Fracture Resistance of 3D-Printed Hybrid Abutment Crowns Made from a Tooth-Colored Ceramic Filled Hybrid Composite: A Pilot Study
Abstract
1. Introduction
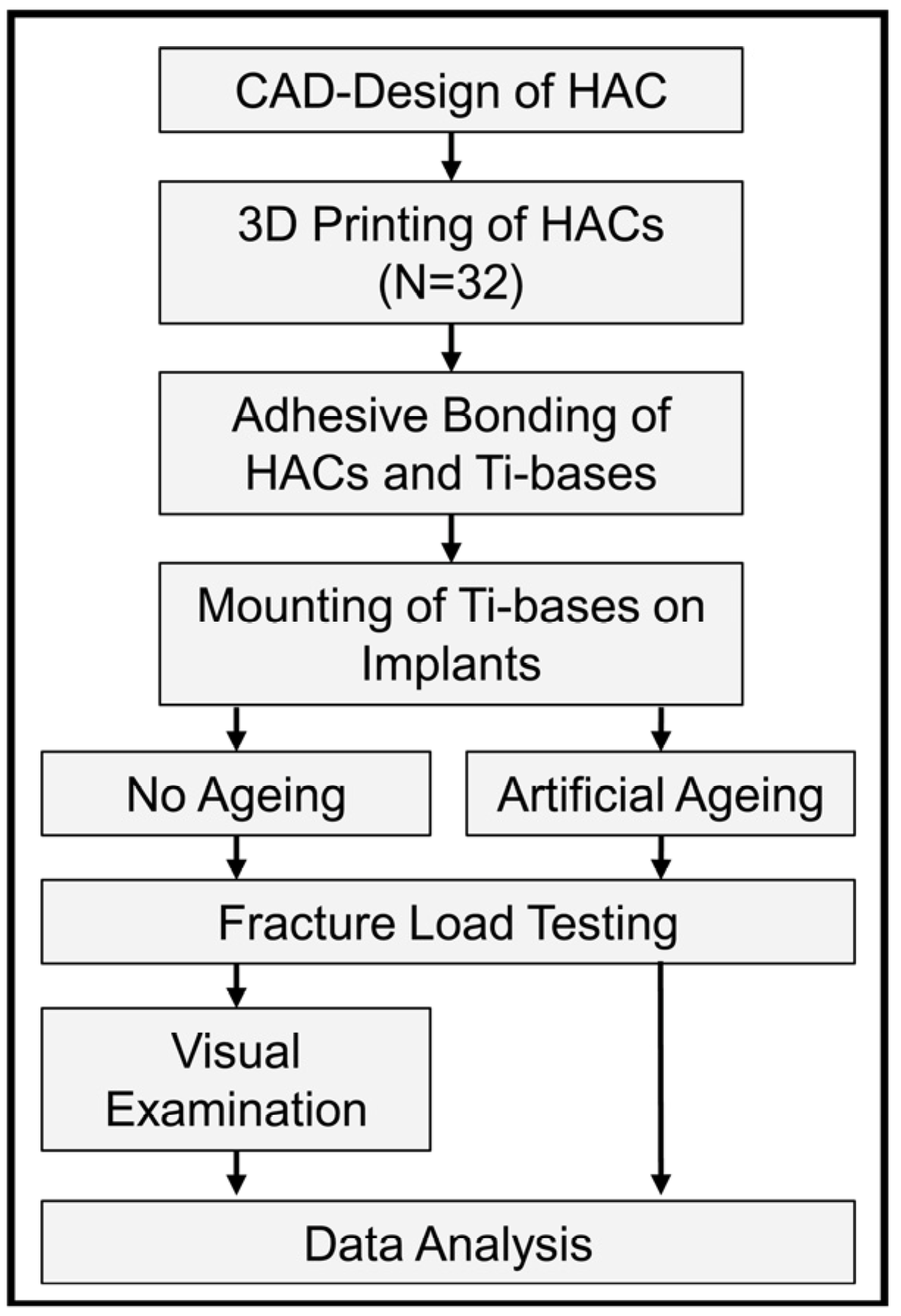
2. Materials and Methods
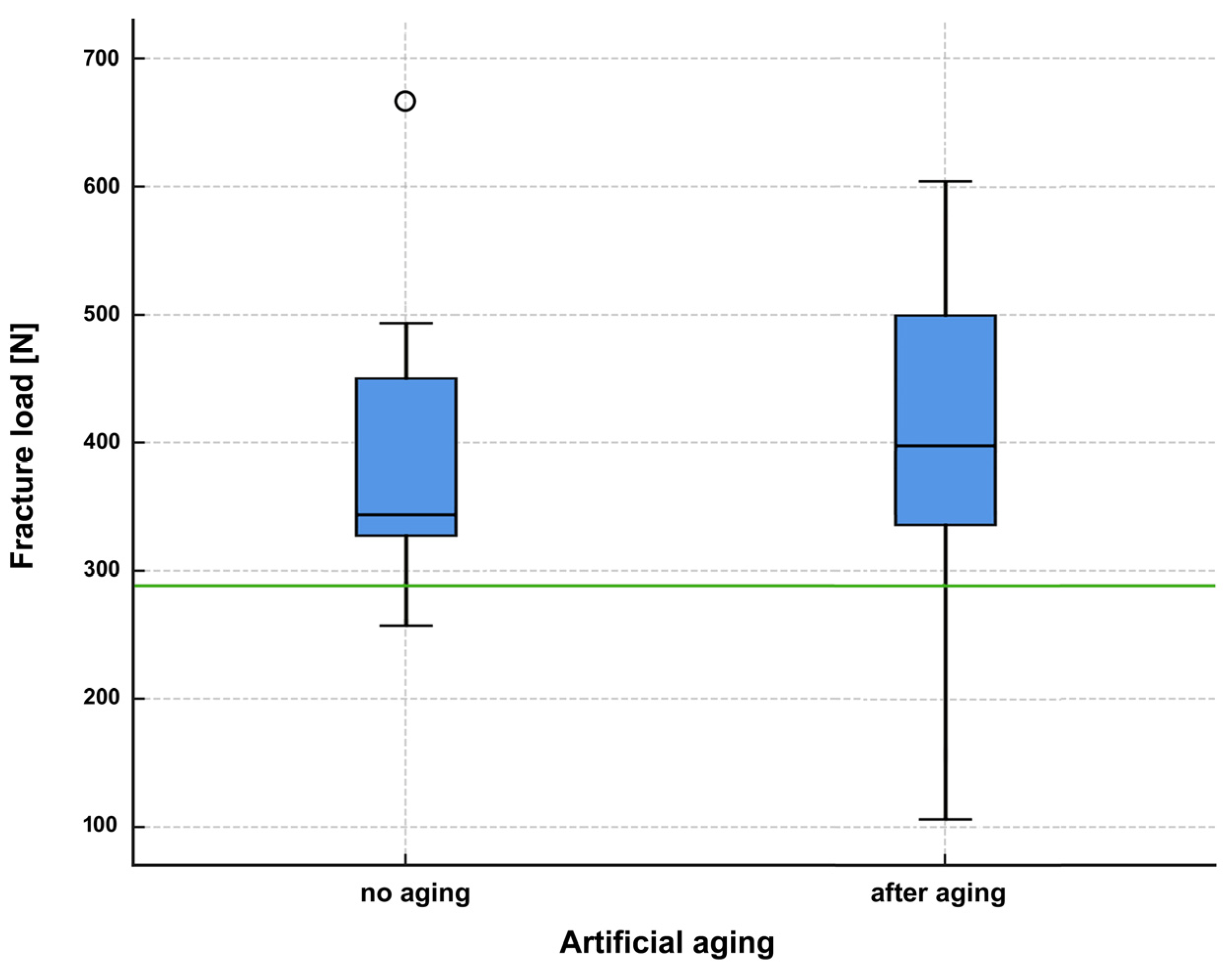
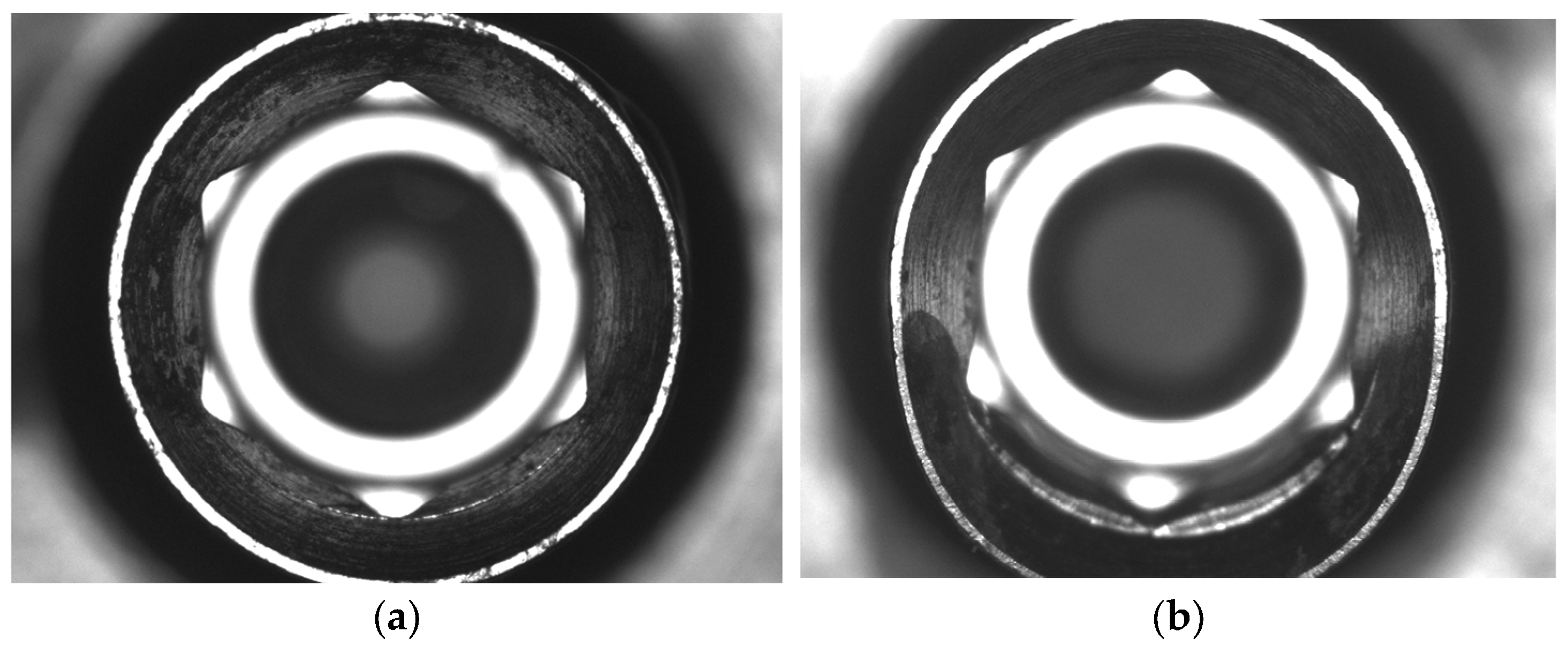
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.K.; Nishimura, G.H.; Pozzi, A.; Danda, A.K. Single implants in dorsal areas—A systematic review. Eur. J. Oral Implantol. 2016, 9 (Suppl. 1), S163–S172. [Google Scholar]
- Pjetursson, B.E.; Sailer, I.; Latyshev, A.; Rabel, K.; Kohal, R.J.; Karasan, D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral Implant. Res. 2021, 32 (Suppl. S21), 254–288. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Joda, T.; Bragger, U. Time-Efficiency Analysis Comparing Digital and Conventional Workflows for Implant Crowns: A Prospective Clinical Crossover Trial. Int. J. Oral Maxillofac. Implant. 2015, 30, 1047–1053. [Google Scholar] [CrossRef]
- Joda, T.; Bragger, U. Time-efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clin. Oral Implant. Res. 2016, 27, 1401–1406. [Google Scholar] [CrossRef]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Pitta, J.; Hicklin, S.P.; Fehmer, V.; Boldt, J.; Gierthmuehlen, P.C.; Sailer, I. Mechanical stability of zirconia meso-abutments bonded to titanium bases restored with different monolithic all-ceramic crowns. Int. J. Oral Maxillofac. Implant. 2019, 34, 1091–1097. [Google Scholar] [CrossRef]
- Roberts, E.E.; Bailey, C.W.; Ashcraft-Olmscheid, D.L.; Vandewalle, K.S. Fracture Resistance of Titanium-Based Lithium Disilicate and Zirconia Implant Restorations. J. Prosthodont. 2018, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Schubert, O.; Goob, J.; Schweiger, J.; Guth, J.F.; Edelhoff, D.; Graf, T. Clinical performance of monolithic lithium disilicate hybrid abutment crowns over at least 3.5 years. J. Prosthodont. 