Effects of Implant Silver Coatings on Bone Formation in Animal Models: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Question
2.2. Search Strategies
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Risk of Bias in Individual Studies
2.8. Data Analysis
3. Results
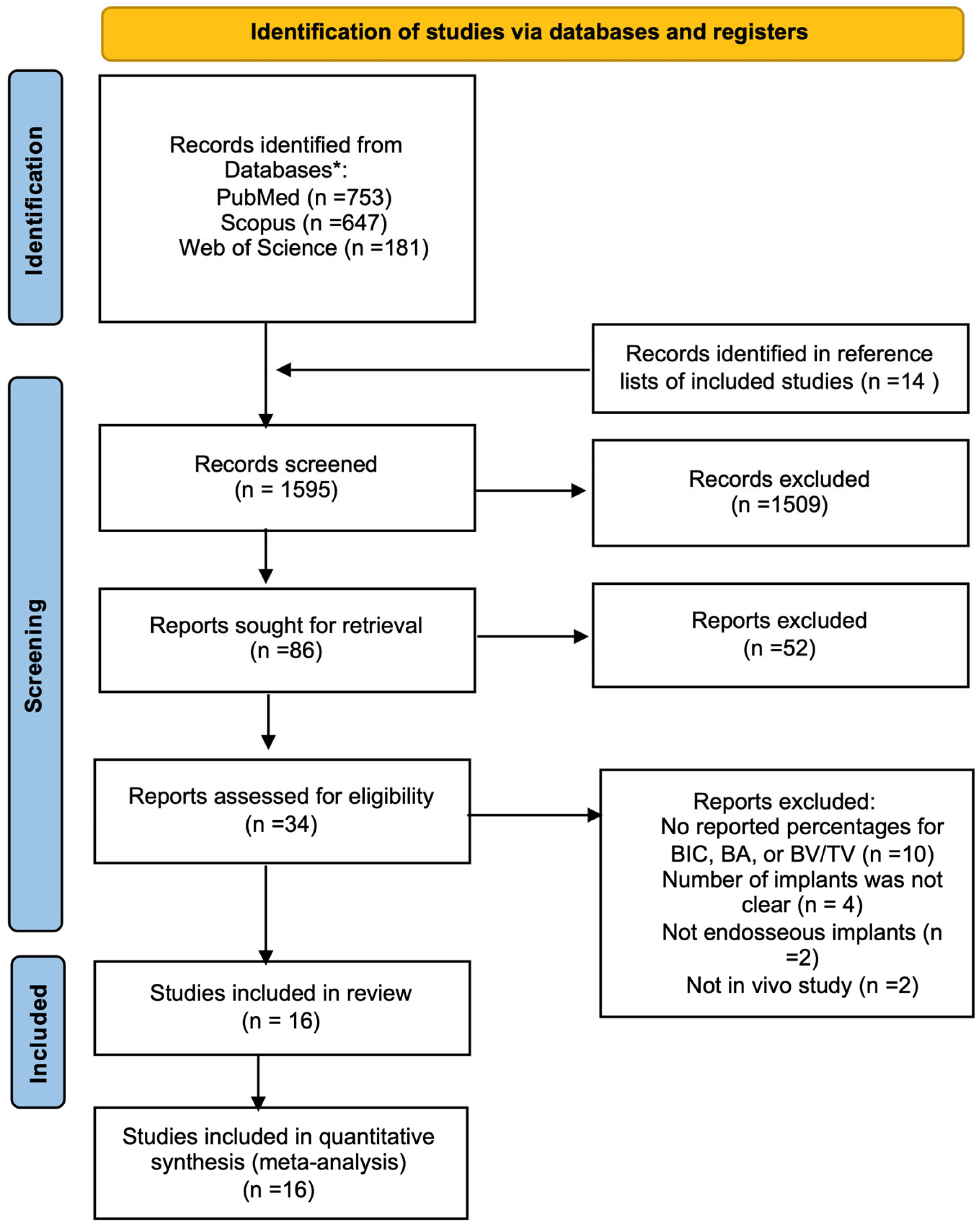
3.1. Literature Search
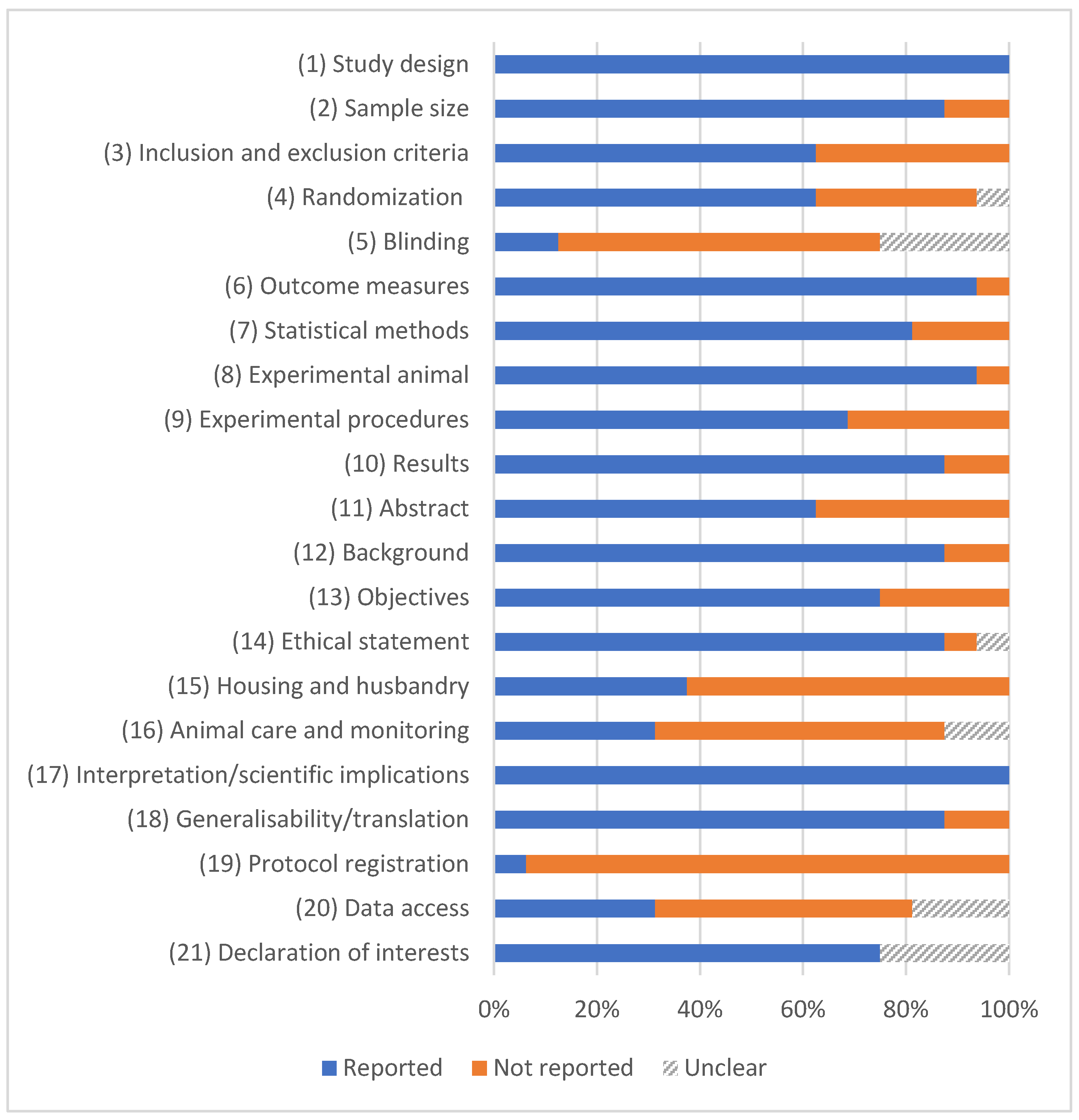
3.2. Quality Assessment
3.3. Risk of Bias
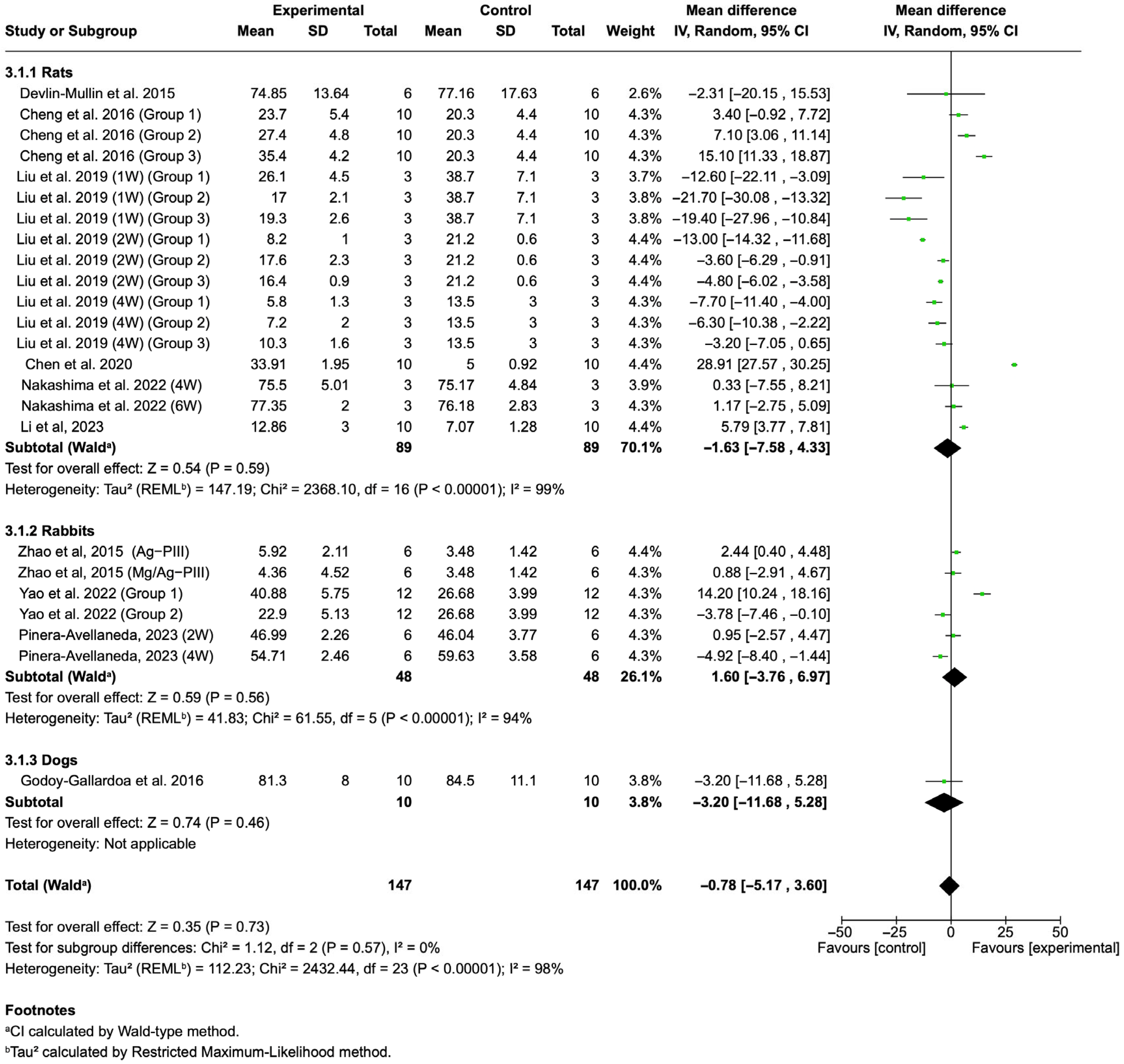
3.4. Meta-Analysis of Bone Formation Outcomes
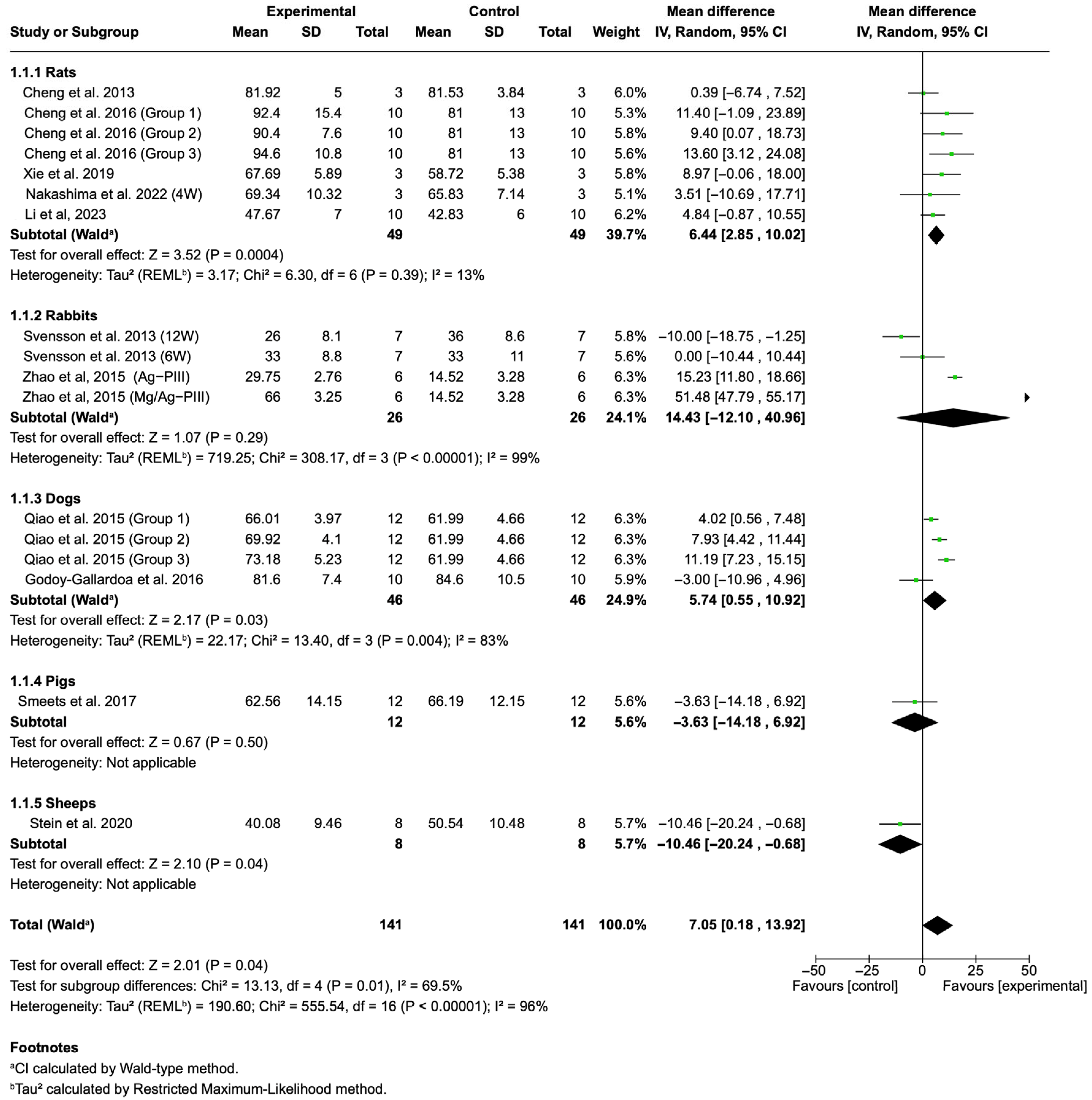
3.4.1. BIC
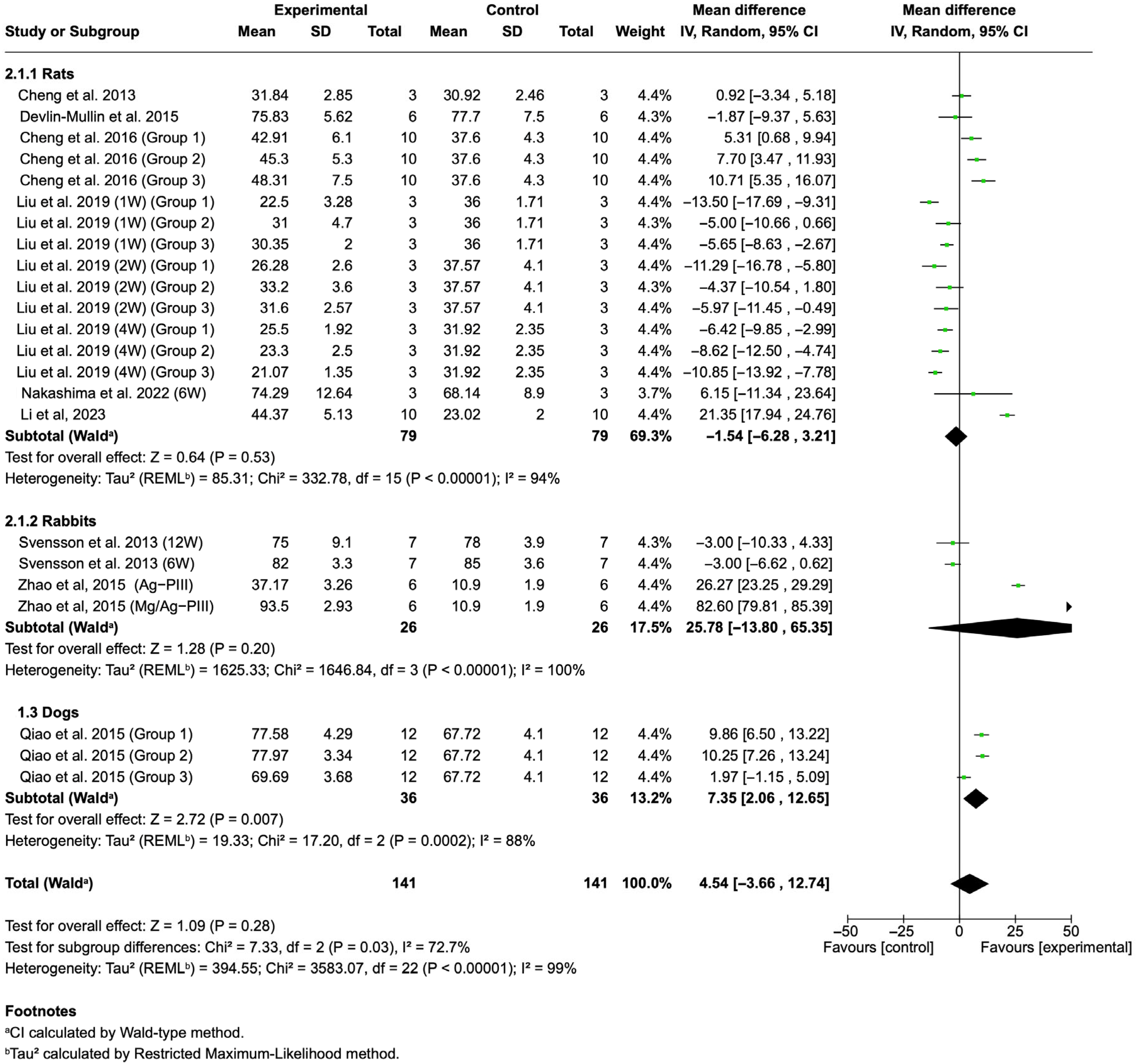
3.4.2. BA
3.4.3. BV/TV
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIC | bone–implant contact |
| BA | bone area |
| BV/TV | bone volume |
References
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- Busenlechner, D.; Fürhauser, R.; Haas, R.; Watzek, G.; Mailath, G.; Pommer, B. Long-term implant success at the Academy for Oral Implantology: 8-year follow-up and risk factor analysis. J. Periodontal Implant. Sci. 2014, 44, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Maghaireh, H.; Worthington, H.V. Interventions for replacing missing teeth: Different times for loading dental implants. Cochrane Database Syst. Rev. 2013, 2013, Cd003878. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implants Res. 2015, 26 (Suppl. 11), 15–44. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S246–S266. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Petzold, M.; Berglundh, T. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Teulé-Trull, M.; Altuna, P.; Arregui, M.; Rodriguez-Ciurana, X.; Aparicio, C. Antibacterial coatings for dental implants: A systematic review. Dent. Mater. 