Autologous Tooth-Derived Biomaterials in Alveolar Bone Regeneration: A Systematic Review of Clinical Outcomes and Histological Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Processing
2.2. Inclusion and Exclusion Criteria
2.3. PICO Question
2.4. Data Processing
2.5. Grouping of Studies for Synthesis
2.6. Effect Measures
2.7. Synthesis Methods
2.8. Certainty Assessment
3. Results
3.1. Study Selection and Characteristics
3.2. Study Characteristics
3.3. Clinical and Radiographic Outcomes
3.4. Histological Outcomes
3.5. Implant Outcomes
3.6. Complications
| Author (Year) | Design | Country | Purpose | Sample Size (I/C) | Mean Age (I/C) | Population | Intervention (I) | Comparator (C) | Outcome (O) | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Elfana et al. (2021) [66] | RCT | Egypt | Evaluate efficacy of whole-tooth vs demineralized dentin graft in ARP | 20/20 | 42.6 ± 6.2/41.9 ± 5.8 | Patients undergoing tooth extraction | Autogenous whole-tooth graft for ARP | Demineralized dentin graft | Dimensional changes of the alveolar ridge, radiographic and clinical evaluation | At 6 months: ridge width loss 1.2 mm vs. 2.4 mm (p < 0.05). New bone: 48.9% vs. 21.5% (p < 0.001). Conclusion: demineralized dentin is superior. |
| Elraee et al. (2022) [59] | RCT | Egypt | Compare dentin vs autogenous bone block for ridge augmentation | 18/18 | 41.2 ± 6.9/42.1 ± 7.0 | Patients requiring horizontal ridge augmentation | Autogenous dentin block graft for ridge augmentation | Autogenous bone block graft | Clinical and histological evaluation of horizontal ridge width gain, bone quality, and implant feasibility | At 6 months: horizontal gain 3.52 mm vs. 2.24 mm (p < 0.05). Histology: 42% new bone both groups, residual dentin 30%. Conclusion: dentin maintains volume longer. |
| Hussain et al. (2023) [62] | RCT | Iraq | Evaluate autogenous dentin biomaterial in socket preservation | 29/29 | 39.8 ± 8.2/40.1 ± 7.9 | Patients undergoing single tooth extraction | Autogenous dentin graft | Natural healing (no graft) | Radiographic evaluation of bone height and width preservation | At 3 months: horizontal bone loss 0.9 mm vs. 2.5 mm (p < 0.01). Histology: vital bone, minimal remnants. Conclusion: ADB is safe and effective. |
| Li et al. (2018) [65] | RCT | China | Compare DDM vs Bio-Oss in GBR with immediate implants | 20/20 | 40.6 ± 5.8/41.1 ± 6.1 | Patients requiring immediate implant placement in periodontal post-extraction sites | Autogenous DDM in GBR | Bio-Oss granules in GBR | Radiographic assessment of bone volume around implants | At 6 months: implant ISQ stability similar; new bone 45% vs. 38% (NS). Implant survival 95.6%. Conclusion: DDM is comparable to xenograft. |
| López Sacristán et al. (2024) [61] | Split-mouth clinical study | Spain | Compare ATDG with spontaneous healing in bilateral sockets | 15/15 | 46.2 ± 7.5/45.8 ± 7.1 | Patients requiring bilateral tooth extraction | ATDG in post-extraction socket | Contralateral socket left to heal naturally | Radiological and histological analysis of bone regeneration and socket preservation | At 4 months: ridge width loss 12.8% vs. 26% (p < 0.05). Histology: osteogenesis and integration. Conclusion: ATDG is effective. |
| Oguić et al. (2023) [67] | RCT | Croatia | Assess autologous dentin graft vs bovine/autologous mix in esthetic zone | 22/22 | 44.9 ± 7.4/45.6 ± 7.3 | Patients requiring grafting in the esthetic zone | Autologous dentin graft | Bovine xenograft mixed with autologous bone | Radiographic, histological, and immunohistochemical evaluation of osteogenesis and graft integration | At 6 months: bone fill is similar. Immunohistochemistry: lower TNF-α, BMP-4 in dentin group. Conclusion: favorable remodeling, reduced inflammation |
| Ouyang et al. (2024) [63] | RCT | China | Compare APDDM vs DBBM in orthodontic patients with alveolar deficiency | 30/30 | 37.9 ± 9.1/38.4 ± 8.8 | Orthodontic patients with alveolar bone deficiency (n = 60) | Partially demineralized dentin matrix(APDDM) | Deproteinized bovine bone mineral (DBBM) | Dimensional gain in bone width and height, reduced postoperative pain and swelling, similar long-term bone stability | At 6 months: gain width 3.5 ± 0.8 mm vs. 2.2 ± 0.6 mm (p = 0.002). At 24 months: resorption higher in APDDM. Conclusion: faster turnover, less discomfort. |
| Pohl (2017) [64] | Open prospective clinical study | Austria | Evaluate untreated tooth grafts for ridge augmentation | 20 | 49.7 ± 10.2 | Patients requiring lateral ridge augmentation or intraosseous defect filling (n = 20) | Autogenous unaltered tooth material (blocks and particulate) | No direct comparator; descriptive clinical cohort | Implant survival rate (96.4%), peri-implant bone loss (0.58 mm at 2 years), probing depth (1.7 mm), no inflammation | At 24 months: implant survival 96.4%; marginal bone loss 0.58 mm. Conclusion: tooth graft is effective, osteoconductive. |
