Improving the Biocompatibility of Plant-Derived Scaffolds for Tissue Engineering Using Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
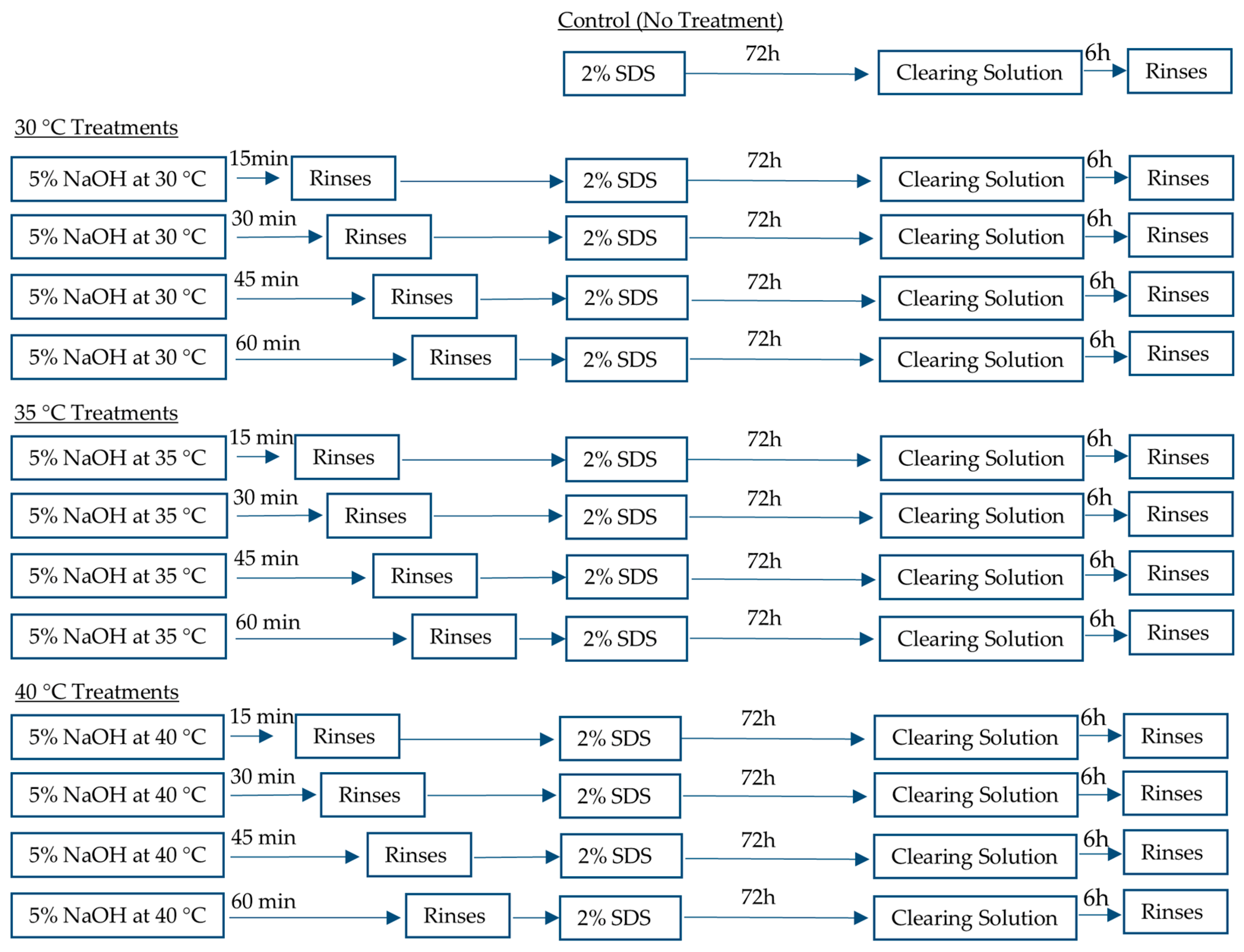
2.1. Heat Treatment and Decellularization of Plant Leaves
2.2. DNA Quantification
2.3. Tensile Testing
2.4. Burst Pressure Testing
2.5. Scanning Electron Microscopy
2.6. Histological Staining
2.7. White Blood Cell Assay
2.8. Recellularization of Plant-Derived Scaffolds
2.9. Statistical Analysis
3. Results
3.1. Assessment of Decellularization
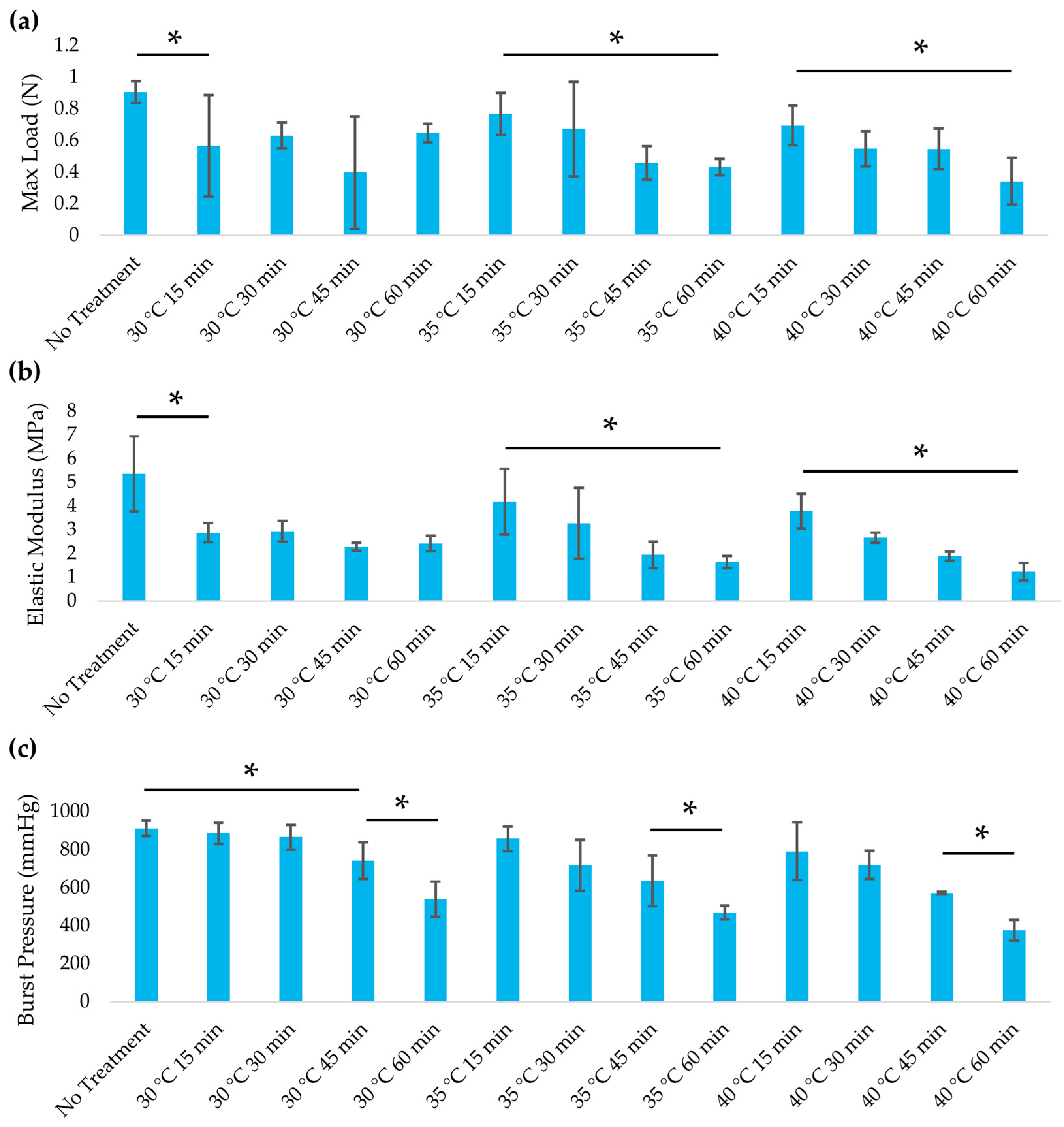
3.2. Mechanical Properties
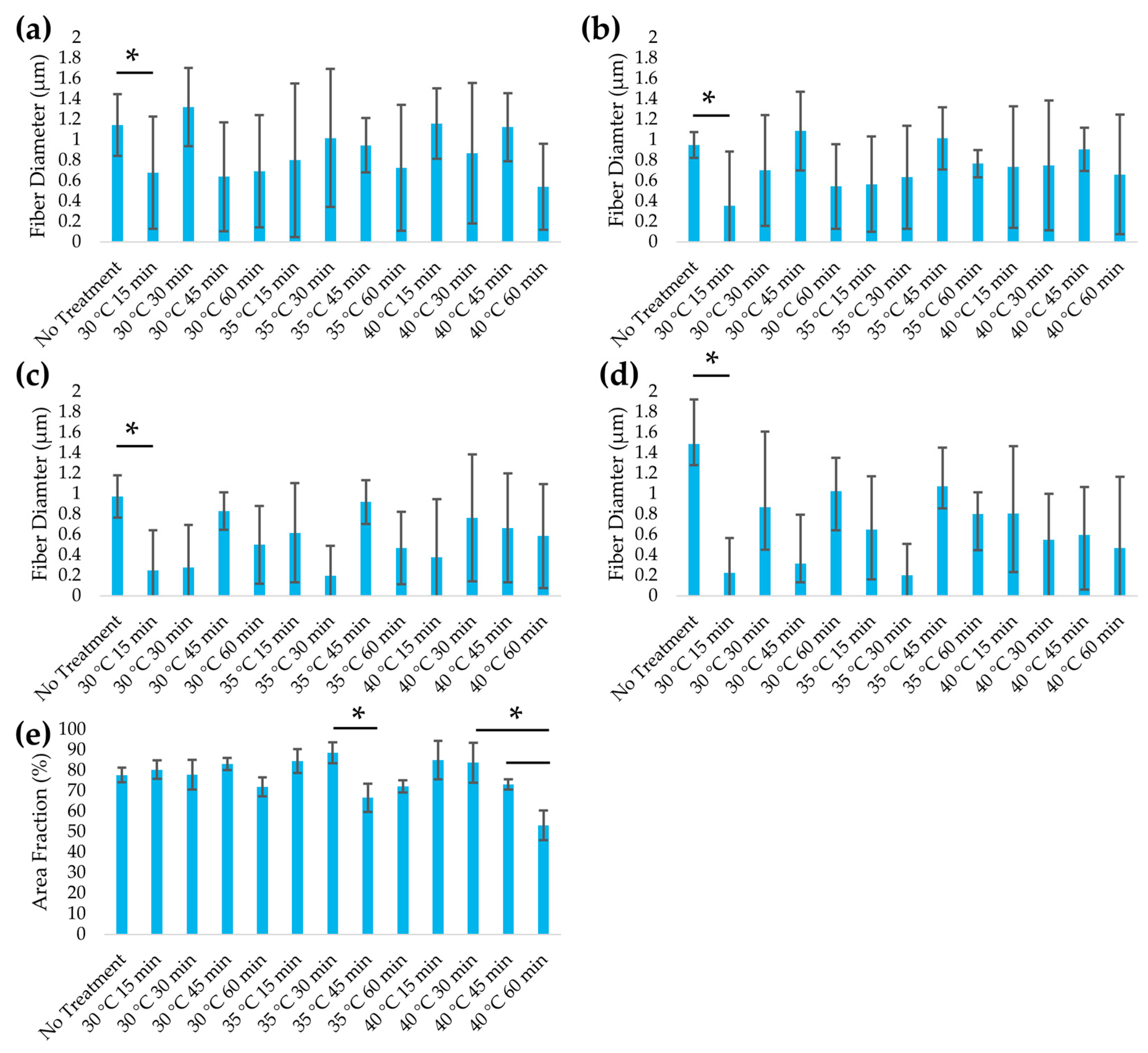
3.3. Structural Analysis
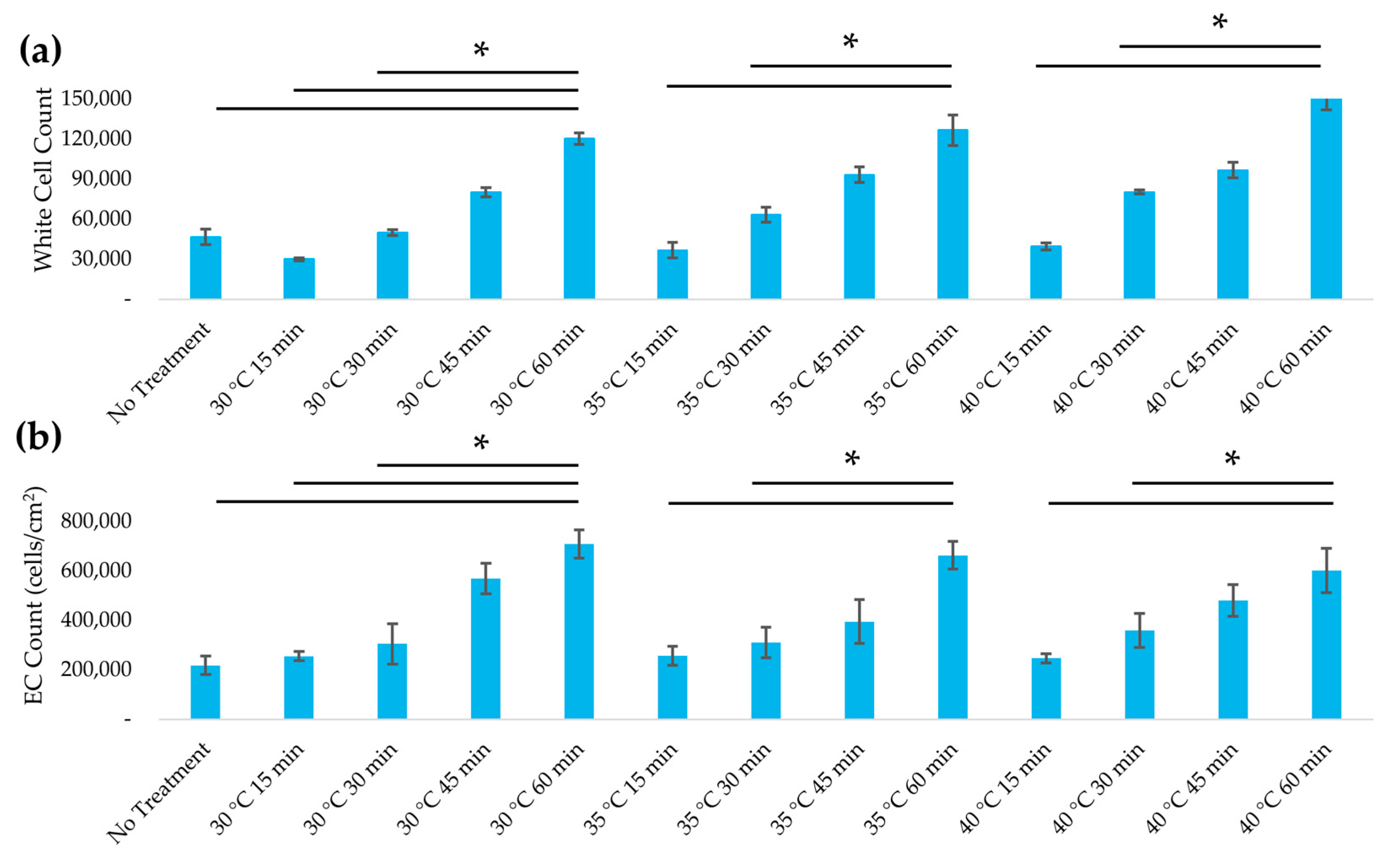
3.4. Assessment of Immunogenicity
3.5. Recellularization of Decellularized Leatherleaf with ECs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaOH | Sodium Hydroxide |
| ePTFE | Expanded polytetrafluoroethylene |
| EC | Endothelial cell |
| SEM | Scanning electron microscopy |
| ECM | Extracellular matrix |
| WBC | White blood cell |
| PBS | Phosphate-buffered saline |
| ANOVA | Analysis of variance |
| SDS | Sodium dodecyl sulfate |
References
- Li, Q.; Ding, X.; Chen, C.; Zhang, K.; Dong, R. An overview of small diameter vascular grafts: From materials to fabrication. Mater. Adv. 2025, 6, 6221–6242. [Google Scholar] [CrossRef]
- Li, M.-X.; Wei, Q.-Q.; Mo, H.-L.; Ren, Y.; Zhang, W.; Lu, H.-J.; Joung, Y.K. Challenges and advances in materials and fabrication technologies of small-diameter vascular grafts. Biomater. Res. 2023, 27, 58. [Google Scholar] [CrossRef] [PubMed]