2025, 41, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, A.; Naito, Y.; Andersson, M.; Chrcanovic, B.R.; Wennerberg, A.; Jimbo, R. Characteristics of 2 Different Commercially Available Implants with or without Nanotopography. Int. J. Dent. 2013, 2013, 769768. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Makhtari, S.; Ahmadi, S.; Hussain, B. The Antibacterial and Cytotoxic Effects of Silver Nanoparticles Coated Titanium Implants: A Narrative Review. Materials 2022, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Choi, S.; Jo, Y.H.; Han, J.S.; Yoon, H.I.; Lee, J.H.; Yeo, I.L. Antibacterial activity and biocompatibility of silver coating via aerosol deposition on titanium and zirconia surfaces. Int. J. Implant Dent. 2023, 9, 24. [Google Scholar] [CrossRef]
- Xu, L.; Pei, H.; Qian, Q.; Shi, X.; Bang, L.T.; Li, B.; Wang, Y. Fabrication of silver-containing antibacterial coating on titanium by alkaline-heat treatment and hydrothermal sterilization. J. Mater. Res. Technol. 2024, 33, 5863–5873. [Google Scholar] [CrossRef]
- Sycińska-Dziarnowska, M.; Ziąbka, M.; Cholewa-Kowalska, K.; Klesiewicz, K.; Spagnuolo, G.; Lindauer, S.J.; Park, H.S.; Woźniak, K. Antibacterial and Antibiofilm Activity of Layers Enriched with Silver Nanoparticles on Orthodontic Microimplants. J. Funct. Biomater. 2025, 16, 78. [Google Scholar] [CrossRef]
- Nakashima, T.; Morimoto, T.; Hashimoto, A.; Kii, S.; Tsukamoto, M.; Miyamoto, H.; Todo, M.; Sonohata, M.; Mawatari, M. Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats. JOR Spine 2023, 6, e1236. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial Titanium Implants Biofunctionalized by Plasma Electrolytic Oxidation with Silver, Zinc, and Copper: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhang, T.; Gong, Y.; He, Y. Silver-Containing Biomaterials for Biomedical Hard Tissue Implants. Adv. Healthc. Mater. 