| Radoczy-Drajko et al. (2021) [60] | Prospective clinical study | Hungary | Assess autologous tooth particulate graft in ridge preservation | 25/25 | 51.3 ± 7.1/50.9 ± 6.8 | Patients with extraction defect score class 3–4 post-extraction defects | Autogenous tooth-derived particulate graft for ridge preservation | No graft (natural healing) | Clinical, radiographic and histological evaluation of bone regeneration and ridge preservation | At 6 months: horizontal reduction 15% vs 26% (p < 0.05). New bone 56% vs. 42%. Conclusion: graft improved preservation |
3.7. Risk of Bias Assessment
3.7.1. ROBINS-I Domains (Non-Randomized Studies) (Table 3)
- Bias due to confounding
- Bias in the selection of participants
- Bias in the classification of interventions
- Bias due to deviations from intended interventions
- Bias due to missing data
- Bias in the measurement of outcomes
- Bias in the selection of the reported result
3.7.2. RoB-2 Domains (Randomized Studies) (Table 4)
- Bias arising from the randomization process
- Bias due to deviations from intended interventions
- Bias due to missing outcome data
- Bias in the measurement of the outcome
- Bias in the selection of the reported result
- Low-risk studies employed appropriate randomization, maintained blinding when applicable, and reported outcomes completely and transparently.
- Moderate risk studies lacked blinding or had some methodological limitations but retained informative value.
- High-risk studies showed clear issues with randomization, management of deviations, or incomplete outcome data, limiting the internal validity of results.
4. Discussion
4.1. Methodological Limitations and Critical Issues
4.2. Clinical Implications and Future Perspectives
- Multicenter randomized clinical trials, with large sample sizes and a minimum follow-up of 24 months to assess long-term tissue stability and implant survival rates;
- Subjective outcome evaluations, such as pain, functional recovery, patient satisfaction, and perceived aesthetic outcomes; and
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADB | Autogenous Dentin Biomaterial |
| ADG | Autogenous Demineralized Dentin Graft |
| ABR | Alveolar Bone Regeneration |
| APDDM | Autologous Partially Demineralized Dentin Matrix |
| ARA | Alveolar Ridge Augmentation |
| ARP | Alveolar Ridge Preservation |
| ATDG(s) | Autologous Tooth-Derived Graft(s) |
| BMP(s) | Bone Morphogenetic Protein(s) |
| CBCT | Cone Beam Computed Tomography |
| DBBM | Deproteinized Bovine Bone Mineral |
| DDM | Demineralized Dentin Matrix |
| DMP-1 | Dentin Matrix Protein 1 |
| GBR | GBR Guided Bone Regeneration |
| HA | Hydroxyapatite |
| IGF-II | Insulin-like Growth Factor II |
| ISQ | Implant Stability Quotient |
| PRF | Platelet-Rich Fibrin |
| RCT(s) | Randomized Controlled Trial(s) |
| TGF-β | Transforming Growth Factor Beta |
References
- Ohnishi, H.; Fujii, N.; Futami, T.; Taguchi, N.; Kusakari, H.; Maeda, T. A Histochemical Investigation of the Bone Formation Process by Guided Bone Regeneration in Rat Jaws. Effect of PTFE Membrane Application Periods on Newly Formed Bone. J. Periodontol. 2000, 71, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Nampo, T.; Watahiki, J.; Enomoto, A.; Taguchi, T.; Ono, M.; Nakano, H.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Maki, K. A New Method for Al veolar Bone Repair Using Extracted Teeth for the Graft Material. J. Periodontol. 2010, 81, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.-P.; Buser, D. Ridge Alterations Post-Extraction in the Esthetic Zone. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zeng, H.; Fan, W.; Wu, T.; Sun, J.; Yan, Q.; Shi, B. Ridge Preservation of a Novel Extraction Socket Applying Bio-Oss® Collagen: An Experimental Study in Dogs. J. Dent. Sci. 2021, 16, 831–839. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A Systematic Review of Post-Extractional Alveolar Hard and Soft Tissue Dimensional Changes in Humans. Clin. Oral Implant. Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult Human Dental Pulp Stem Cells Differentiate toward Functionally Active Neurons under Appropriate Environmental. Cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced Platelet-Rich Fibrin and Freeze-Dried Bone Allograft for Ridge Preservation: A Randomized Controlled Clinical Trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef]
- Development of a Novel Bone Grafting Material Using Autogenous Teeth-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20060336/ (accessed on 22 July 2025).