- Golovina, V.; Panfilov, V.; Seliverstov, E.; Erechkanova, D.; Zolotukhin, I. Availability of the great saphenous veins as conduits for arterial bypass surgery in patients with varicose veins. J. Clin. Med. 2024, 13, 7747. [Google Scholar] [CrossRef] [PubMed]
- McGevna, M.A.; Ratner, M.; Speranza, G.; Garg, K.; Teter, K.; Jacobowitz, G.R.; Maldonado, T.S.; Sadek, M.; Rockman, C.B. Availability of a suitable single-segment great saphenous vein in patients with severe peripheral arterial disease. Ann. Vasc. Surg. 2025. [Google Scholar] [CrossRef]
- de Ceniga, M.V.; Gutiérrez, M.; Ormaechevarria, A.; Cabezuelo, X.; Estallo, L. Availability of great saphenous vein for infrainguinal bypass. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 787–788. [Google Scholar] [CrossRef]
- Ding, K.; Yu, X.; Wang, D.; Wang, X.; Li, Q. Small diameter expanded polytetrafluoroethylene vascular graft with differentiated inner and outer biomacromolecules for collaborative endothelialization, anti-thrombogenicity and anti-inflammation. Colloids Surf. B Biointerfaces 2023, 229, 113449. [Google Scholar] [CrossRef]
- Yao, Y.; Pohan, G.; Cutiongco, M.F.A.; Jeong, Y.; Kunihiro, J.; Zaw, A.M.; David, D.; Shangguan, H.; Yu, A.C.H.; Yim, E.K.F. In vivo evaluation of compliance mismatch on intimal hyperplasia formation in small diameter vascular grafts. Biomater. Sci. 2023, 11, 3297–3307. [Google Scholar] [CrossRef]
- Gorbenko, N.; Rinaldi, G.; Sanchez, A.; Merna, N. Small-caliber vascular grafts engineered from decellularized leaves and cross-linked gelatin. Tissue Eng. Part A 2023, 29, 397–409. [Google Scholar] [CrossRef]
