Comparison of Wound Healing Efficiency Between Bacterial Cellulose Dry Membrane and Commercial Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cellulose Membrane Production
2.2. Scanning Electron Microscopy (SEM)
2.3. Cell Culture, BC Extraction, and Cytotoxic Assay
2.4. S. aureus-Containing Poloxamer 407 Hydrogel Preparation
2.5. Animal Experiment
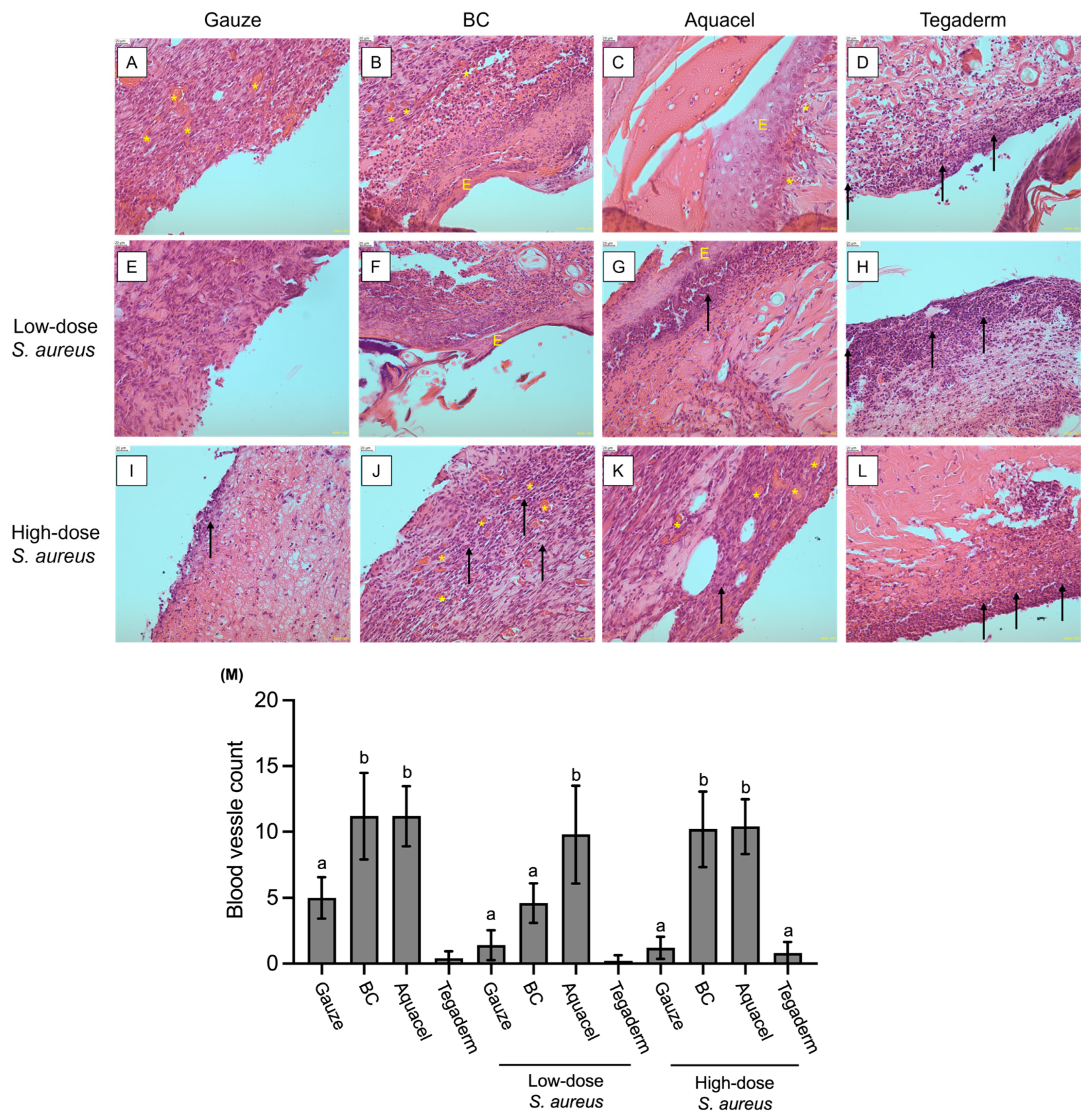
2.6. Histological Analysis and Blood Vessel Count
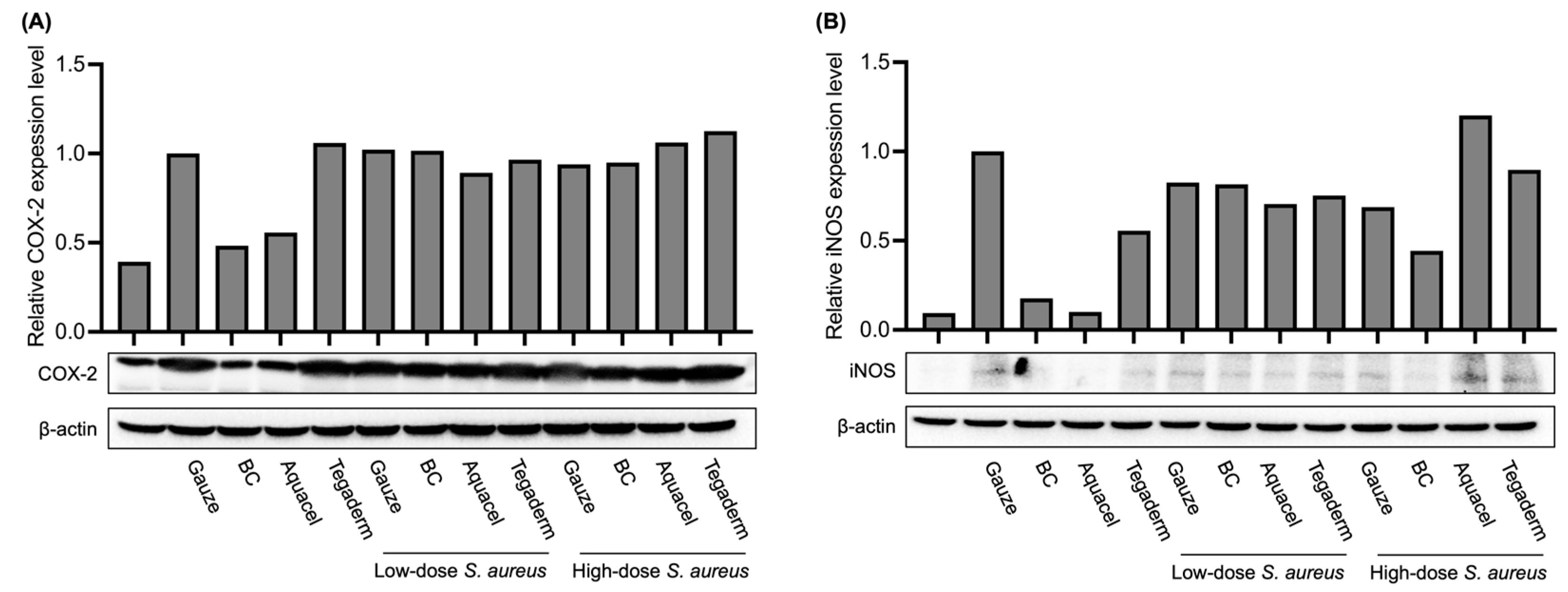
2.7. Protein Extraction and Western Blot
2.8. Statistical Analysis
3. Results
3.1. BC Membrane Structure and Cytotoxic Assay
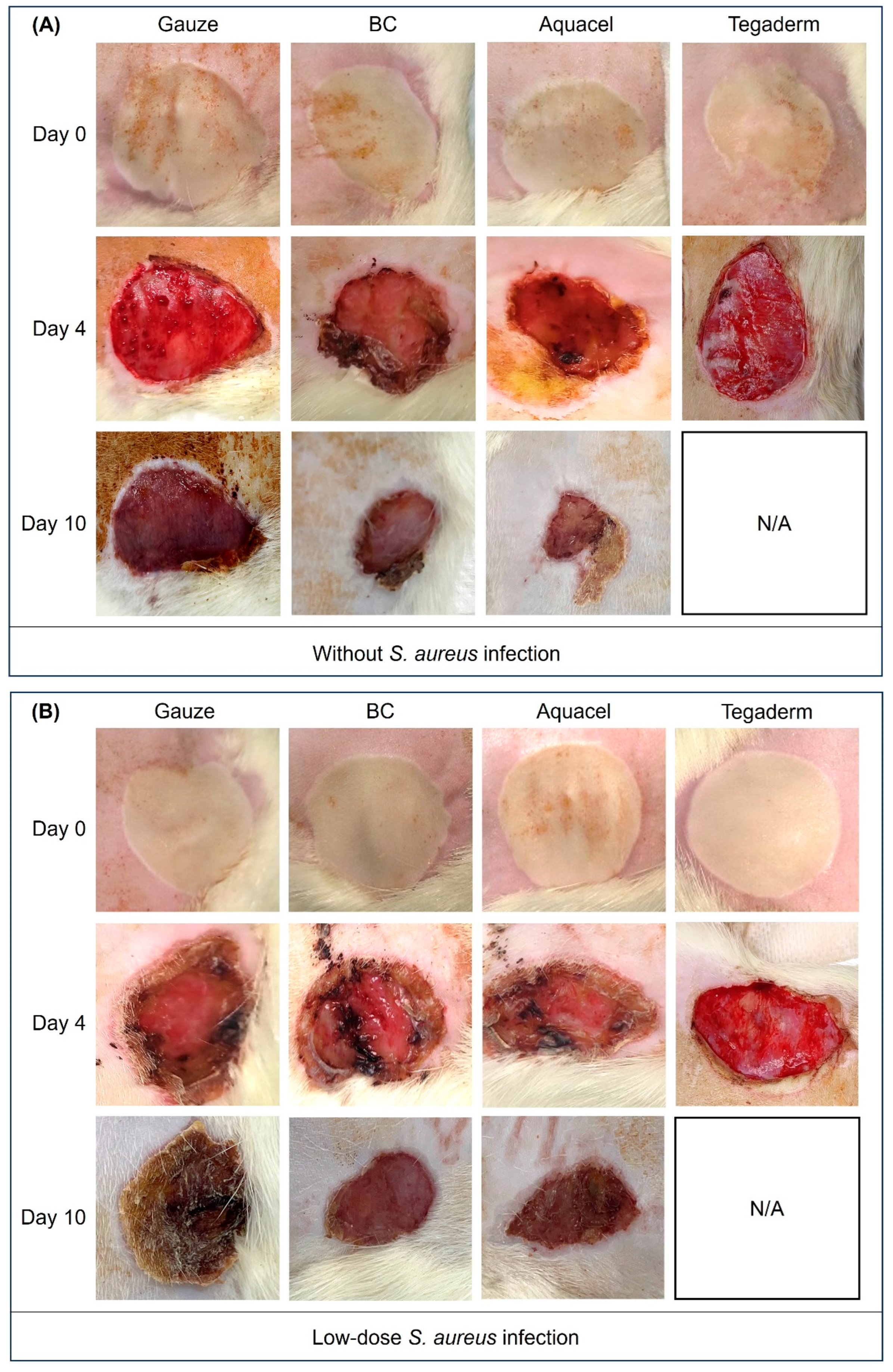
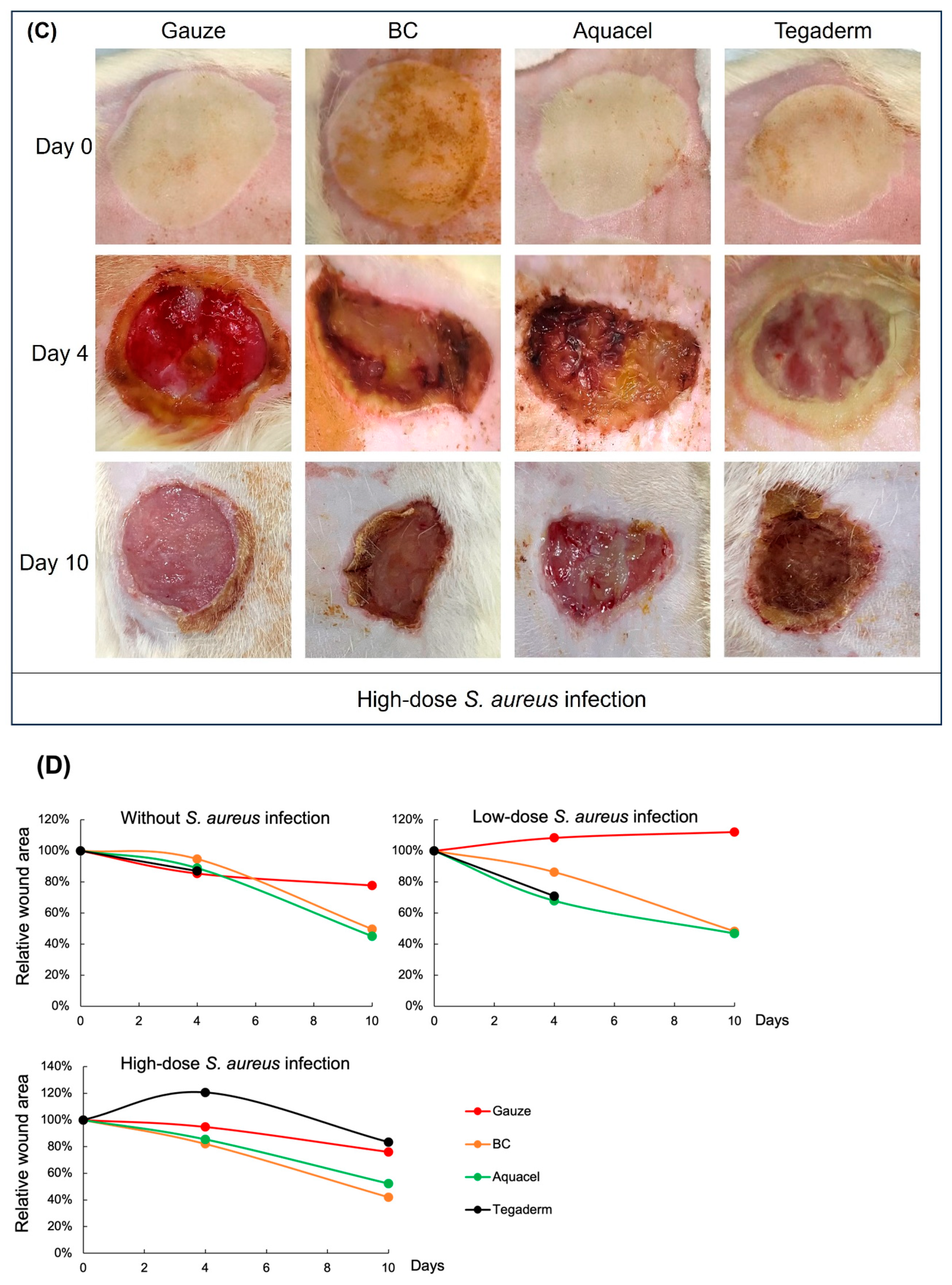
3.2. Wound Healing Activity Between Different Treatments
3.3. Comparison of Different Dressing Treatments by Histology Analysis
