Influence of Different Biomaterials Extracted from Autologous Blood on the Cell Migration of Stem Cells from Dental Pulp
Abstract
1. Introduction
2. Materials and Methods
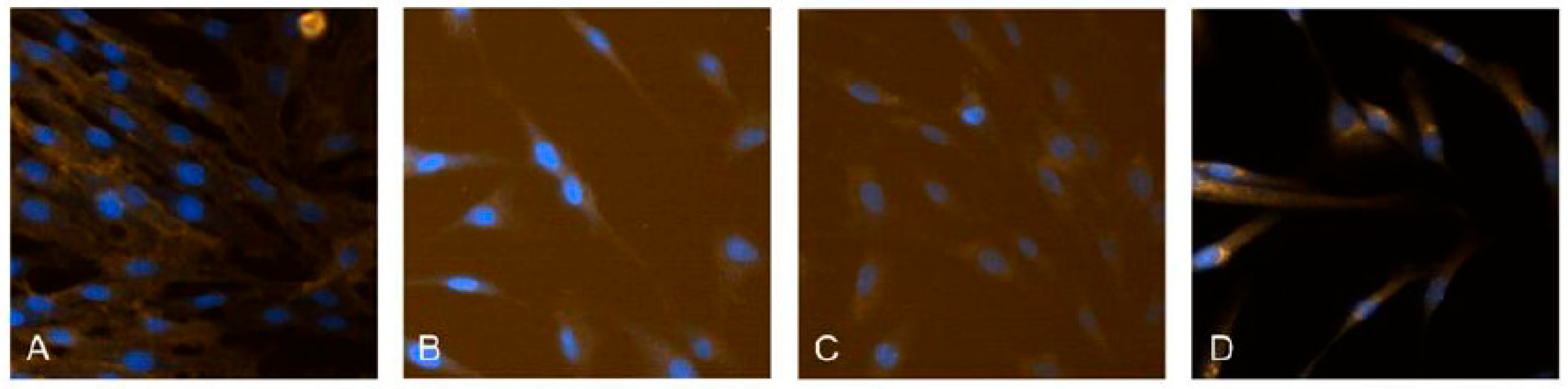
2.1. Isolation and Cultivation of hDPSC
2.2. Preparation of Biomaterials from Autologous Blood (AB Biomaterial)
2.2.1. Donor Inclusion and Exclusion Criteria
2.2.2. Type of Centrifuges Used to Obtain Biomaterials from PRF
- Centrifuge with fixed angle of the slots: DUO Quattro PRF centrifuge (Process for PRF, Nice, France) with the corresponding tubes provided according to the specifications of the same manufacturer with a red cap A-PRF (A-P by Choukroun, Process for PRF, Nice, France) and a green cap S-PRF (S Process for PRF, Nice, France) and are anticoagulant-free. The tubes differ in the internal treatment of their walls and are free from anticoagulants. The AB biomaterials A-PRF+ and Gel A-PRF+ were obtained according to the protocols of Choukroun J [10].
- Centrifuge with horizontal arrangement of the slots during operation: Bio-PRF Horizontal Centrifuge with the corresponding tubes provided according to the specifications of the same manufacturer. Bio-PRF Red Glass Tubes, 100% glass (Bio-PRF, Jupiter, FL, USA). The test tubes differ in the internal treatment of their walls and are free of anticoagulants. The AB biomaterial Solid PRF was obtained according to Miron’s protocols [15].