- Cevik, M.; Dikici, S. Development of tissue-engineered vascular grafts from decellularized parsley stems. Soft Matter 2023, 20, 338–350. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef]
- Varhama, K.; Oda, H.; Shima, A.; Takeuchi, S. Decellularized plant leaves for 3D cell culturing. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Republic of Korea, 27–31 January 2019; IEEE: New York, NY, USA, 2019; pp. 226–228. [Google Scholar] [CrossRef]
- Merna, N. Engineering vascular grafts from decellularized plants: Advances and challenges. Histol. Histopathol. 2025, 40, 18934. [Google Scholar] [CrossRef]
- Couvrette, L.J.; Walker, K.L.; Bui, T.V.; Pelling, A.E. Plant cellulose as a substrate for 3D neural stem cell culture. Bioengineering 2023, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Robertson, S.; Kim, C.; Suzuki, M.; Murphy, W.L.; Gopalan, P. Aligned skeletal muscle assembly on a biofunctionalized plant leaf scaffold. Acta Biomater. 2023, 171, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Maparu, A.K.; Mishra, M.; Rai, B.; Sivakumar, S. In vitro evaluation of decellularized floral scaffold with surface nanotopography for skin tissue engineering. Mater. Today Commun. 2024, 41, 111056. [Google Scholar] [CrossRef]
- Latour, M.L.; Pelling, A.E. Mechanosensitive osteogenesis on native cellulose scaffolds for bone tissue engineering. J. Biomech. 2022, 135, 111030. [Google Scholar] [CrossRef]
- Latour, M.L.; Tarar, M.; Hickey, R.J.; Cuerrier, C.M.; Catelas, I.; Pelling, A.E. Decellularized apple-derived scaffolds for bone tissue engineering in vitro and in vivo. J. Vis. Exp. 2024, 204, e65226. [Google Scholar] [CrossRef]