3.4. Comparison of Inflammation Marker Levels Under Different Treatments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brassolatti, P.; Bossini, P.S.; Kido, H.W.; Derencio Oliveira, M.C.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; Retto da Silva de Avó, L.; Araújo-Moreira, F.M.; Parizotto, N.A. Photobiomodulation and bacterial cellulose membrane in the treatment of third-degree burns in rats. J. Tissue Viability 2018, 27, 249–256. [Google Scholar] [CrossRef]
- Meng, E.; Chen, C.L.; Liu, C.C.; Liu, C.C.; Chang, S.J.; Cherng, J.H.; Wang, H.H.; Wu, S.T. Bioapplications of bacterial cellulose polymers conjugated with Resveratrol for epithelial defect regeneration. Polymers 2019, 11, 1048. [Google Scholar] [CrossRef]
- Plucknett, D.L.; Smith, N.J. Agricultural research and Third World food production. Science 1982, 217, 215–220. [Google Scholar] [CrossRef]
- Khalid, A.; Ullah, H.; Ul-Islam, M.; Khan, R.; Khan, S.; Ahmad, F.; Khan, T.; Wahid, F. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662–47668. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Nikolaeva, E.D.; Kirichenko, A.K.; Shishatskaya, E.I. Biotechnological wound dressings based on bacterial cellulose and degradable copolymer P(3HB/4HB). Int. J. Biol. Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Novaes, A.B. Soft tissue management for primary closure in guided bone regeneration: Surgical technique and case report. Int. J. Oral. Maxillofac. Implant. 1997, 12, 84–87. [Google Scholar]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial cellulose-based materials as dressings for wound healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef]
- Lin, S.-P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Moraes, P.R.F.d.S.; Saska, S.; Barud, H.; Lima, L.R.d.; Martins, V.d.C.A.; Plepis, A.M.d.