2023, 12, 2300932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Lansdown, A.B. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.; Suska, F.; Emanuelsson, L.; Palmquist, A.; Norlindh, B.; Trobos, M.; Bäckros, H.; Persson, L.; Rydja, G.; Ohrlander, M.; et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine 2013, 9, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653–664. [Google Scholar] [CrossRef]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E.; et al. Atomic Layer Deposition of a Silver Nanolayer on Advanced Titanium Orthopedic Implants Inhibits Bacterial Colonization and Supports Vascularized de Novo Bone Ingrowth. Adv. Healthc. Mater. 2017, 6, 33. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, H.; Qin, H.; Cheng, T.; Qian, S.; Cheng, M.; Peng, X.; Wang, J.; Zhang, Y.; Jin, G.; et al. Balancing the Osteogenic and Antibacterial Properties of Titanium by Codoping of Mg and Ag: An in Vitro and in Vivo Study. ACS Appl. Mater. Interfaces 2015, 7, 17826–17836. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Smeets, R.; Precht, C.; Hahn, M.; Jung, O.; Hartjen, P.; Heiland, M.; Gröbe, A.; Holthaus, M.G.; Hanken, H. Biocompatibility and Osseointegration of Titanium Implants with a Silver-Doped Polysiloxane Coating: An In Vivo Pig Model. Int. J. Oral Maxillofac. Implant. 2017, 32, 1338–1345. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhang, H.; Tian, A.; You, J.; Wu, L.; Lei, Z.; Li, X.; Bai, X.; Chen, S. Biocompatibility evaluation of antibacterial Ti-Ag alloys with nanotubular coatings. Int. J. Nanomed. 2019, 14, 457–468. [Google Scholar] [CrossRef]