- Hammad, M.; Dugué, J.; Maubert, E.; Baugé, C. Decellularized apple hypanthium as a plant-based biomaterial for cartilage regeneration in vitro: A comparative study of progenitor cell types and environmental conditions. J. Biol. Eng. 2025, 19, 38. [Google Scholar] [CrossRef]
- Zamudio-Ceja, R.B.; Garcia-Contreras, R.; Chavez-Granados, P.A.; Aranda-Herrera, B.; Alvarado-Garnica, H.; Jurado, C.A.; Fischer, N.G. Decellularized scaffolds of nopal (Opuntia Ficus-indica) for bioengineering in regenerative dentistry. J. Funct. Biomater. 2023, 14, 252. [Google Scholar] [CrossRef]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized Alstroemeria flower stem modified with chitosan for tissue engineering purposes: A cellulose/chitosan scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef]
- Gorbenko, N.; Vaccaro, J.C.; Fagan, R.; Cerro, R.A.; Khorrami, J.M.; Galindo, L.; Merna, N. Perfusion bioreactor conditioning of small-diameter plant-based vascular grafts. Tissue Eng. Regen. Med. 2024, 21, 1189–1201. [Google Scholar] [CrossRef]
- Imeidopf, G.; Khaimov, D.; John, S.; Merna, N. Optimization and standardization of plant-derived vascular scaffolds. Int. J. Mol. Sci. 2025, 26, 2752. [Google Scholar] [CrossRef]
- Fazal, F.; Raghav, S.; Callanan, A.; Koutsos, V.; Radacsi, N. Recent advancements in the bioprinting of vascular grafts. Biofabrication 2021, 13, 32003. [Google Scholar] [CrossRef]
- ANSI/ISO 7198:2016; Cardiovascular Implants and Extracorporeal Systems—Vascular Prostheses—Tubular Vascular Grafts and Vascular Patches. International Organization for Standardization: Geneva, Switzerland, 2016.