- Group 1. AB biomaterial Gel A-PRF+
- Group 2. AB biomaterial Solid PRF
- Group 3. AB biomaterial A-PRF+
2.2.3. Protocols for AB Biomaterial: Gel A-PRF+; Solid PRF and A-PRF+
Protocol for Autologous Blood Biomaterial A-PRF+
Protocol for Autologous Blood Biomaterial Gel A-PRF+
Protocol for Autologous Blood Biomaterial Solid PRF
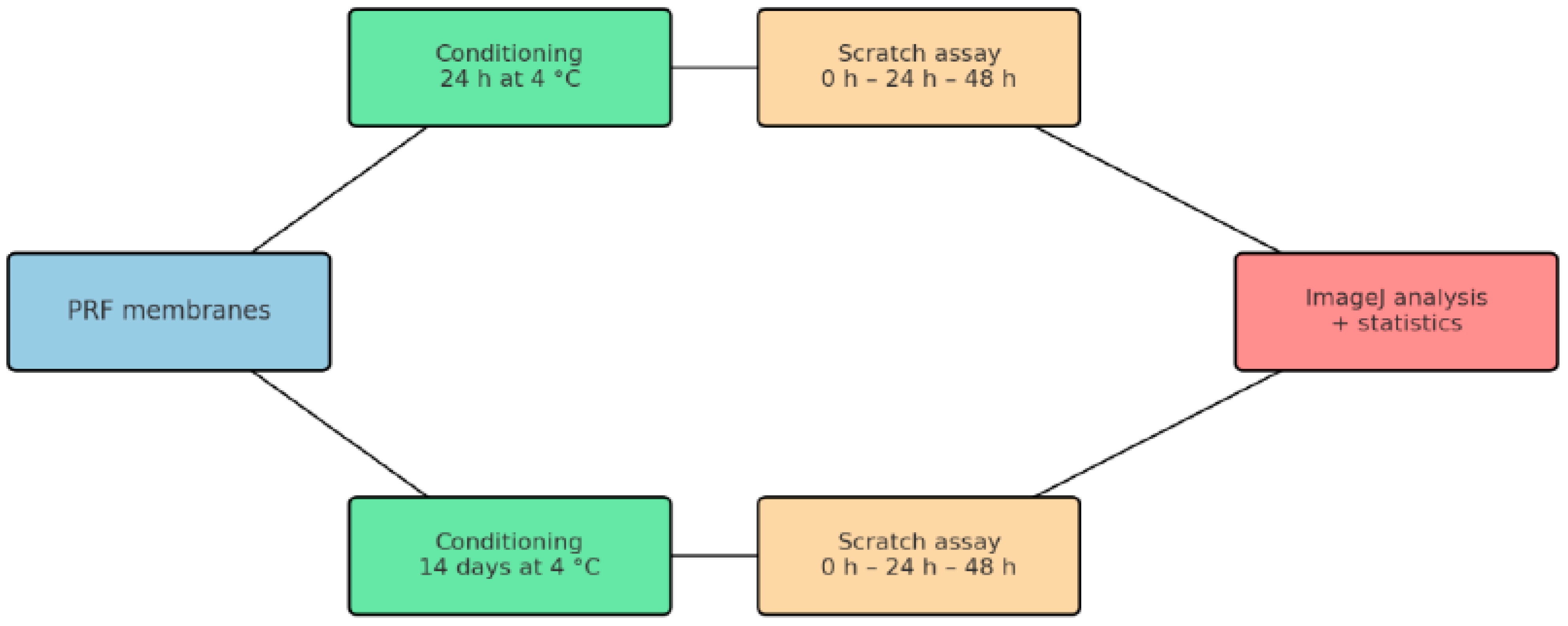
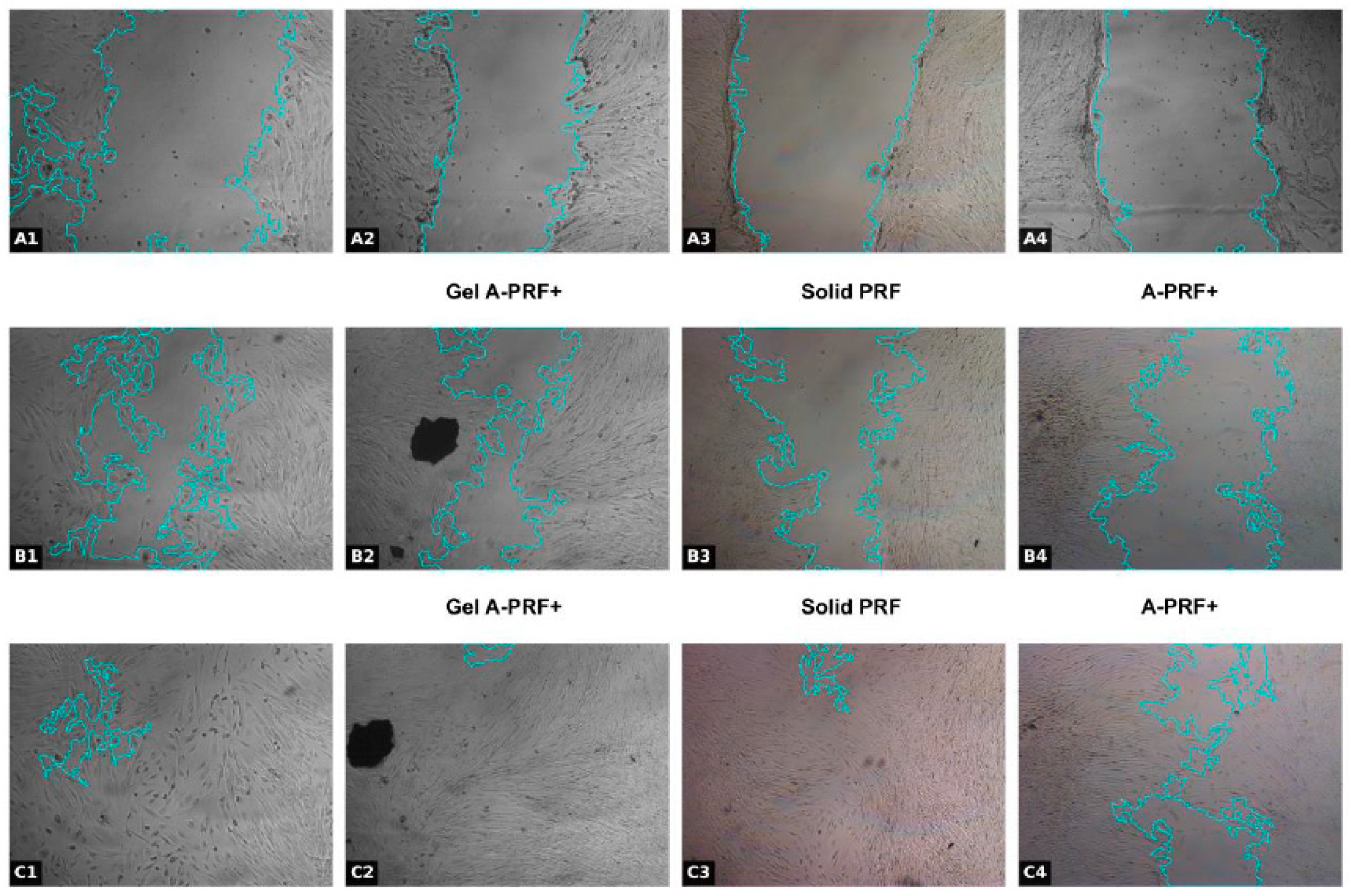
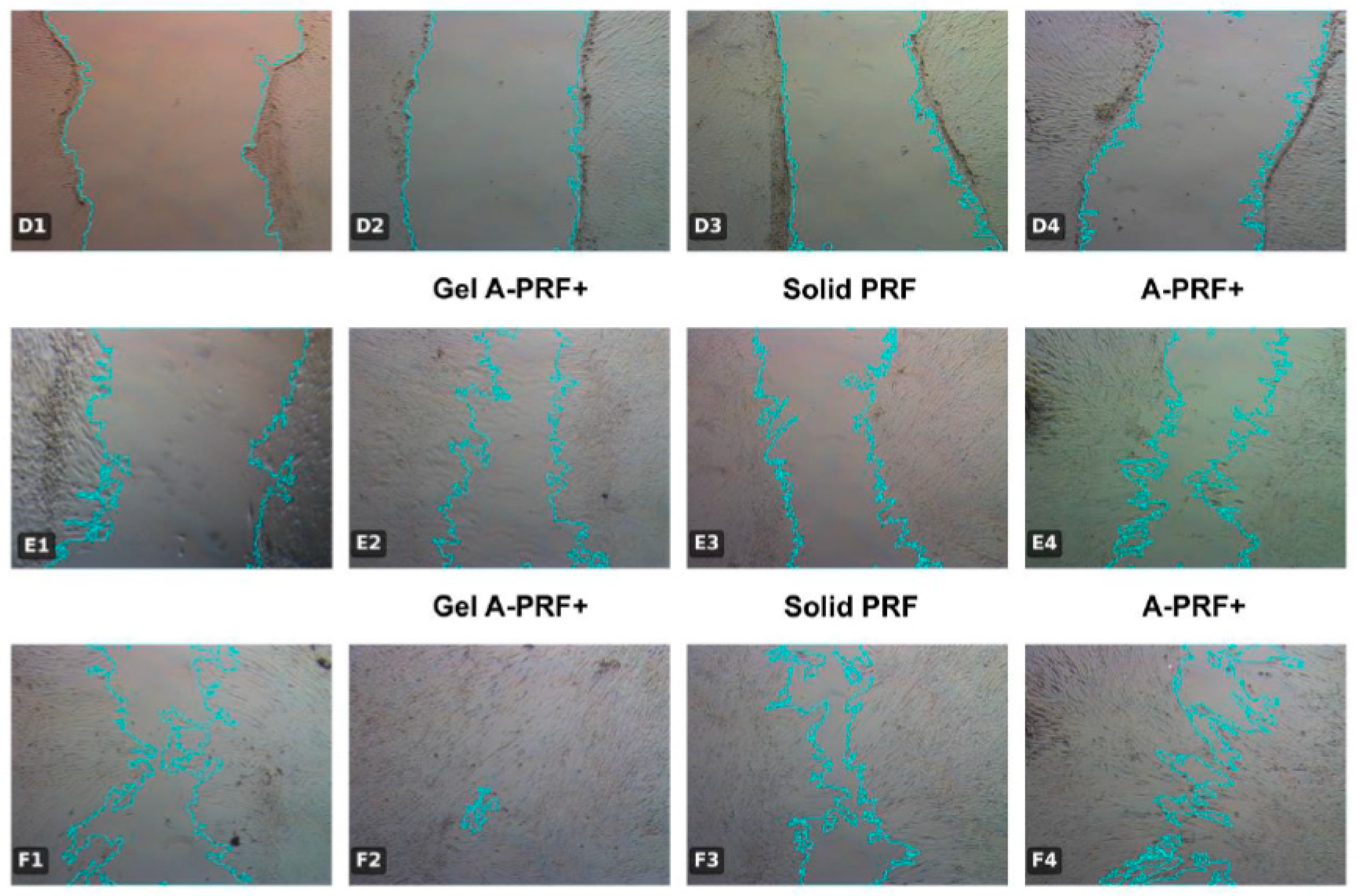
2.3. Test Method: Scratch Wound Healing Assay
3. Results
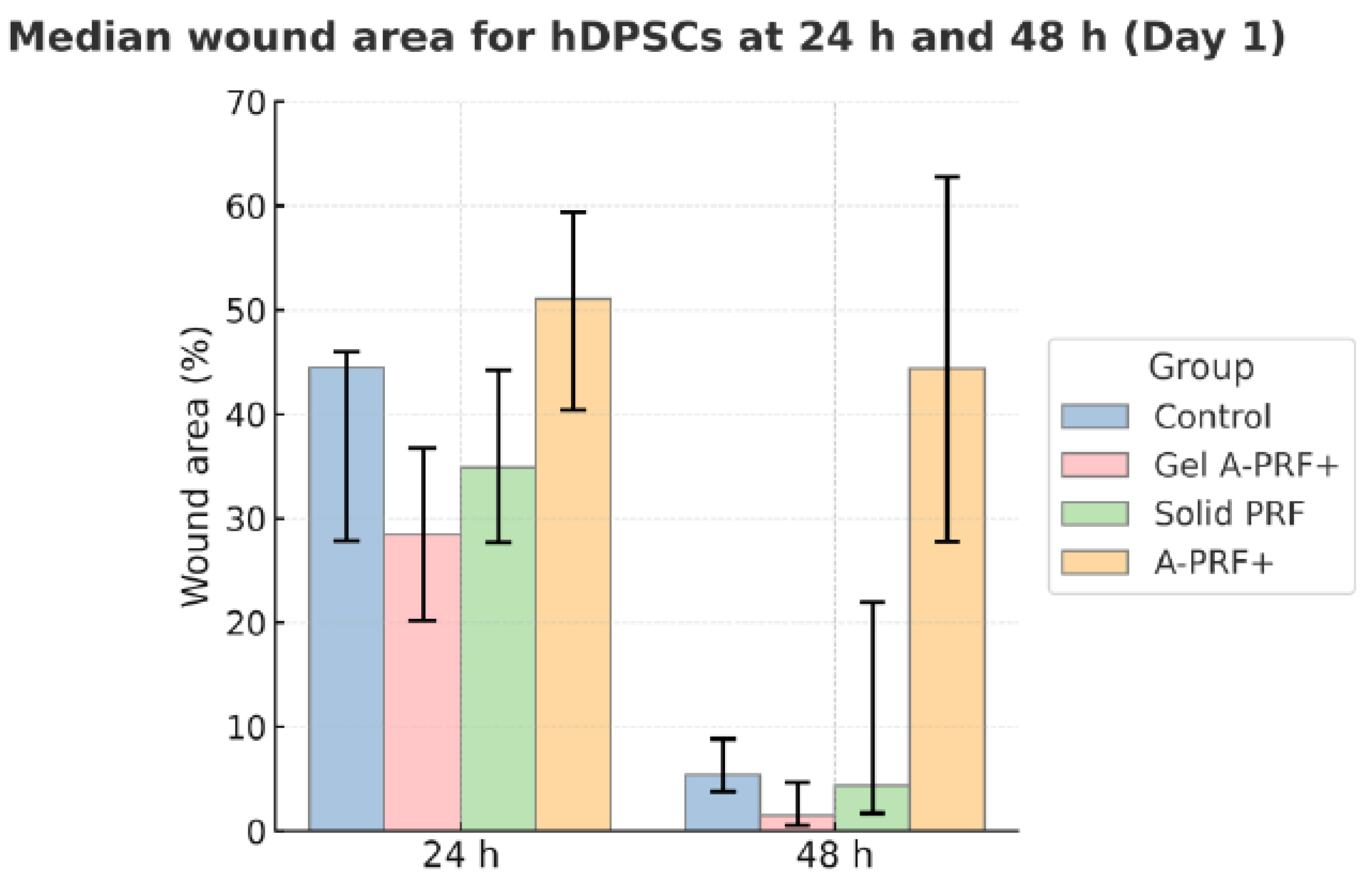
3.1. Results from the First Day of the Study
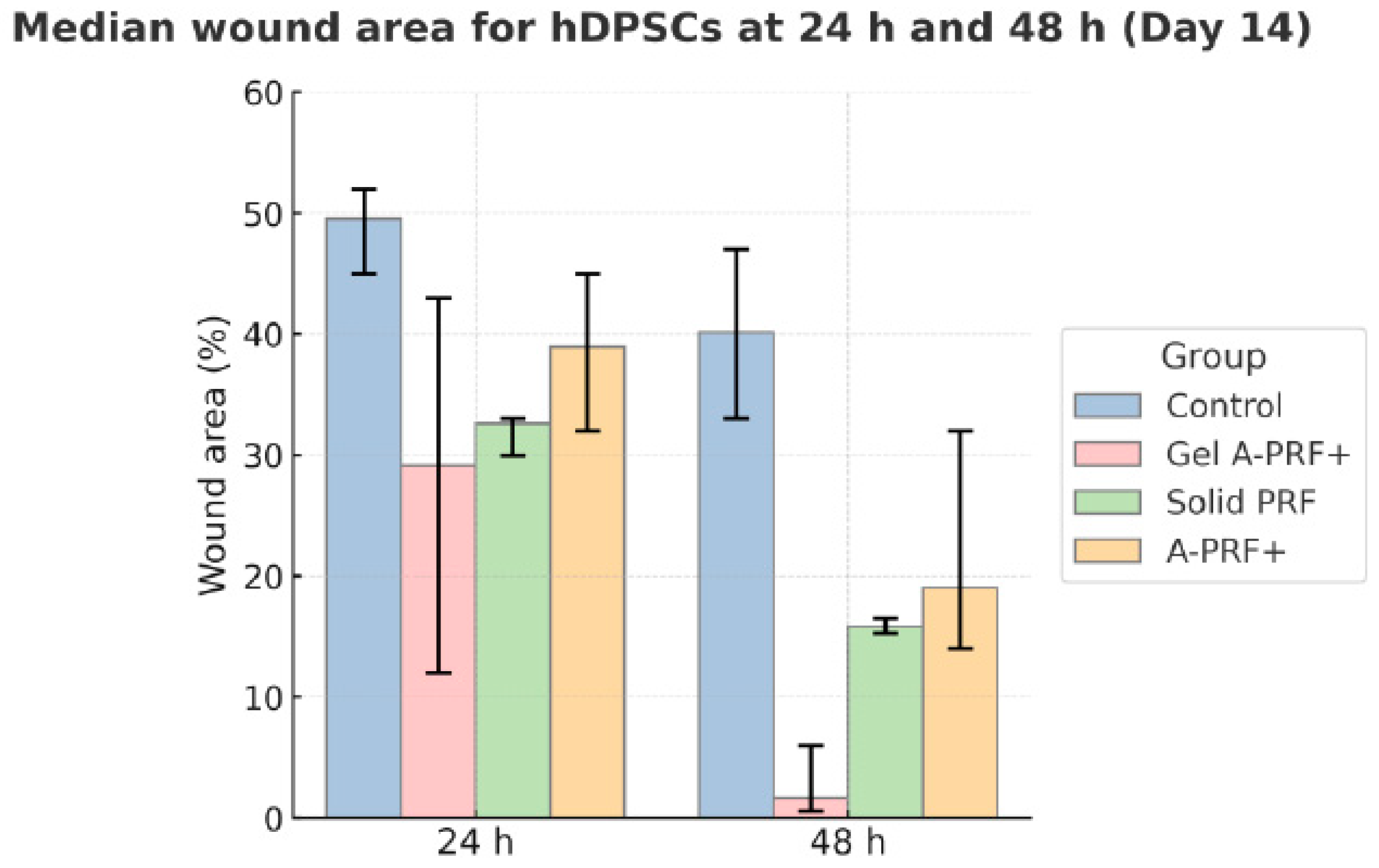
3.2. Results from the Fourteenth Day of the Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | Autologous Blood |
| A-PRF+ | Advanced Platelet-Rich Fibrin Plus |
| CD44 | Cluster of Differentiation 44 |
| CGF | Concentrated Growth Factors |
| CK19 | Cytokeratin 19 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DSPP | Dentin Sialophosphoprotein |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal Bovine Serum |
| Gel A-PRF+ | Gel form of Advanced Platelet-Rich Fibrin Plus |
| hDPSCs | Human Dental Pulp Stem Cells |
| IL-1β | Interleukin-1 beta |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| PBS | Phosphate-Buffered Saline |
| PDGF | Platelet-Derived Growth Factor |
| PRF | Platelet-Rich Fibrin |
| SD | Standard Deviation |