- Xie, K.; Zhou, Z.; Guo, Y.; Wang, L.; Li, G.; Zhao, S.; Liu, X.; Li, J.; Jiang, W.; Wu, S.; et al. Long-Term Prevention of Bacterial Infection and Enhanced Osteoinductivity of a Hybrid Coating with Selective Silver Toxicity. Adv. Healthc. Mater. 2019, 8, e1801465. [Google Scholar] [CrossRef]
- Stein, S.; Kruck, L.; Warnecke, D.; Seitz, A.; Dürselen, L.; Ignatius, A. Osseointegration of titanium implants with a novel silver coating under dynamic loading. Eur. Cell Mater. 2020, 39, 249–259. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, M.; Ren, R.; Gao, C.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO(2) Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, H.; Li, L.; Cao, Z.; Dong, Y.; Yao, L.; Lou, W.; Zheng, S.; Shi, Y.; Shen, X.; et al. Development and evaluation of osteogenesis and antibacterial properties of strontium/silver-functionalized hierarchical micro/nano-titanium implants. Mater. Des. 2022, 224, 111425. [Google Scholar] [CrossRef]
- Li, K.; Tang, Z.; Song, K.; Fischer, N.G.; Wang, H.; Guan, Y.; Deng, Y.; Cai, H.; Hassan, S.U.; Ye, Z.; et al. Multifunctional nanocoating for enhanced titanium implant osseointegration. Colloids Surf. B Biointerfaces 2023, 232, 113604. [Google Scholar] [CrossRef] [PubMed]
- Piñera-Avellaneda, D.; Buxadera-Palomero, J.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Surface competition between osteoblasts and bacteria on silver-doped bioactive titanium implant. Biomater. Adv. 2023, 146, 213311. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis--a review. Head. Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, D.; Birke, U.; Ghanem, M.; Prietzel, T.; Josten, C.; Roth, A.; Fakler, J.K.M. Silver-coated modular Megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss—A pilot study of 34 patients. BMC Musculoskelet. Disord. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Liu, X.; Sun, J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int. J. Nanomed. 2012, 7, 875–884. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Dizaj, S.M. Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef]
- Sopchenski, L.; Popat, K.; Soares, P. Bactericidal activity and cytotoxicity of a zinc doped PEO titanium coating. Thin Solid. Films 2018, 660, 477–483. [Google Scholar] [CrossRef]
- Pfau, E.A.; Avila-Campos, M.J. Prevotella intermedia and Porphyromonas gingivalis isolated from osseointegrated dental implants: Colonization and antimicrobial susceptibility. Braz. J. Microbiol. 2005, 36, 281–285. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration-A Review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, S.; Yang, K.; Ren, L.; Dai, K. Antibacterial activity of copper-bearing 316L stainless steel for the prevention of implant-related infection. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef]
- Xie, H.; Kang, Y.J. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.; Bettenga, M.; Agrawal, C.; Bumgardner, J.; Ong, J. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Page, M.J.; McKenziem, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Author | Silver Coating Technique | Silver Form | Animal Model | Bone Formation Outcome(s) | Evaluation Method(s) | Evaluated Groups | Healing Period |
|---|---|---|---|---|---|---|---|
| Cheng, 2013 [43] | Silver nanoparticle (Ag NPs) incorporated nanostructured Ti coating | Ag NPs | SD rats | BIC and BA | Histological evaluation | Control: Ti oxide nanotubes (TiO2-NT); test group: NT-Ag | 8 weeks |