- Iezzi, G.; Aprile, G.; Tripodi, D.; Scarano, A.; Piattelli, A.; Perrotti, V. Implant surface topographies analyzed using fractal dimension. Implant. Dent. 2011, 20, 131–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schutte, R.J.; Parisi-Amon, A.; Reichert, W.M. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J. Biomed. Mater. Res. A 2009, 88, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, K.; Duprey, A.; Zainal, M.; Schlicht, M.; Williams, D.; Berguer, R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J. Thorac. Cardiovasc. Surg. 2011, 142, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef]
- Bouchet, M.; Gauthier, M.; Maire, M.; Ajji, A.; Lerouge, S. Towards compliant small-diameter vascular grafts: Predictive analytical model and experiments. Mater. Sci. Eng. C 2019, 100, 715–723. [Google Scholar] [CrossRef]
- Jørgensen, C.S.; Paaske, W.P. Physical and mechanical properties of ePTFE stretch vascular grafts determined by time-resolved scanning acoustic microscopy. Eur. J. Vasc. Endovasc. Surg. 1998, 15, 416–422. [Google Scholar] [CrossRef][Green Version]
- Montini-Ballarin, F.; Calvo, D.; Caracciolo, P.C.; Rojo, F.; Frontini, P.M.; Abraham, G.A.; Guinea, G.V. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 2016, 60, 220–233. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Merna, N. Autofluorescence quenching in decellularized plant scaffolds for tissue engineering. Ann. Biomed. Eng. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng. Part C Methods 2013, 19, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Diaz-Rodriguez, P.; Balouch, B.; Paulsen, S.; Wu, S.; Miller, J.; Hahn, M.; Cosgriff-Hernandez, E. Elucidating the role of graft compliance mismatch on intimal hyperplasia using an ex vivo organ culture model. Acta Biomater. 2019, 89, 84–94. [Google Scholar] [CrossRef]