| Solid PRF | Solid Platelet-Rich Fibrin |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TNF-α | Tumor Necrosis Factor alpha |
| VEGF | Vascular Endothelial Growth Factor |
References
- Goldberg, M. Pulp healing and regeneration: More questions than answers. Adv. Dent. Res. 2011, 23, 270–274. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal stem cell migration and tissue repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Bartold, P.M.; Miura, M.; Seo, B.M.; Robey, P.G.; Gronthos, S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac. Res. 2005, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Chai, J.; Jin, R.; Yuan, G.; Kanter, V.; Miron, R.J.; Zhang, Y. Effect of liquid platelet-rich fibrin and platelet-rich plasma on the regenerative potential of dental pulp cells cultured under inflammatory conditions: A comparative analysis. J. Endod. 2019, 45, 1000–1008. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef]
- Kirilova, J.N.; Kosturkov, D. Direct Pulp Capping with Advanced Platelet-Rich Fibrin: A Report of Two Cases. Medicina 2023, 59, 225. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Ozsagir, Z.B.; Saglam, E.; Sen Yilmaz, B.; Choukroun, J.; Tunali, M. Injectable platelet-rich fibrin and microneedling for gingival augmentation in thin periodontal phenotype: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zuleika, P.; Saleh, I.; Murti, K.; Liberty, I.A.; Legiran; Irfanuddin; Surono, A. Platelet-Rich Fibrin: A Systematic Review of Its Action. J. Nat. Sci. Med. 2024, 7, 242–252. [Google Scholar] [CrossRef]
