIVO Clinical Study). J. Vasc. Interv. Radiol. 2024, 35, 834–845. [Google Scholar] [CrossRef]
- Black, S.; Sapoval, M.; Dexter, D.J.; Gibson, K.; Kolluri, R.; Razavi, M.; deFreitas, D.J.; Wang, H.; Brucato, S.; Murphy, E.; et al. Three-Year Outcomes of the Abre Venous Self-Expanding Stent System in Patients with Symptomatic Iliofemoral Venous Outflow Obstruction. J. Vasc. Interv. Radiol. 2024, 35, 664–675.e5. [Google Scholar] [CrossRef]
- Razavi, M.; Lichtenberg, M.; Desai, K.; Dexter, D.; Soukas, P.; Shammas, N.; Lodha, A.; Gagne, P.; Nordell, A.; Kolluri, R.; et al. The VIVID trial 12-month outcomes of the venous stent for the iliofemoral vein using the Duo venous stent system. J. Vasc. Surg. Venous Lymphat. Disord. 2025, 13, 101995. [Google Scholar] [CrossRef]
- Neglen, P.; Hollis, K.C.; Olivier, J.; Raju, S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 2007, 46, 979–990. [Google Scholar] [CrossRef]
- Jayaraj, A.; Noel, C.; Kuykendall, R.; Raju, S. Long-term outcomes following use of a composite Wallstent-Z stent approach to iliofemoral venous stenting. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 393–400.e2. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.D.S.; Bertanha, M.; El Dib, R.; Moura, R. Association between deep vein thrombosis and stent patency in symptomatic iliac vein compression syndrome: Systematic review and meta-analysis. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Badesha, A.S.; Bains, P.R.S.; Bains, B.R.S.; Khan, T. A systematic review and meta-analysis of the treatment of obstructive chronic deep venous disease using dedicated venous stents. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 267–282.e4. [Google Scholar] [CrossRef] [PubMed]
- Majeed, G.M.; Lodhia, K.; Carter, J.; Kingdon, J.; Morris, R.I.; Gwozdz, A.; Saratzis, A.; Saha, P. A Systematic Review and Meta-Analysis of 12-Month Patency After Intervention for Iliofemoral Obstruction Using Dedicated or Non-Dedicated Venous Stents. J. Endovasc. Ther. 2022, 29, 478–492. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Zhao, J.; Li, C.; Yan, Y.; Shi, C. Iliac vein stenting outcomes in non-thrombotic and thrombotic diseases: A systematic review and meta-analysis. Biomol. Biomed. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Lu, X.; Li, W.; Huang, Y.; Huang, X.; Lu, M.; Jiang, M. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J. Vasc. Interv. Radiol. 2012, 23, 497–502. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Peng, Z.; Yin, M.; Lu, X.; Ye, K. Outcomes of endovenous laser ablation with additional iliac vein stenting of nonthrombotic lesions in patients presenting with active venous ulcers. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1517–1525. [Google Scholar] [CrossRef]
- Hong, L.; Wang, X.; Fang, Z.; Sun, X.; Ge, X.; Chen, C.; Feng, H.; Hu, H. Editor’s Choice—Clinical Efficacy of Venastent—A Novel Iliac Vein Stent for Non-Thrombotic Iliac Vein Lesions: A Multi-Centre Randomised Controlled Trial. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 883–889. [Google Scholar] [CrossRef]
- Ramirez Ortega, M.; Tejero, O.T.; Benages, E.M.; Griggs, A.; Segbefia, E.; Mallagray, E.P. Real-world outcomes of Zilver Vena(R) Venous Self Expanding Stent placement for thrombotic and non-thrombotic indications in Spain. Phlebology 2025, 40, 496–507. [Google Scholar] [CrossRef]
- Porter, J.M.; Moneta, G.L. Reporting standards in venous disease: An update. International Consensus Committee on Chronic Venous Disease. J. Vasc. Surg. 1995, 21, 635–645. [Google Scholar] [CrossRef]
- Stoner, M.C.; Calligaro, K.D.; Chaer, R.A.; Dietzek, A.M.; Farber, A.; Guzman, R.J.; Hamdan, A.D.; Landry, G.J.; Yamaguchi, D.J.; Society for Vascular Surgery. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J. Vasc. Surg. 2016, 64, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.H.; Kambara, A.M.; Izukawa, N.M.; Rodrigues, T.O.; Rossi, C.B.; Sousa, A.G.; Metzger, P.B.; Thorpe, P.E. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Shekarchian, S.; Van Laanen, J.; Esmaeil Barbati, M.; Vleugels, M.J.; Nelemans, P.; Razavi, M.K.; Mees, B.; Jacobs, M.J.; Jalaie, H. Editor’s Choice—Quality of Life after Stenting for Iliofemoral Venous Obstruction: A Randomised Controlled Trial with One Year Follow Up. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.R.; Sabri, S.S.; Elias, S.; Gagne, P.J.; Garcia, M.J.; Gibson, K.; Kiguchi, M.M.; Mathews, S.J.; Murphy, E.H.; Secemsky, E.A.; et al. Consensus Statement on the Management of Nonthrombotic Iliac Vein Lesions From the VIVA Foundation, the American Venous Forum, and the American Vein and Lymphatic Society. Circ. Cardiovasc Interv. 2024, 17, e014160. [Google Scholar] [CrossRef]