2023, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Majeed-Saidan, A.; Albagami, H.H.; Khan, A.S.; Ahmad, S.; Collares, F.M.; Della Bona, A.; et al. Three-dimensional (3D) printing in dental practice: Applications, areas of interest, and level of evidence. Clin. Oral Investig. 2023, 27, 2465–2481. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Gmeiner, R.; Schonherr, J.A.; Stampfl, J. Stereolithography-based additive manufacturing of lithium disilicate glass ceramic for dental applications. Mater. Sci. Eng. C 2020, 116, 111180. [Google Scholar] [CrossRef]
- Branco, A.C.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. Recent Advances on 3D-Printed Zirconia-Based Dental Materials: A Review. Materials 2023, 16, 1860. [Google Scholar] [CrossRef]
- Güth, J.F.; Schweiger, J.; Graf, T.; Stimmelmayr, M.; Schubert, O.; Erdelt, K. Short communication: In vitro pilot study: Are monolithic 3Y-TZP zirconia crowns too strong for titanium Implants? Int. J. Prosthodont. 2022, 35, 509–511. [Google Scholar] [CrossRef]
- Baumgart, P.; Kirsten, H.; Haak, R.; Olms, C. Biomechanical properties of polymer-infiltrated ceramic crowns on one-piece zirconia implants after long-term chewing simulation. Int. J. Implant. Dent. 2018, 4, 16. [Google Scholar] [CrossRef]
- Preis, V.; Hahnel, S.; Behr, M.; Bein, L.; Rosentritt, M. In-vitro fatigue and fracture testing of CAD/CAM-materials in implant-supported molar crowns. Dent. Mater. 2017, 33, 427–433. [Google Scholar] [CrossRef]
- Duarte, S., Jr.; Phark, J.H. Advances in Dental Restorations: A Comprehensive Review of Machinable and 3D-Printed Ceramic-Reinforced Composites. J. Esthet. Restor. Dent. 2025, 37, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, H.; Izumita, K.; Yoda, N.; Egusa, H.; Sasaki, K. Comparison of the accuracy of resin-composite crowns fabricated by three-dimensional printing and milling methods. Dent. Mater. J. 2022, 41, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Abad-Coronel, C.; Freire Bonilla, C.; Vidal, S.; Rosero, F.; Encalada Abad, C.; Mena Córdova, N.; Paltan, A.; Fajardo, J.I.; Aliaga, P. Evaluating a Novel 3D-Printed Resin for Dental Restorations: Fracture Resistance of Restorations Fabricated by Digital Press Stereolithography. Polymers 2025, 17, 2322. [Google Scholar] [CrossRef]