- Pan, J.; Luo, L.; Jiang, Z.; Huang, H.; Jiang, B. The effect of injectable platelet-rich fibrin and platelet-rich fibrin in regenerative endodontics: A comparative in vitro study. J. Appl. Oral Sci. Rev. FOB 2024, 32, e20230449. [Google Scholar] [CrossRef] [PubMed]
- Margono, A.; Bagio, D.A.; Yulianto, I.; Dewi, S.U. Changes in migratory speed rate of human dental pulp stromal cells cultured in advanced platelet-rich fibrin. Eur. J. Dent. 2023, 17, 91–96. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Evaluation of 24 Protocols for the Production of Platelet-Rich Fibrin. BMC Oral Health 2020, 20, 310. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stemcells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.M.; Ishkitiev, N.; Yaegaki, K.; Calenic, B.; Ishikawa, H.; Nakahara, T.; Mitev, V.; Haapasalo, M. Expression of multiple stem cell markers in dental pulp cells cultured in serum-free media. J. Endod. 2010, 36, 1139–1144. [Google Scholar] [CrossRef]
- Miteva, M.; Mihaylova, Z.; Mitev, V.; Zagorchev, P.; Niwa, T.; Okawa, R.; Takeuchi, A.; Kawada, H.; Naka, K.; Komatsu, N.; et al. A Review of Stem Cell Attributes Derived from the Oral Cavity. Int. Dent. J. 2024, 74, 1129–1141. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital Pulp Therapy: Histopathology and Histobacteriology-Based Guidelines to Treat Teeth with Deep Caries and Pulp Exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef]
- Le, S.H.; Nguyen, S.H. The in vitro efficacy of advanced platelet-rich fibrin plus versus injectable platelet-rich fibrin on the proliferation, migration, and differentiation of stem cells from apical papilla. J. Dent. Sci. 2024, 19, 1580–1590. [Google Scholar] [CrossRef]
- Zwittnig, K.; Kirnbauer, B.; Jakse, N.; Schlenke, P.; Mischak, I.; Ghanaati, S.; Al-Maawi, S.; Végh, D.; Payer, M.; Zrnc, T.A. Growth Factor Release within Liquid and Solid PRF. J. Clin. Med. 2022, 11, 5070. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Optimization of Platelet-Rich Fibrin. Periodontology 2000 2023, 93, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Song, G.; Chai, J.; Gou, X.; Yuan, G.; Chen, Z. Effects of Concentrated Growth Factor on Proliferation, Migration, and Differentiation of Human Dental Pulp Stem Cells In Vitro. J. Tissue Eng. 2018, 9, 2041731418817505. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Ghanaati, S. Reduction of Relative Centrifugation Force within Injectable Platelet-Rich Fibrin (PRF) Concentrates Advances Patients’ Own Inflammatory Cells, Platelets and Growth Factors: The First Introduction to the Low Speed Centrifugation Concept. Eur. J. Trauma. Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, Y.; Lin, Y.; Jiang, H. Platelet-Rich Fibrin and Concentrated Growth Factors as Novel Platelet Concentrates for Chronic Hard-to-Heal Skin Ulcers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Dermatol. Treat. 2020, 33, 613–621. [Google Scholar] [CrossRef]
| Parameter | Fixed-Angle (“Vertical”, Choukroun) | Horizontal (Swing-Out, Miron) |
|---|---|---|
| Rotor orientation | Tubes fixed ~25–45°; separation along an inclined column | Buckets swing to true horizontal; separation across a short vertical column |
| RCF field | Steeper radial RCF gradient along tube length | More uniform in-tube RCF distribution |
| Cell distribution | Sharper phase demarcation; higher g/longer spins may drive cells toward RBC layer | Broader interface; often higher platelet/leukocyte recovery at matched RCF/time |
| Typical PRF protocols * | A-PRF/A-PRF+: lower g, longer time (≈few hundred g for ~8–14 min) → looser fibrin, better cell preservation | Solid/liquid PRF variants: multiple validated protocols across a range of g and durations |
| Tubes/consumables | Prefer glass/plain, no anticoagulant | Same; tube material/coatings can modulate fibrin polymerization |
| Temperature/braking | Room temperature; gentle braking; start timing after target speed | Same |