- Lin, W.L.; McCulloch, C.A.; Cho, M.I. Differentiation of Periodontal Ligament Fibroblasts into Osteoblasts during Socket Healing after Tooth Extraction in the Rat. Anat. Rec. 1994, 240, 492–506. [Google Scholar] [CrossRef]
- Mancini, A.; Laforgia, A.; Dipalma1, G.; Inchingolo, A.D.; Chieppa, S.; Colonna, V.; Tartaglia, F.C.; Corsalini, M.; Palermo, A.; Bordea, I.R.; et al. Difficulties And Perspectives Regarding Botulinum Injection And Bruxism. 2024. Available online: https://ricerca.uniba.it/handle/11586/530499 (accessed on 29 January 2024).
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation Procedures for the Rehabilitation of Deficient Edentulous Ridges with Oral Implants. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 136–159. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous Bone Grafts in Oral Implantology-Is It Still a “Gold Standard”? A Consecutive Review of 279 Patients with 456 Clinical Procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Removal of the Alveolar Processes of Both Jaws. Med. Chir. Rev. 1826, 5, 288–289. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5106719/?page=1 (accessed on 22 July 2025).
- Ling, Y.; Chen, D.; Li, P.; Zeng, X.; Xu, W. Repairing Alveolar Bone Defect Using Demineralized Dentin Grafts: A Meta-Analysis of Randomized Controlled Trials. BMC Oral Health 2024, 24, 1368. [Google Scholar] [CrossRef]
- Valerio, C.S.; e Alves, C.A.; Manzi, F.R. Reproducibility of Cone-Beam Computed Tomographic Measurements of Bone Plates and the Interdental Septum in the Anterior Mandible. Imaging Sci. Dent. 2019, 49, 9–17. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Qorri, E.; Inchingolo, F.; Isola, G.; Dongiovanni, P.; Tartaglia, G.M.; Scarano, A. Research Efficacy of Gaseous Ozone Therapy as an Adjuvant to Periodontal Treatment on Oxidative Stress Mediators in Patients with Type 2 Diabetes: A Randomized Clinical Trial. BMC Oral Health 2023, 23, 278. [Google Scholar] [CrossRef]
- Schliephake, H.; Dard, M.; Planck, H.; Hierlemann, H.; Stern, U. Alveolar Ridge Repair Using Resorbable Membranes and Autogenous Bone Particles with Simultaneous Placement of Implants: An Experimental Pilot Study in Dogs. Int. J. Oral Maxillofac. Implant. 2000, 15, 364–373. [Google Scholar]
- Walter, C.; Schmidt, J.C.; Rinne, C.A.; Mendes, S.; Dula, K.; Sculean, A. Cone Beam Computed Tomography (CBCT) for Diagnosis and Treatment Planning in Periodontology: Systematic Review Update. Clin. Oral Investig. 2020, 24, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current Bone Substitutes for Implant Dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.-I.; Takashi, I.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef]
- Kawai, T.; Urist, M.R. Bovine Tooth-Derived Bone Morphogenetic Protein. J. Dent. Res. 1989, 68, 1069–1074. [Google Scholar] [CrossRef]
- Almagro, M.I.; Roman-Blas, J.A.; Bellido, M.; Castañeda, S.; Cortez, R.; Herrero-Beaumont, G. PTH [1-34] Enhances Bone Response around Titanium Implants in a Rabbit Model of Osteoporosis. Clin. Oral Implant. Res. 2013, 24, 1027–1034. [Google Scholar] [CrossRef]
- Bessho, K.; Tagawa, T.; Murata, M. Purification of Rabbit Bone Morphogenetic Protein Derived from Bone, Dentin, and Wound Tissue after Tooth Extraction. J. Oral Maxillofac. Surg. 1990, 48, 162–169. [Google Scholar] [CrossRef]