| Svensson, 2013 [44] | Wet chemical plating process | Nanostructured coating | New Zealand White rabbits | BIC and BA | Histological evaluation | Control: non-coated implants; test group: coated implants | 6 and 12 weeks |
| Qiao, 2015 [45] | Plasma immersion ion implantation of silver (Ag-PIII) | Ag NPs | Labrador dogs | BA and BIC | Micro-CT and histological evaluation | Control: sandblasted and acid-etched (SLA) implants; test groups: implants treated for (1) 30 min with 30 Ag PIII, (2) 60 min with 30 Ag PIII, and (3) 90 min with 30 Ag PIII | 3 months |
| Devlin-Mullin, 2015 [46] | Silver nanolayer deposition via atomic layer deposition | Ag Nanolayer | Wistar rats | BA | Micro-CT analysis | Control: Ti; test group: Ti-Ag | 12 weeks |
| Zhao, 2015 [47] | Plasma immersion ion implantation of silver (Ag-PIII) | Ag NPs | New Zealand White rabbits | BIC, BA, and BV/TV | Micro-CT and histological evaluation | Control: Ti; test groups: (1) Ag-PIII and (2) Mg/Ag-PIII | 8 weeks |
| Godoy-Gallardoa, 2016 [48] | Implants with silver electrodeposition | Ag electrodeposition | Beagle dogs | BIC and BV/TV | Micro-CT and histological evaluation | Control: Ti; test group: Ti-Ag | 2 months |
| Cheng, 2016 [49] | Silver (Ag)-loaded nanotubular structures | Ag NPs | Rats | BIC, BA, and BV/TV | Micro-CT and histological evaluation | Control: TiO2-NTs; test groups: (1) NT10-Ag1, (2) NT10-Ag2, and (3) NT40-Ag1 | 6 weeks |
| Smeets, 2017 [50] | Silver-doped polysiloxane coating | Ag particles | Pigs | BIC | Histological evaluation | Control: grit-blasted and acid-etched Ti implants coated with polysiloxane; test group: silver-doped polysiloxane | 3 months |
| Liu, 2019 [51] | Ti-Ag alloy nanotubular coatings (TNT) | Ag ions | Sprague–Dawley rats | BA | Micro-CT and histological evaluation | Control: TNT; test groups: (1) Ti1% Ag-NT, (2) Ti2% Ag-NT, and (3) Ti4% Ag-NT | (1, 2, and 4 weeks) |