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial cellulose/collagen hydrogel for wound healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M.; et al. Recent advances in cellulose-based structures as the wound-healing biomaterials: A clinically oriented review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Calum, H.; Moser, C.; Jensen, P.; Christophersen, L.; Maling, D.S.; van Gennip, M.; Bjarnsholt, T.; Hougen, H.P.; Givskov, M.; Jacobsen, G.K.; et al. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin. Exp. Immunol. 2009, 156, 102–110. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound dressing modifications for accelerated healing of infected wounds. Int. J. Mol. Sci. 2023, 24, 7193. [Google Scholar] [CrossRef]
- Omar, S.; Asif, A.; Douha, S.; Joshua, B. Antimicrobial dressings for improving wound healing. In Wound Healing-New Insights Into Ancient Challenges; IntechOpen: Rijeka, Croatia, 2016; Volume 17, pp. 374–398. [Google Scholar] [CrossRef]
- Woodford, N.; Livermore, D.M. Infections caused by Gram-positive bacteria: A review of the global challenge. J. Infect. 2009, 59 (Suppl. 1), S4–S16. [Google Scholar] [CrossRef]
- Forng, E.R.; Anderson, S.M.; Cannon, R.E. Synthetic medium for Acetobacter xylinum that can be used for isolation of auxotrophic mutants and study of cellulose biosynthesis. Appl. Environ. Microbiol. 1989, 55, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Ryan, A.J.; Squires, S.; Strutt, H.L.; Johnson, R.T. Camptothecin cytotoxicity in mammalian cells is associated with the induction of persistent double strand breaks in replicating DNA. Nucleic Acids Res. 1991, 19, 3295–3300. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organ-ization for Standardization: Geneva, Switzerland, 2009.