- Vazquez-Padron, R.I.; Duque, J.C.; Tabbara, M.; Salman, L.H.; Martinez, L. Intimal hyperplasia and arteriovenous fistula failure: Looking beyond size differences. Kidney360 2021, 2, 1360–1372. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Jiang, Y.; Zhang, B.; Li, K.; Li, Q. Suture retention strength of P (LLA-CL) tissue-engineered vascular grafts. RSC Adv. 2019, 9, 21258–21264. [Google Scholar] [CrossRef]
- Lee, M.K.; Song, J.Y.; Kim, T.Y.; Kim, J.H.; Choi, J.B.; Kuh, J.H. Simple anastomotic techniques for coronary artery bypass surgery in patients with small coronary arteries or a marked size discrepancy between the coronary artery and graft. Korean J. Thorac. Cardiovasc. Surg. 2016, 49, 485. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Tolba, E.; Dorweiler, B.; Schröder, H.C.; Link, T.; Diehl-Seifert, B.; Müller, W.E.G. Modular small diameter vascular grafts with bioactive functionalities. PLoS ONE 2015, 10, e0133632. [Google Scholar] [CrossRef]
- Solan, A.; Dahl, S.L.; Niklason, L.E. Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant. 2009, 18, 915–921. [Google Scholar] [CrossRef]
- Kumar, V.A.; Brewster, L.P.; Caves, J.M.; Chaikof, E.L. Tissue engineering of blood vessels: Functional requirements, progress, and future challenges. Cardiovasc. Eng. Technol. 2011, 2, 137–148. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep. 2017, 7, 3615. [Google Scholar] [CrossRef]