- Nart, J.; Barallat, L.; Jimenez, D.; Mestres, J.; Gómez, A.; Carrasco, M.A.; Violant, D.; Ruíz-Magaz, V. Radiographic and Histological Evaluation of Deproteinized Bovine Bone Mineral vs. Deproteinized Bovine Bone Mineral with 10% Collagen in Ridge Preservation. A Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2017, 28, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Beretta, M.; Salina, S.; Santoro, F. Reduction of Autogenous Bone Graft Resorption by Means of Bio-Oss Coverage: A Prospective Study. Int. J. Periodontics Restor. Dent. 2005, 25, 19–25. [Google Scholar]
- Schwarz, F.; Sahin, D.; Becker, K.; Sader, R.; Becker, J. Autogenous Tooth Roots for Lateral Extraction Socket Augmentation and Staged Implant Placement. A Prospective Observational Study. Clin. Oral Implant. Res. 2019, 30, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Vermesan, D.; Inchingolo, A.D.; Malcangi, G.; Santacroce, L.; Scacco, S.; Benagiano, V.; Girolamo, F.; Cagiano, R.; Caprio, M.; et al. Bedsores Successfully Treated with Topical Phenytoin. Acta Biomed. 2017, 88, 45–48. Available online: https://pubmed.ncbi.nlm.nih.gov/28467333/ (accessed on 22 July 2025).
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Kim, J.-S.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and Microstructural Analysis of Osteopromotive Property of Allogenic Demineralized Dentin Matrix. Int. J. Oral Maxillofac. Implant. 2013, 28, 1655–1662. [Google Scholar] [CrossRef]
- Ye, L.; MacDougall, M.; Zhang, S.; Xie, Y.; Zhang, J.; Li, Z.; Lu, Y.; Mishina, Y.; Feng, J.Q. Deletion of Dentin Matrix Protein-1 Leads to a Partial Failure of Maturation of Predentin into Dentin, Hypomineralization, and Expanded Cavities of Pulp and Root Canal during Postnatal Tooth Development. J. Biol. Chem. 2004, 279, 19141–19148. [Google Scholar] [CrossRef]
- Linde, A. Dentin Matrix Proteins: Composition and Possible Functions in Calcification. Anat. Rec. 1989, 224, 154–166. [Google Scholar] [CrossRef]
- Longerich, U.; Crismani, A.; Mayr, A.; Walch, B.; Kolk, A. Development of a New Ramus Anterior Vertical Reference Line for the Evaluation of Skeletal and Dental Changes as a Decision Aid for the Treatment of Crowding in the Lower Jaw: Extraction vs. Nonextraction. J. Clin. Med. 2025, 14, 2884. [Google Scholar] [CrossRef]
- Shamsoddin, E.; Houshmand, B.; Golabgiran, M. Biomaterial Selection for Bone Augmentation in Implant Dentistry: A Systematic Review. J. Adv. Pharm. Technol. Res. 2019, 10, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Terheyden, H. Bone Augmentation Procedures in Localized Defects in the Alveolar Ridge: Clinical Results with Different Bone Grafts and Bone-Substitute Materials. Int. J. Oral Maxillofac. Implant. 2009, 24 (Suppl. 2), 218–236. [Google Scholar]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, L.H.; Secreto, F.; Jensen, E.D.; Li, X.; Kahler, R.A.; Westendorf, J.J. Runx2 and Bone Morphogenic Protein 2 Regulate the Expression of an Alternative Lef1 Transcript during Osteoblast Maturation. J. Cell Physiol. 2009, 221, 480–489. [Google Scholar] [CrossRef]
- Adibrad, M.; Shahabuei, M.; Sahabi, M. Significance of the Width of Keratinized Mucosa on the Health Status of the Supporting Tissue around Implants Supporting Overdentures. J. Oral Implant. 2009, 35, 232–237. [Google Scholar] [CrossRef]
- Le, B.T.; Borzabadi-Farahani, A. Simultaneous Implant Placement and Bone Grafting with Particulate Mineralized Allograft in Sites with Buccal Wall Defects, a Three-Year Follow-up and Review of Literature. J. Craniomaxillofac Surg. 2014, 42, 552–559. [Google Scholar] [CrossRef]