| Days of Investigation Comparison Between Group | 24 h/Day 1 | 48 h/Day 1 | ||||
|---|---|---|---|---|---|---|
| p-Value Mann–Whitney | p-Bonferroni | Effect Size (r) | p-Value Mann–Whitney | p-Bonferroni | Effect Size (r) | |
| Gel-A-PRF+ vs. Control | 0.0181 | 0.1084 | 0.506 *** | 0.0006 * | 0.0036 * | 0.731 ** |
| Solid-PRF vs. Control | 0.067 | 0.4022 | 0.394 | 0.8157 | 1 | 0.056 |
| A-PRF+ vs. Control | 0.067 | 0.4022 | 0.394 | 0.0001 * | 0.0004 * | 0.843 *** |
| Gel-A-PRF+ vs. Solid-PRF | 0.1028 | 0.6168 | 0.372 | 0.0252 | 0.1512 | 0.507 *** |
| A-PRF+ vs. Gel-A-PRF+ | 0.0002 * | 0.001 * | 0.845 ** | 0.0002 * | 0.0010 * | 0.845 ** |
| Solid-PRF vs. A-PRF+ | 0.011 | 0.0661 | 0.575 *** | 0.0002 * | 0.0010 * | 0.845 ** |
| Days of Investigation Comparison Between Group | 24 h/Day 14 | 48 h/Day 14 | ||||
|---|---|---|---|---|---|---|
| p-Value Mann–Whitney | p-Bonferroni | Effect Size (r) | p-Value Mann–Whitney | p-Bonferroni | Effect Size (r) | |
| Gel-A-PRF+ vs. Solid-PRF | 0.3027 | 1 | 0.237 | 0.0015 * | 0.0092 * | 0.710 ** |
| Gel-A-PRF+ vs. A-PRF+ | 0.1028 | 0.6168 | 0.372 | 0.0006 * | 0.0033 * | 0.778 ** |
| Solid-PRF vs. A-PRF+ | 0.0042 * | 0.0252 * | 0.642 * | 0.0243 | 0.1459 | 0.507 *** |
| Gel-A-PRF+ vs. Control | 0.0006 * | 0.0036 * | 0.731 ** | 0.0001 * | 0.0004 * | 0.843 *** |
| Solid-PRF vs. Control | 0.0001 * | 0.0004 * | 0.843 *** | 0.0001 * | 0.0004 * | 0.843 *** |