- Fusco, D.; Meissner, F.; Podesser, B. Small-diameter bacterial cellulose-based vascular grafts for coronary artery bypass grafting in a pig model. Front. Cardiovasc. Med. 2022, 9, 881557. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Yao, Y.; Yim, E.K. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater. Sci. 2020, 8, 4383–4395. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, D.L.; Kleinert, L.B.; Berman, S.S.; Williams, S.K. The effects of porosity on endothelialization of ePTFE implanted in subcutaneous and adipose tissue. J. Biomed. Mater. Res. 1997, 34, 463–476. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef]
- Skośkiewicz-Malinowska, K.; Mysior, M.; Rusak, A.; Kuropka, P.; Kozakiewicz, M.; Jurczyszyn, K. Application of texture and fractal dimension analysis to evaluate subgingival cement surfaces in terms of biocompatibility. Materials 2021, 14, 5857. [Google Scholar] [CrossRef]
- Massai, D.; Pennella, F.; Gentile, P.; Gallo, D.; Ciardelli, G.; Bignardi, C.; Audenino, A.; Morbiducci, U. Image-based three-dimensional analysis to characterize the texture of porous scaffolds. Biomed Res. Int. 2014, 2014, 161437. [Google Scholar] [CrossRef]
- Ya, J.; Bayraktutan, U. Donor variability alters the characteristics of human brain microvascular endothelial cells. Curr. Issues Mol. Biol. 2025, 47, 73. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-4:2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization: Geneva, Switzerland, 2017.
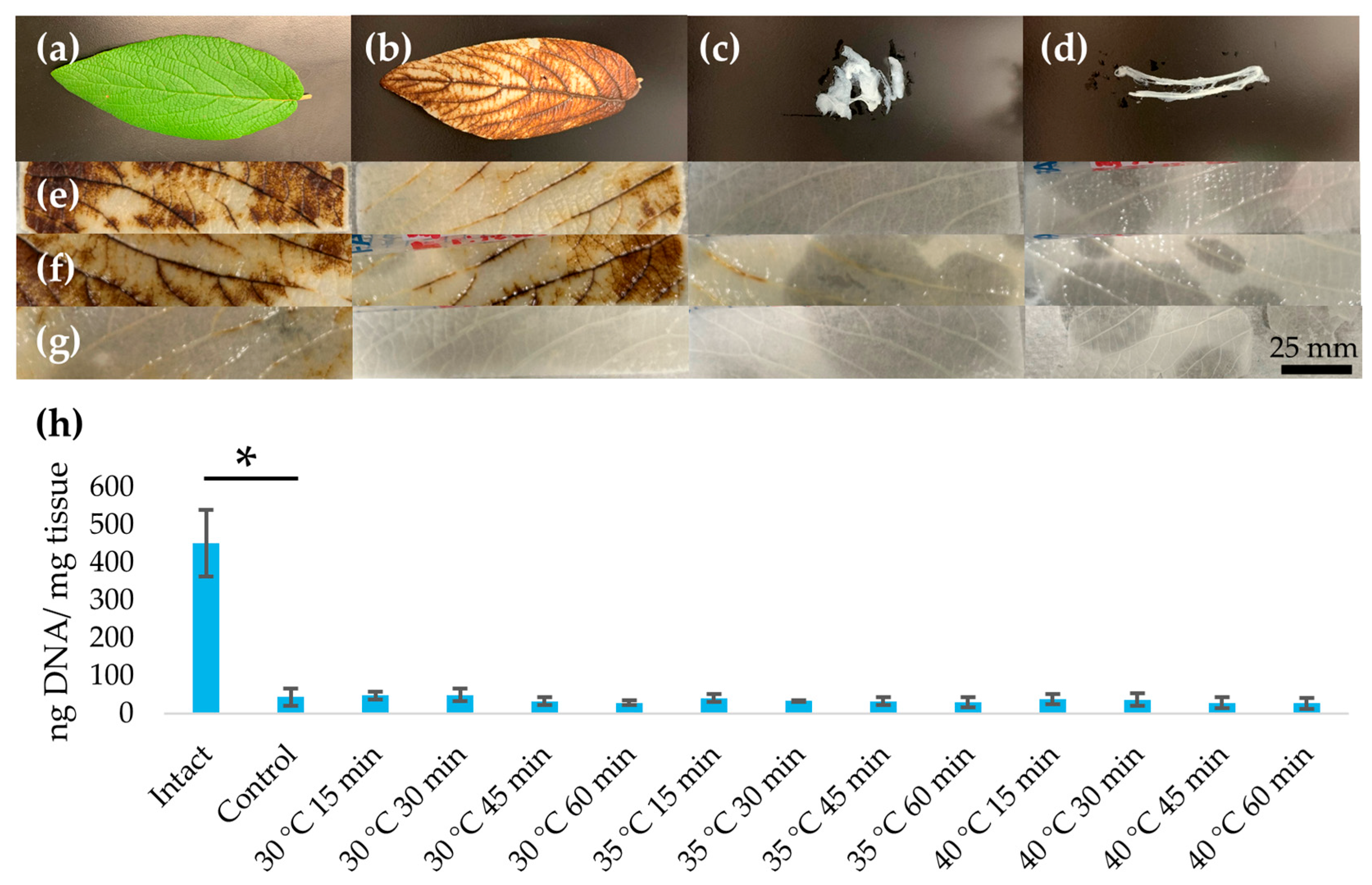


| Condition. | DNA (ng/mg Tissue, Mean ± SD) |
|---|---|
| Intact | 452.0 ± 88.1 |
| Control | 44.5 ± 23.5 |
| 30 °C 15 min | 48.6 ± 10.4 |
| 30 °C 30 min | 49.3 ± 16.6 |
| 30 °C 45 min | 33.1 ± 10.3 |
| 30 °C 60 min | 28.8 ± 6.0 |
| 35 °C 15 min | 41.8 ± 10.6 |