- Ritchie, H.H.; Ritchie, D.G.; Wang, L.H. Six Decades of Dentinogenesis Research. Historical and Prospective Views on Phosphophoryn and Dentin Sialoprotein. Eur. J. Oral Sci. 1998, 106 (Suppl. 1), 211–220. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone Healing and Soft Tissue Contour Changes Following Single-Tooth Extraction: A Clinical and Radiographic 12-Month Prospective Study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Reddi, A.H. Bone Matrix in the Solid State: Geometric Influence on Differentiation of Fibroblasts. Adv. Biol. Med. Phys. 1974, 15, 1–18. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef]
- Morrison, S.J.; White, P.M.; Zock, C.; Anderson, D.J. Prospective Identification, Isolation by Flow Cytometry, and in Vivo Self-Renewal of Multipotent Mammalian Neural Crest Stem Cells. Cell 1999, 96, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Bonino, F.; Steffensen, B.; Natto, Z.; Hur, Y.; Holtzman, L.P.; Weber, H.-P. Prospective Study of the Impact of Peri-Implant Soft Tissue Properties on Patient-Reported and Clinically Assessed Outcomes. J. Periodontol. 2018, 89, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Handschin, A.E.; Egermann, M.; Trentz, O.; Wanner, G.A.; Kock, H.-J.; Zünd, G.; Trentz, O.A. Cbfa-1 (Runx-2) and Osteocalcin Expression by Human Osteoblasts in Heparin Osteoporosis in Vitro. Clin. Appl. Thromb. Hemost. 2006, 12, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Vivatbutsiri, P.; Morriss-Kay, G.; Saga, Y.; Iseki, S. Cell Lineage in Mammalian Craniofacial Mesenchyme. Mech. Dev. 2008, 125, 797–808. [Google Scholar] [CrossRef]
- Fontana, F.; Santoro, F.; Maiorana, C.; Iezzi, G.; Piattelli, A.; Simion, M. Clinical and Histologic Evaluation of Allogeneic Bone Matrix versus Autogenous Bone Chips Associated with Titanium-Reinforced e-PTFE Membrane for Vertical Ridge Augmentation: A Prospective Pilot Study. Int. J. Oral Maxillofac. Implant. 2008, 23, 1003–1012. [Google Scholar]
- Cl Clinical Application of Auto-Tooth Bone Graft Material. Available online: https://www.jkaoms.org/journal/view.html?volume=38&number=1&page=2 (accessed on 22 July 2025).
- Araújo, M.G.; Lindhe, J. Dimensional Ridge Alterations Following Tooth Extraction. An Experimental Study in the Dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Al-Quisi, A.F.; Mohammed Aldaghir, O.; Al-Jumaily, H.A. Comparison between Rolled and Non-rolled U-Shaped Flap in the Second Stage of Dental Implant Surgery: A Randomized Clinical Trial. Int. J. Dent. 2022, 2022, 1329468. [Google Scholar] [CrossRef]
- Bessho, K.; Tagawa, T.; Murata, M. Comparison of Bone Matrix-Derived Bone Morphogenetic Proteins from Various Animals. J. Oral Maxillofac. Surg. 1992, 50, 496–501. [Google Scholar] [CrossRef]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 4987437. [Google Scholar] [CrossRef]
- Checchi, V.; Gasparro, R.; Pistilli, R.; Canullo, L.; Felice, P. Clinical Classification of Bone Augmentation Procedure Failures in the Atrophic Anterior Maxillae: Esthetic Consequences and Treatment Options. BioMed Res. Int. 2019, 2019, 4386709. [Google Scholar] [CrossRef]
- Jeong, K.-I.; Kim, S.-G.; Kim, Y.-K.; Oh, J.-S.; Jeong, M.-A.; Park, J.-J. Clinical Study of Graft Materials Using Autogenous Teeth in Maxillary Sinus Augmentation. Implant. Dent. 2011, 20, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Cicciù, M.; Lo Russo, L. Combination of Bone Graft and Resorbable Membrane for Alveolar Ridge Preservation: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. J. Periodontol. 2018, 89, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Elraee, L.; Abdel Gaber, H.K.; Elsayed, H.H.; Adel-Khattab, D. Autogenous Dentin Block versus Bone Block for Horizontal Alveolar Ridge Augmentation and Staged Implant Placement: A Randomized Controlled Clinical Trial Including Histologic Assessment. Clin. Oral Implant. Res. 2022, 33, 723–734. [Google Scholar] [CrossRef]