| A-PRF+ vs. Control | 0.0038 * | 0.0226 * | 0.619 * | 0.0038 * | 0.0226 * | 0.619 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirilova, J.N.; Vladova, R.Z.; Petrova, V.P.; Yantcheva, S.; Deliverska, E.G.; Ishkitiev, N.D. Influence of Different Biomaterials Extracted from Autologous Blood on the Cell Migration of Stem Cells from Dental Pulp. J. Funct. Biomater. 2025, 16, 398. https://doi.org/10.3390/jfb16110398
Kirilova JN, Vladova RZ, Petrova VP, Yantcheva S, Deliverska EG, Ishkitiev ND. Influence of Different Biomaterials Extracted from Autologous Blood on the Cell Migration of Stem Cells from Dental Pulp. Journal of Functional Biomaterials. 2025; 16(11):398. https://doi.org/10.3390/jfb16110398
Chicago/Turabian StyleKirilova, Janet N., Rositsa Z. Vladova, Viktoria P. Petrova, Sevda Yantcheva, Elitsa G. Deliverska, and Nikolay D. Ishkitiev. 2025. "Influence of Different Biomaterials Extracted from Autologous Blood on the Cell Migration of Stem Cells from Dental Pulp" Journal of Functional Biomaterials 16, no. 11: 398. https://doi.org/10.3390/jfb16110398
APA StyleKirilova, J. N., Vladova, R. Z., Petrova, V. P., Yantcheva, S., Deliverska, E. G., & Ishkitiev, N. D. (2025). Influence of Different Biomaterials Extracted from Autologous Blood on the Cell Migration of Stem Cells from Dental Pulp. Journal of Functional Biomaterials, 16(11), 398. https://doi.org/10.3390/jfb16110398