- Rossi, F.H.; Kambara, A.M.; Rodrigues, T.O.; Rossi, C.B.O.; Izukawa, N.M.; Pinto, I.M.F.; Thorpe, P.E. Comparison of computed tomography venography and intravascular ultrasound in screening and classification of iliac vein obstruction in patients with chronic venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 413–422. [Google Scholar] [CrossRef]
- Saleem, T.; Raju, S. Comparison of intravascular ultrasound and multidimensional contrast imaging modalities for characterization of chronic occlusive iliofemoral venous disease: A systematic review. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1545–1556.e2. [Google Scholar] [CrossRef]
- Dean, S.M.; Valenti, E.; Hock, K.; Leffler, J.; Compston, A.; Abraham, W.T. The clinical characteristics of lower extremity lymphedema in 440 patients. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Kibbe, M.R.; Ujiki, M.; Goodwin, A.L.; Eskandari, M.; Yao, J.; Matsumura, J. Iliac vein compression in an asymptomatic patient population. J. Vasc. Surg. 2004, 39, 937–943. [Google Scholar] [CrossRef]
- Oguzkurt, L.; Ozkan, U.; Ulusan, S.; Koc, Z.; Tercan, F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J. Vasc. Interv. Radiol. 2008, 19, 366–370, quiz 371. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Chen, W.; Zhou, K.; Xu, Z.; Xu, M.; Sun, Z. Novel typing of iliac vein compression in asymptomatic individuals evaluated by contrast enhanced CT. Surg. Radiol. Anat. 2021, 43, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Kwan, K.J.S.; Chan, Y.C.; Wulamu, W.; Cheng, S.W. Prevalence and predictors of radiological left common iliac vein compression in asymptomatic patients. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101661. [Google Scholar] [CrossRef]
- Gagne, P.J.; Tahara, R.W.; Fastabend, C.P.; Dzieciuchowicz, L.; Marston, W.; Vedantham, S.; Ting, W.; Iafrati, M.D. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 678–687. [Google Scholar] [CrossRef]
- Lau, I.; Png, C.Y.M.; Eswarappa, M.; Miller, M.; Kumar, S.; Tadros, R.; Vouyouka, A.; Marin, M.; Faries, P.; Ting, W. Defining the utility of anteroposterior venography in the diagnosis of venous iliofemoral obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 514–521.e4. [Google Scholar] [CrossRef]
- Montminy, M.L.; Thomasson, J.D.; Tanaka, G.J.; Lamanilao, L.M.; Crim, W.; Raju, S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Rodriguez, P.P.; Abualanain, E.; Li, Y.; Khalil, M.S.; Aboloyoun, H.; Perez Lozada, J.C.; Aboian, E.; Attaran, R.; Ochoa Chaar, C.I. The Use of Intravascular Ultrasound During Deep Venous Interventions in a Tertiary Care Center. Vasc. Endovasc. Surg. 2025, 59, 505–512. [Google Scholar] [CrossRef]
- Jayaraj, A.; Noel, C.; Raju, S. Contralateral limb improvement after unilateral iliac vein stenting argues against simultaneous bilateral stenting. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 565–571. [Google Scholar] [CrossRef]
- Kassab, G.; Raju, S. Grading venous stenosis is different from arterial lesions. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, A.; Powell, T.; Raju, S. Utility of the 50% stenosis criterion for patients undergoing stenting for chronic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Buck, W.J.; Crim, W.; Jayaraj, A. Optimal sizing of iliac vein stents. Phlebology 2018, 33, 451–457. [Google Scholar] [CrossRef]
- Jayaraj, A.; Thaggard, D.; Lucas, M. Technique of stent sizing in patients with symptomatic chronic iliofemoral venous obstruction-the case for intravascular ultrasound-determined inflow channel luminal area-based stenting and associated long-term outcomes. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 634–641. [Google Scholar] [CrossRef]
- Raju, S.; Darcey, R.; Neglen, P. Unexpected major role for venous stenting in deep reflux disease. J. Vasc. Surg. 2010, 51, 401–408; discussion 408. [Google Scholar] [CrossRef] [PubMed]
- Pergamo, M.; Kabnick, L.S.; Jacobowitz, G.R.; Rockman, C.B.; Maldonado, T.S.; Berland, T.L.; Blumberg, S.; Sadek, M. Relationship between iliofemoral venous stenting and femoropopliteal deep venous reflux. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 346–350. [Google Scholar] [CrossRef]