- Radoczy-Drajko, Z.; Windisch, P.; Svidro, E.; Tajti, P.; Molnar, B.; Gerber, G. Clinical, Radiographical and Histological Evaluation of Alveolar Ridge Preservation with an Autogenous Tooth-Derived Particulate Graft in EDS Class 3-4 Defects. BMC Oral Health 2021, 21, 63. [Google Scholar] [CrossRef]
- López Sacristán, H.; Del Canto Pingarrón, M.; Alobera Gracia, M.A.; de Elío Oliveros, J.; Díaz Pedrero, R.; Seco-Calvo, J. Use of Autologous Tooth-Derived Material as a Graft in the Post-Extraction Socket. Split-Mouth Study with Radiological and Histological Analysis. BMC Oral Health 2024, 24, 832. [Google Scholar] [CrossRef]
- Hussain, A.A.; Al-Quisi, A.F.; Abdulkareem, A.A. Efficacy of Autogenous Dentin Biomaterial on Alveolar Ridge Preservation: A Randomized Controlled Clinical Trial. BioMed Res. Int. 2023, 2023, 7932432. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, J.; Dong, Y.; Li, J.; Jin, F.; Luo, Y.; Wang, R.; Wang, S. Comparison of Clinical Efficacy between Autologous Partially Demineralized Dentin Matrix and Deproteinized Bovine Bone Mineral for Bone Augmentation in Orthodontic Patients with Alveolar Bone Deficiency: A Randomized Controlled Clinical Trial. BMC Oral Health 2024, 24, 984. [Google Scholar] [CrossRef]
- Pohl, V.; Pohl, S.; Sulzbacher, I.; Fuerhauser, R.; Mailath-Pokorny, G.; Haas, R. Alveolar Ridge Augmentation Using Dystopic Autogenous Tooth: 2-Year Results of an Open Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 870–879. [Google Scholar] [CrossRef]
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss Granules in GBR for Immediate Implantation in Periodontal Postextraction Sites: A Prospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2018, 20, 923–928. [Google Scholar] [CrossRef]
- Elfana, A.; El-Kholy, S.; Saleh, H.A.; Fawzy El-Sayed, K. Alveolar Ridge Preservation Using Autogenous Whole-Tooth versus Demineralized Dentin Grafts: A Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2021, 32, 539–548. [Google Scholar] [CrossRef]
- Oguić, M.; Čandrlić, M.; Tomas, M.; Vidaković, B.; Blašković, M.; Jerbić Radetić, A.T.; Zoričić Cvek, S.; Kuiš, D.; Cvijanović Peloza, O. Osteogenic Potential of Autologous Dentin Graft Compared with Bovine Xenograft Mixed with Autologous Bone in the Esthetic Zone: Radiographic, Histologic and Immunohistochemical Evaluation. Int. J. Mol. Sci. 2023, 24, 6440. [Google Scholar] [CrossRef]
- Amer, O.; Shemais, N.; Fawzy El-Sayed, K.; Saleh, H.A.; Darhous, M. Does Injectable Platelet-Rich Fibrin Combined With Autogenous Demineralized Dentine Enhance Alveolar Ridge Preservation? A Randomized Controlled Trial. Clin. Oral Implant. Res. 2025, 36, 166–177. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of Bone Tissue Formation in Tooth Extraction Sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of Alveolar Ridge Preservation Interventions Following Tooth Extraction: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 195–223. [Google Scholar] [CrossRef] [PubMed]