- Güney, B.; Nalbant, A.D.; Bankoğlu Güngör, M. Comparison of the Fracture Resistance of Provisional Crowns and Fixed Partial Dentures Manufactured with Conventional, Milling, and 3D-Printing Techniques. Appl. Sci. 2025, 15, 6539. [Google Scholar] [CrossRef]
- Prause, E.; Hey, J.; Beuer, F.; Yassine, J.; Hesse, B.; Weitkamp, T.; Gerber, J.; Schmidt, F. Microstructural investigation of hybrid CAD/CAM restorative dental materials by micro-CT and SEM. Dent. Mater. 2024, 40, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Graf, T.; Erdelt, K.J.; Guth, J.F.; Edelhoff, D.; Schubert, O.; Schweiger, J. Influence of Pre-Treatment and Artificial Aging on the Retention of 3D-Printed Permanent Composite Crowns. Biomedicines 2022, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Grzebieluch, W.; Kowalewski, P.; Grygier, D.; Rutkowska-Gorczyca, M.; Kozakiewicz, M.; Jurczyszyn, K. Printable and Machinable Dental Restorative Composites for CAD/CAM Application—Comparison of Mechanical Properties, Fractographic, Texture and Fractal Dimension Analysis. Materials 2021, 14, 4919. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Ozer, N.E.; Yiotakiotaciota, C.; Kiotaliotacarslan, M.A. Mechanical Characteristics of Composite Resins Produced by Additive and Subtractive Manufacturing. Eur. J. Prosthodont. Restor. Dent. 2023, 31, 278–285. [Google Scholar] [CrossRef]
- de Souza, F.A.; Blois, M.C.; Collares, K.; dos Santos, M.B.F. 3D-printed and conventional provisional single crown fabrication on anterior implants: A randomized clinical trial. Dent. Mater. 2024, 40, 340–347. [Google Scholar] [CrossRef]