| 35 °C 30 min | 33.8 ± 1.4 |
| 35 °C 45 min | 33.2 ± 10.3 |
| 35 °C 60 min | 29.8 ± 13.5 |
| 40 °C 15 min | 38.3 ± 13.6 |
| 40 °C 30 min | 37.3 ± 16.3 |
| 40 °C 45 min | 28.6 ±14.5 |
| 40 °C 60 min | 28.2 ± 14.6 |
| Property | Treated Plant Scaffold | Saphenous Vein | Coronary Artery | Synthetic Grafts |
|---|---|---|---|---|
| Burst pressure (mmHg) | ≥820 | 1599 [30] | 2031 [31] | ≥3500 [32] |
| Elastic modulus (MPa) | 2.3–5.3 | 1.5–4 [33] | 0.5–3 [33] | 17.4 [31] |
| Compliance (% per 100 mmHg) | 2.72 | 1.77 [25] | 4.06 [25] | 1.63 [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsamooj, A.; Gorbenko, N.; Olivares, C.; John, S.; Merna, N. Improving the Biocompatibility of Plant-Derived Scaffolds for Tissue Engineering Using Heat Treatment. J. Funct. Biomater. 2025, 16, 380. https://doi.org/10.3390/jfb16100380
Ramsamooj A, Gorbenko N, Olivares C, John S, Merna N. Improving the Biocompatibility of Plant-Derived Scaffolds for Tissue Engineering Using Heat Treatment. Journal of Functional Biomaterials. 2025; 16(10):380. https://doi.org/10.3390/jfb16100380
Chicago/Turabian StyleRamsamooj, Arvind, Nicole Gorbenko, Cristian Olivares, Sashane John, and Nick Merna. 2025. "Improving the Biocompatibility of Plant-Derived Scaffolds for Tissue Engineering Using Heat Treatment" Journal of Functional Biomaterials 16, no. 10: 380. https://doi.org/10.3390/jfb16100380
APA StyleRamsamooj, A., Gorbenko, N., Olivares, C., John, S., & Merna, N. (2025). Improving the Biocompatibility of Plant-Derived Scaffolds for Tissue Engineering Using Heat Treatment. Journal of Functional Biomaterials, 16(10), 380. https://doi.org/10.3390/jfb16100380