- Jayaraj, A.; Luke, C.; Robinson, J.; Burr, B. Role of lower extremity fasciectomy plus fasciotomy for patients with persistent leg pain after stenting for chronic iliofemoral venous obstruction. J. Vasc. Surg. Cases Innov. Tech. 2022, 8, 616–619. [Google Scholar] [CrossRef]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef]
- Sayed, M.H.; Salem, M.; Desai, K.R.; O’Sullivan, G.J.; Black, S.A. A review of the incidence, outcome, and management of venous stent migration. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 482–490. [Google Scholar] [CrossRef]
- Smith, S.; Butts, H.; Owens, J.; Matheson, S.; Dickerson, M.M.; Jayaraj, A. Outcomes following stenting for symptomatic chronic iliofemoral venous stenosis—A comparison of three stent types. J. Vasc. Surg. Venous Lymphat. Disord. 2025, 13, 102208. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Gamas, L.; Rocha-Neves, J.P.; Pereira-Neves, A.; Dias-Neto, M.; Baekgaard, N. Contralateral deep vein thrombosis after stenting across the iliocaval confluence in chronic venous disease—A systematic review. Phlebology 2020, 35, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Pappas, K.; Mahadevan, S.; Sulakvelidze, L.; Kennedy, R.; Lakhanpal, G.; Lakhanpal, S.; Pappas, P.J. The impact of stent protrusion into the inferior vena cava or jailing of the contralateral iliac vein on the incidence of contralateral deep vein thrombosis following venous stenting. Phlebology 2025, 40, 80–87. [Google Scholar] [CrossRef]
- Jayaraj, A.; Fuller, R.; Raju, S.; Stafford, J. In-stent restenosis and stent compression following stenting for chronic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 42–51. [Google Scholar] [CrossRef] [PubMed]
| Author-Year | Type of Study | ‘n’ Limbs | NIVL (%) | Comparator | Diagnosis | Stent Types | Follow Up | Symptom Improvement | Signs | Score Improvement | Qol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neglen 2007, [15] | R | 982 | 53 | None | Venography and IVUS | Wallstent; Nitinol | Mean—22 months (94%) | Grade of swelling improvement (1.7 to 0.8 p < 0.0001) VAS pain score improvement (3.7 to 0.8 p < 0.0001) | Recurrence free ulcer healing rate—63% | CIVIQ—20 score improvement (p < 0.001) | |
| Ye 2012, [21] | R | 224 | 100 | None | CTV or CF vengraphy | Wallstent | Mean—50 (±36) months | Grade of swelling improvement (1.7 to 0.6 p < 0.01) VAS pain score improvement (4.3 to 0.4 p < 0.01) | 48 month recurrence free ulcer healing rate—82.3% | CIVIQ score improvement (p < 0.001) | |
| Jayaraj 2021, [16] | R | 545 | 19 | None | Venography and IVUS | Wallstent + Z stent | Median—26 months | Grade of swelling improvement (3 to 1 p < 0.0001) VAS pain score improvement (5 to 2 p < 0.0001) | Recurrence free ulcer healing rate—66% | VCSS improvement post stenting 6 to 4 (p < 0001) | Global CIVIQ—20 score improvement 60 to 36 (p < 0.0001) |
| Yang 2021, [22] | R | 157 | 100 | EVLA of incompetent superficial and/or perforator vein(s) with sclerotherapy/phlebectomy of varicose veins | CF venography | Wallstent | Median—24 months | Stenting + EVLA vs. EVLA: Mean VAS pain score improvement 2.3 vs. 1.1 (p = 0.01) | Stenting + EVLA vs. EVLA: Ulcer healing 82.8% vs. 68.8% (p = 0.03) | Stenting + EVLA vs. EVLA: Mean VCSS post stenting 8.3 vs. 11.7 (p = 0.01) | |
| Hong 2022, [23] | RCT | 246 | 100 | Venastent vs. Zilver Vena | Venography | Venastent or Zilver Vena | 12 months | Ulcer healing rate (91% vs. 83% p = 0.99) | rVCSS improvement at 12 months (4.4 vs. 5) | ||
| Ortega 2025, [24] | R | 219 | 70 | None | Venography + TAUS | Zilver Vena | Mean—41 (±22) months | Mean VCSS pain score improvement 6.9 to 1.4 (NIVL limbs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaraj, A. Outcomes Following Iliac Vein Stenting for Non-Thrombotic Iliac Vein Lesions—A Narrative Review Based on Large Sample Studies. J. Funct. Biomater. 2025, 16, 427. https://doi.org/10.3390/jfb16120427
Jayaraj A. Outcomes Following Iliac Vein Stenting for Non-Thrombotic Iliac Vein Lesions—A Narrative Review Based on Large Sample Studies. Journal of Functional Biomaterials. 2025; 16(12):427. https://doi.org/10.3390/jfb16120427
Chicago/Turabian StyleJayaraj, Arjun. 2025. "Outcomes Following Iliac Vein Stenting for Non-Thrombotic Iliac Vein Lesions—A Narrative Review Based on Large Sample Studies" Journal of Functional Biomaterials 16, no. 12: 427. https://doi.org/10.3390/jfb16120427
APA StyleJayaraj, A. (2025). Outcomes Following Iliac Vein Stenting for Non-Thrombotic Iliac Vein Lesions—A Narrative Review Based on Large Sample Studies. Journal of Functional Biomaterials, 16(12), 427. https://doi.org/10.3390/jfb16120427