- Graf, T.; Schweiger, J.; Stimmelmayr, M.; Erdelt, K.; Schubert, O.; Guth, J.F. Influence of monolithic restorative materials on the implant-abutment interface of hybrid abutment crowns: An in vitro investigation. J. Prosthodont. Res. 2023, 67, 450–459. [Google Scholar] [CrossRef]
- Othman, A.; Sandmair, M.; Alevizakos, V.; von See, C. The fracture resistance of 3D-printed versus milled provisional crowns: An in vitro study. PLoS ONE 2023, 18, e0285760. [Google Scholar] [CrossRef]
- Donmez, M.B.; Okutan, Y. Marginal gap and fracture resistance of implant-supported 3D-printed definitive composite crowns: An in vitro study. J. Dent. 2022, 124, 104216. [Google Scholar] [CrossRef]
- DIN EN ISO 14801:2017-03; Dentistry—Implants—Dynamic fatigue test for endosseous dental implants. International Organization for Standardization: Berlin, Germany, 2017.
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lima, V.P.; Machado, J.B.; Zhang, Y.; Loomans, B.A.C.; Moraes, R.R. Laboratory methods to simulate the mechanical degradation of resin composite restorations. Dent. Mater. 2022, 38, 214–229. [Google Scholar] [CrossRef]
- Gök, T.; Durdu, E.; Atik, M.R.; Konuş, F.; Gök, A. Evaluation of Fracture Resistance and Failure Modes of Maxillary Premolars Restored with Different Coronal Designed Fiber Posts: In Vitro Study. Eur. Endod. J. 2025, 10, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Naveau, A.; Rignon-Bret, C.; Wulfman, C. Zirconia abutments in the anterior region: A systematic review of mechanical and esthetic outcomes. J. Prosthet. Dent. 2019, 121, 775–781.e1. [Google Scholar] [CrossRef] [PubMed]
- Tete, S.; Zizzari, V.L.; Borelli, B.; De Colli, M.; Zara, S.; Sorrentino, R.; Scarano, A.; Gherlone, E.; Cataldi, A.; Zarone, F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Moser, M.M.; Kleinheinz, J.; Happe, A. Biocompatibility of Lithium Disilicate and Zirconium Oxide Ceramics with Different Surface Topographies for Dental Implant Abutments. Int. J. Mol. Sci. 2021, 22, 7700. [Google Scholar] [CrossRef]