- Mirmohammadsadegh, N.; Shakoori, M.; Moghaddam, H.N.; Farhadi, R.; Shahverdi, A.R.; Amin, M. Wound healing and anti-inflammatory effects of bacterial cellulose coated with Pistacia atlantica fruit oil. DARU J. Pharm. Sci. 2022, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Yin, H.Q.; Liu, S.; Heggers, J.P.; Langford, R.E.; Olson, M.E.; Hart, D.A.; Burrell, R.E. Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen. 2005, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M., Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Walker, M.; Hobot, J.A.; Newman, G.R.; Bowler, P.G. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL) and alginate dressings. Biomaterials 2003, 24, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Parsons, D. Physico-chemical characterisation of carboxymethylated spun cellulose fibres. Biomaterials 2001, 22, 903–912. [Google Scholar] [CrossRef]
- Moshakis, V.; Fordyce, M.J.; Griffiths, J.D.; McKinna, J.A. Tegadern versus gauze dressing in breast surgery. Br. J. Clin. Pract. 1984, 38, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Rivera, A.E.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef]
- Strecker-McGraw, M.K.; Jones, T.R.; Baer, D.G. Soft tissue wounds and principles of healing. Emerg. Med. Clin. N. Am. 2007, 25, 1–22. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Zayed, H.S.; Saleh, S.; Omar, A.E.; Saleh, A.K.; Salama, A.; Tolba, E. Development of collagen-chitosan dressing gel functionalized with propolis-zinc oxide nanoarchitectonics to accelerate wound healing. Int. J. Biol. Macromol. 2024, 261, 129665. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Chen, X.; Jiang, T.; Song, P.; Wang, B.; Zhao, X. Mussel-inspired nanocomposite hydrogel based on alginate and antimicrobial peptide for infected wound repair. Int. J. Biol. Macromol. 2022, 219, 1087–1099. [Google Scholar] [CrossRef]
- Sadlik, J.; Kosińska, E.; Słota, D.; Niziołek, K.; Tomala, A.; Włodarczyk, M.; Piątek, P.; Skibiński, J.; Jampilek, J.; Sobczak-Kupiec, A. Bioactive hydrogel based on collagen and Hyaluronic acid enriched with freeze-dried sheep placenta for wound healing support. Int. J. Mol. Sci. 2024, 25, 1687. [Google Scholar] [CrossRef]

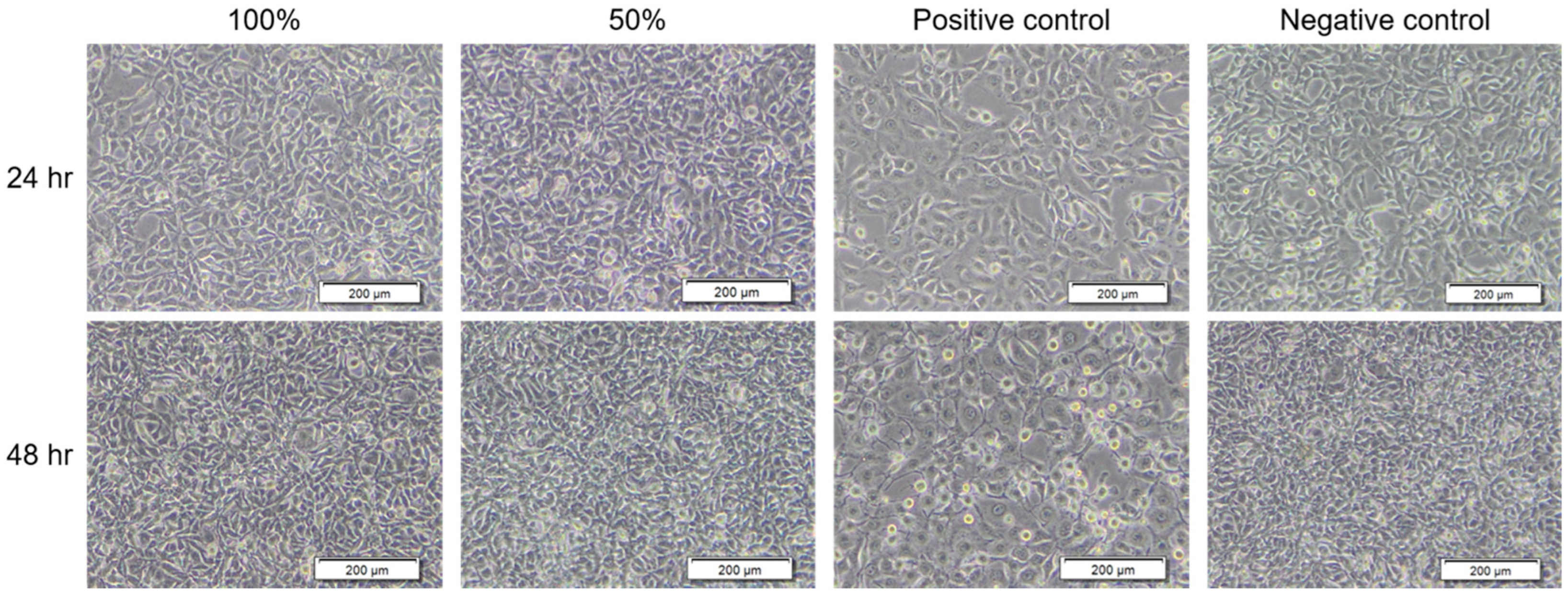
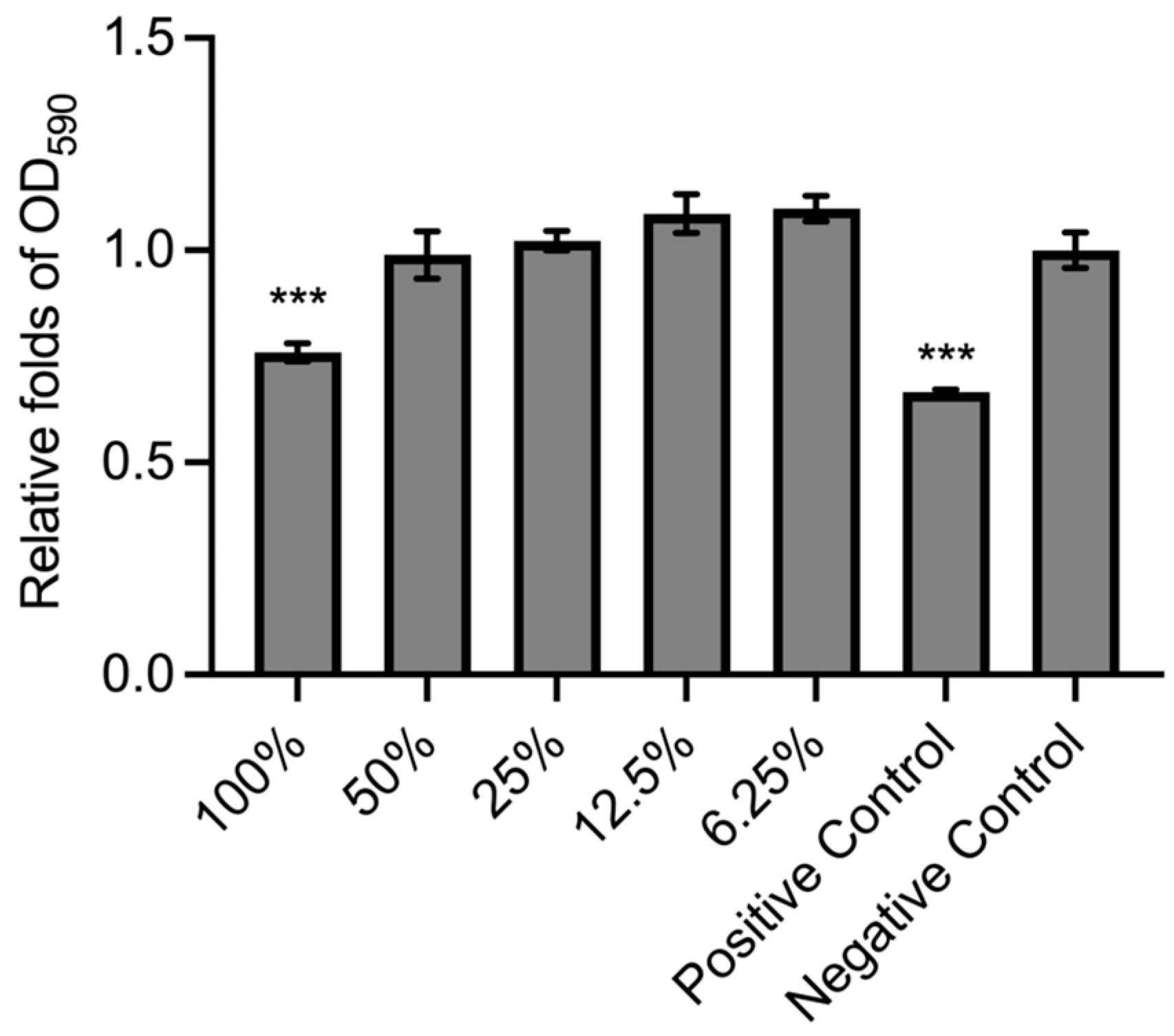
| Group | Length of Treatment Time (h) | Cell Morphology | Grading |
|---|---|---|---|
| BC extract | 0 | No cell lysis and reduction of cell growth | 0 |
| Negative control | 0 | No cell lysis and reduction of cell growth | 0 |
| Positive control | 0 | No cell lysis and reduction of cell growth | 0 |
| BC extract | 24 | No cell lysis and reduction of cell growth | 0 |
| Negative control | 24 | No cell lysis and reduction of cell growth | 0 |
| Positive control | 24 | Not more than 20% of the cells are round, only slight growth inhibition observable. | 1 |