| Xie, 2019 [52] | Coating consisting of hydroxyapatite (HA), AgNPs, and chitosan (CS) | Ag NPs | SD rats | BIC | Micro-CT and histological evaluation | Control: Ti implants; test group: HA/Ag/CS-coated implants | 12 weeks |
| Stein, 2020 [53] | Titanium–vanadium implants (Ti6Al4V) coated with a polysiloxane layer with embedded elementary silver particles | Ag particles | Merino sheep | BIC | Histological evaluation | Control: uncoated Ti implants; test group: silver-coated implants | 6 months |
| Chen, 2020 [54] | TiO2 nanotubes loaded with silver nanoparticles | Ag NPs | Sprague–Dawley rats | BV/TV | Micro-CT analysis | Control: TiO2 nanotubes (TiO2-NTs); test group: AgTiO2-NTs | 2 weeks |
| Nakashima, 2022 [28] | Silver-containing hydroxyapatite coating | Silver oxide | Sprague–Dawley rats | BIC, BA, and BV/TV | Micro-CT and histological evaluation | Control: HA; test group: Ag-HA | 4 and 8 weeks |
| Yao, 2022 [55] | Magnetron sputtering was used to form uniform Ag and strontium titanate (SrTiO3) coatings on Ti | Ag ions | New Zealand White rabbits | BV/TV | Micro-CT analysis | Control: AH-Ti; test groups: (1) AH-Ti/Ag and (2) AH-Ti/Ag/Sr | 1 month |
| Li, 2023 [56] | Ti implants immersed in AgNP solution | Ag NPs | Sprague–Dawley rats | BIC, BA, and BV/TV | Micro-CT and histological evaluation | Control: Ti; test group: AgNP | 4 weeks |
| Pinera-Avellaneda, 2023 [57] | Ti implants with an Ag-doped calcium–Ti surface layer | Ag ions | New Zealand albino rabbits | BV/TV | Micro-CT analysis | Control: Ti; test group: Ti-Ag | 2 and 4 weeks |
| Authors | Year | Animal Model | Coefficient | Quality |
|---|---|---|---|---|
| Cheng et al. [43] | 2013 | Rat | 0.761 | Average |