- Burkhardt, F.; Sailer, I.; Fehmer, V.; Mojon, P.; Pitta, J. Retention and marginal integrity of CAD/CAM fabricated crowns adhesively cemented to titanium base abutments—Influence of bonding system and restorative material. Int. J. Prosthodont. 2022, 36, e88–e102. [Google Scholar] [CrossRef]
- Joda, T.; Ferrari, M.; Bragger, U. A prospective clinical cohort study analyzing single-unit implant crowns after three years of loading: Introduction of a novel Functional Implant Prosthodontic Score (FIPS). Clin. Oral Implant. Res. 2017, 28, 1291–1295. [Google Scholar] [CrossRef]
- Teichmann, M.; Göckler, F.; Weber, V.; Yildirim, M.; Wolfart, S.; Edelhoff, D. Ten-year survival and complication rates of lithium-disilicate (Empress 2) tooth-supported crowns, implant-supported crowns, and fixed dental prostheses. J. Dent. 2017, 56, 65–77. [Google Scholar] [CrossRef]
- De Angelis, P.; Passarelli, P.C.; Gasparini, G.; Boniello, R.; D’Amato, G.; De Angelis, S. Monolithic CAD-CAM lithium disilicate versus monolithic CAD-CAM zirconia for single implant-supported posterior crowns using a digital workflow: A 3-year cross-sectional retrospective study. J. Prosthet. Dent. 2020, 123, 252–256. [Google Scholar] [CrossRef]
- Pitta, J.; Hjerppe, J.; Burkhardt, F.; Fehmer, V.; Mojon, P.; Sailer, I. Mechanical stability and technical outcomes of monolithic CAD/CAM fabricated abutment-crowns supported by titanium bases: An in vitro study. Clin. Oral Implant. Res. 2021, 32, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Keul, C.; Eichberger, M.; Figge, D.; Edelhoff, D.; Lumkemann, N. Three generations of zirconia: From veneered to monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Morneburg, T.R.; Pröschel, P.A. Measurement of masticatory forces and implant loads: A methodologic clinical study. Int. J. Prosthodont. 2002, 15, 20–27. [Google Scholar] [PubMed]
- DIN EN ISO 6872:2015; Dentistry—Ceramic materials. International Organization for Standardization: Berlin, Germany, 2015.