| BC extract | 48 | No cell lysis and reduction of cell growth | 0 |
| Negative control | 48 | No cell lysis and reduction of cell growth | 0 |
| Positive control | 48 | Not more than 50% growth inhibition observable. | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, W.-W.; Zeng, Y.-J.; Yeh, T.-M.; Chen, Y.-Y.; Hsu, M.-K.; Tseng, S.-P.; Wang, H.-Y. Comparison of Wound Healing Efficiency Between Bacterial Cellulose Dry Membrane and Commercial Dressings. J. Funct. Biomater. 2025, 16, 366. https://doi.org/10.3390/jfb16100366
Sung W-W, Zeng Y-J, Yeh T-M, Chen Y-Y, Hsu M-K, Tseng S-P, Wang H-Y. Comparison of Wound Healing Efficiency Between Bacterial Cellulose Dry Membrane and Commercial Dressings. Journal of Functional Biomaterials. 2025; 16(10):366. https://doi.org/10.3390/jfb16100366
Chicago/Turabian StyleSung, Wei-Wen, Yu-Jing Zeng, Tsung-Ming Yeh, Yao-Yuan Chen, Min-Kung Hsu, Sung-Pin Tseng, and Hsian-Yu Wang. 2025. "Comparison of Wound Healing Efficiency Between Bacterial Cellulose Dry Membrane and Commercial Dressings" Journal of Functional Biomaterials 16, no. 10: 366. https://doi.org/10.3390/jfb16100366
APA StyleSung, W.-W., Zeng, Y.-J., Yeh, T.-M., Chen, Y.-Y., Hsu, M.-K., Tseng, S.-P., & Wang, H.-Y. (2025). Comparison of Wound Healing Efficiency Between Bacterial Cellulose Dry Membrane and Commercial Dressings. Journal of Functional Biomaterials, 16(10), 366. https://doi.org/10.3390/jfb16100366