| Svensson et al. [44] | 2013 | Rabbit | 0.809 | Excellent |
| Qiao et al. [45] | 2015 | Dog | 0.880 | Excellent |
| Devlin-Mullin et al. [46] | 2015 | Rat | 0.785 | Average |
| Zhao et al. [47] | 2015 | Rabbit | 0.714 | Average |
| Godoy-Gallardoa et al. [48] | 2016 | Dog | 0.904 | Excellent |
| Cheng et al. [49] | 2016 | Rat | 0.738 | Average |
| Smeets et al. [50] | 2017 | Pig | 0.904 | Excellent |
| Liu et al. [51] | 2019 | Rat | 0.761 | Average |
| Xie et al. [52] | 2019 | Rat | 0.904 | Excellent |
| Stein et al. [53] | 2020 | Sheep | 0.857 | Excellent |
| Chen et al. [54] | 2020 | Rat | 0.714 | Average |
| Nakashima et al. [28] | 2022 | Rat | 0.928 | Excellent |
| Yao et al. [55] | 2022 | Rabbit | 0.761 | Average |
| Li et al. [56] | 2023 | Rat | 0.857 | Excellent |
| Pinera-Avellaneda et al. [57] | 2023 | Rabbit | 0.761 | Average |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, A. Effects of Implant Silver Coatings on Bone Formation in Animal Models: A Systematic Review and Meta-Analysis. J. Funct. Biomater. 2025, 16, 369. https://doi.org/10.3390/jfb16100369
Alenezi A. Effects of Implant Silver Coatings on Bone Formation in Animal Models: A Systematic Review and Meta-Analysis. Journal of Functional Biomaterials. 2025; 16(10):369. https://doi.org/10.3390/jfb16100369
Chicago/Turabian StyleAlenezi, Ali. 2025. "Effects of Implant Silver Coatings on Bone Formation in Animal Models: A Systematic Review and Meta-Analysis" Journal of Functional Biomaterials 16, no. 10: 369. https://doi.org/10.3390/jfb16100369
APA StyleAlenezi, A. (2025). Effects of Implant Silver Coatings on Bone Formation in Animal Models: A Systematic Review and Meta-Analysis. Journal of Functional Biomaterials, 16(10), 369. https://doi.org/10.3390/jfb16100369