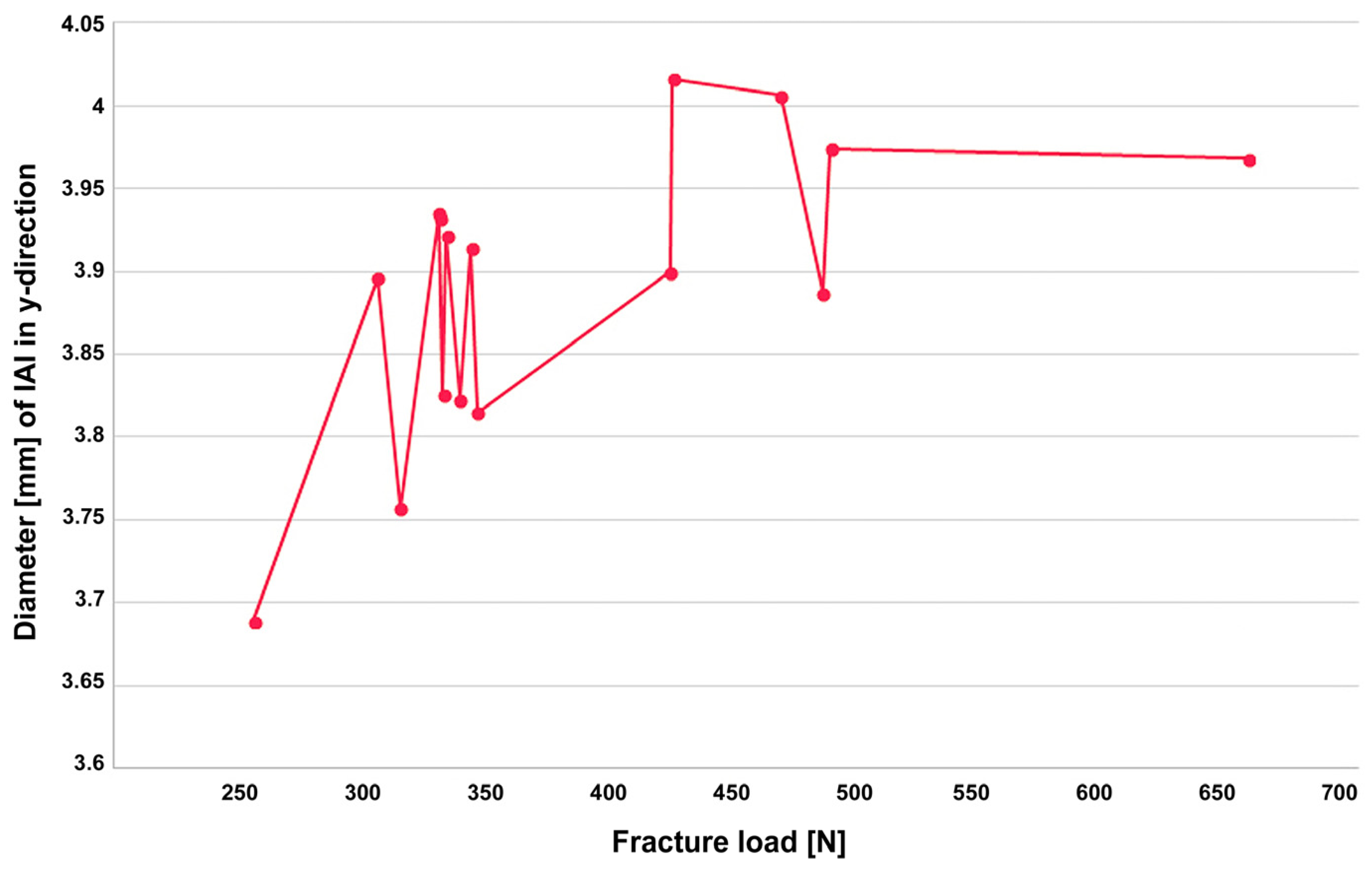

| No | Fracture Load [N] | X [mm] | Y [mm] | Failure Mode |
|---|---|---|---|---|
| 1 | 257.2 | 3.627 | 3.689 | catastrophic crown fracture |
| 2 | 308 | 3.666 | 3.896 | catastrophic crown fracture |
| 3 | 317.2 | 3.64 | 3.757 | catastrophic crown fracture |
| 4 | 332.9 | 3.676 | 3.935 | catastrophic crown fracture |
| 5 | 333.3 | 3.666 | 3.932 | catastrophic crown fracture |
| 6 | 334.8 | 3.694 | 3.826 | catastrophic crown fracture |
| 7 | 336.2 | 3.647 | 3.922 | catastrophic crown fracture |
| 8 | 341.5 | 3.681 | 3.823 | catastrophic crown fracture |
| 9 | 346.5 | 3.664 | 3.914 | catastrophic crown fracture |
| 10 | 348.9 | 3.664 | 3.815 | catastrophic crown fracture |
| 11 | 427.6 | 3.63 | 3.9 | catastrophic crown fracture |
| 12 | 428.9 | 3.648 | 4.016 | screw fracture |
| 13 | 472.99 | 3.628 | 4.006 | screw fracture |
| 14 | 490.07 | 3.641 | 3.887 | catastrophic crown fracture |
| 15 | 493.5 | 3.687 | 3.974 | catastrophic crown fracture |
| 16 | 664.1 | 3.607 | 3.968 | catastrophic crown fracture |
| No | Fracture Load [N] | Failure Mode |
|---|---|---|
| 1 | 522.14 | catastrophic crown fracture |
| 2 | 472.28 | screw fracture |
| 3 | 540.08 | catastrophic crown fracture |
| 4 | 176.02 | catastrophic crown fracture |
| 5 | 357.69 | catastrophic crown fracture |
| 6 | 106.48 | cohesive crown fracture |
| 7 | 313.15 | catastrophic crown fracture |
| 8 | 518.25 | catastrophic crown fracture |
| 9 | 603.89 | screw fracture |
| 10 | 493.01 | catastrophic crown fracture |
| 11 | 376.88 | catastrophic crown fracture |
| 12 | 465.01 | screw fracture |
| 13 | 158.19 | catastrophic crown fracture |
| 14 | 390.81 | catastrophic crown fracture |
| 15 | 378.26 | catastrophic crown fracture |
| 16 | 405.12 | catastrophic crown fracture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweiger, J.; Erdelt, K.-J.; Lente, I.; Edelhoff, D.; Graf, T.; Schubert, O. Fracture Resistance of 3D-Printed Hybrid Abutment Crowns Made from a Tooth-Colored Ceramic Filled Hybrid Composite: A Pilot Study. J. Funct. Biomater. 2025, 16, 375. https://doi.org/10.3390/jfb16100375
Schweiger J, Erdelt K-J, Lente I, Edelhoff D, Graf T, Schubert O. Fracture Resistance of 3D-Printed Hybrid Abutment Crowns Made from a Tooth-Colored Ceramic Filled Hybrid Composite: A Pilot Study. Journal of Functional Biomaterials. 2025; 16(10):375. https://doi.org/10.3390/jfb16100375
Chicago/Turabian StyleSchweiger, Josef, Kurt-Jürgen Erdelt, Isabel Lente, Daniel Edelhoff, Tobias Graf, and Oliver Schubert. 2025. "Fracture Resistance of 3D-Printed Hybrid Abutment Crowns Made from a Tooth-Colored Ceramic Filled Hybrid Composite: A Pilot Study" Journal of Functional Biomaterials 16, no. 10: 375. https://doi.org/10.3390/jfb16100375
APA StyleSchweiger, J., Erdelt, K.-J., Lente, I., Edelhoff, D., Graf, T., & Schubert, O. (2025). Fracture Resistance of 3D-Printed Hybrid Abutment Crowns Made from a Tooth-Colored Ceramic Filled Hybrid Composite: A Pilot Study. Journal of Functional Biomaterials, 16(10), 375. https://doi.org/10.3390/jfb16100375