- Frigério, P.B.; Gomes-Ferreira, P.H.S.; de Souza Batista, F.R.; Moura, J.; Rangel Garcia Júnior, I.; Botticelli, D.; Lisboa-Filho, P.N.; Okamoto, R. Effect of Topical PTH 1-34 Functionalized to Biogran® in the Process of Alveolar Repair in Rats Submitted to Orchiectomy. Materials 2021, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of Autogenous Teeth for the Reconstruction of Alveolar Ridge Deficiencies: A Systematic Review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Schwarz, F.; Hazar, D.; Becker, K.; Sader, R.; Becker, J. Efficacy of Autogenous Tooth Roots for Lateral Alveolar Ridge Augmentation and Staged Implant Placement. A prospective controlled clinical study. J. Clin. Periodontol. 2018, 45, 996–1004. [Google Scholar] [CrossRef]
- Cho, H.-L.; Lee, J.-K.; Um, H.-S.; Chang, B.-S. Esthetic Evaluation of Maxillary Single-Tooth Implants in the Esthetic Zone. J. Periodontal. Implant. Sci. 2010, 40, 188–193. [Google Scholar] [CrossRef]
- Signorini, L.; Ballini, A.; Arrigoni, R.; De Leonardis, F.; Saini, R.; Cantore, S.; De Vito, D.; Coscia, M.F.; Dipalma, G.; Santacroce, L.; et al. Evaluation of a Nutraceutical Product with Probiotics, Vitamin D, Plus Banaba Leaf Extracts (Lagerstroemia speciosa) in Glycemic Control. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1356–1365. [Google Scholar] [CrossRef]
- Chisci, G.; Hatia, A.; Chisci, E.; Chisci, D.; Gennaro, P.; Gabriele, G. Socket Preservation after Tooth Extraction: Particulate Autologous Bone vs. Deproteinized Bovine Bone. Bioengineering 2023, 10, 421. [Google Scholar] [CrossRef]
- Bassir, S.H.; Alhareky, M.; Wangsrimongkol, B.; Jia, Y.; Karimbux, N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int. J. Oral Maxillofac. Implant. 2018, 33, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Jafer, M.A.; Salem, R.M.; Hakami, F.B.; Ageeli, R.E.; Alhazmi, T.A.; Bhandi, S.; Patil, S. Techniques for Extraction Socket Regeneration for Alveolar Ridge Preservation. J. Contemp. Dent. Pract. 2022, 23, 245–250. [Google Scholar] [PubMed]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; Di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The Effectiveness of Autologous Demineralized Tooth Graft for the Bone Ridge Preservation: A Systematic Review of the Literature. J. Biol. Regul. Homeost. Agents 2021, 35, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jones, A.; Heijl, L.; Mellonig, J.T.; Schoolfield, J.; King, G.N. Periodontal Regeneration with a Combination of Enamel Matrix Proteins and Autogenous Bone Grafting. J. Periodontol. 2003, 74, 1269–1281. [Google Scholar] [CrossRef]
- Zhao, L.; Zhan, Y.; Hu, W.; Xu, T.; Wei, Y.; Zhen, M.; Wang, C. Evaluation with Different Measuring Methods for the Alveolar Bone Change of Ridge Preservation in Molar Sites. Beijing Da Xue Xue Bao Yi Xue Ban 2016, 48, 126–132. [Google Scholar]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. Dense Polytetrafluoroethylene Membranes in Vertical Ridge Augmentation around Dental Implants: A Prospective Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2014, 25, 859–866. [Google Scholar] [CrossRef]
- Schwarz, F.; Golubovic, V.; Becker, K.; Mihatovic, I. Extracted Tooth Roots Used for Lateral Alveolar Ridge Augmentation: A Proof-of-Concept Study. J. Clin. Periodontol. 2016, 43, 345–353. [Google Scholar] [CrossRef]
- Feng, J.Q.; Luan, X.; Wallace, J.; Jing, D.; Ohshima, T.; Kulkarni, A.B.; D’Souza, R.N.; Kozak, C.A.; MacDougall, M. Genomic Organization, Chromosomal Mapping, and Promoter Analysis of the Mouse Dentin Sialophosphoprotein (Dspp) Gene, Which Codes for Both Dentin Sialoprotein and Dentin Phosphoprotein. J. Biol. Chem. 1998, 273, 9457–9464. [Google Scholar] [CrossRef]
- Pohl, V.; Fürhauser, L.; Haas, R.; Pohl, S. Gingival Recession Behavior with Immediate Implant Placement in the Anterior Maxilla with Buccal Dehiscence without Additional Augmentation-a Pilot Study. Clin. Oral Investig. 2020, 24, 1455–1464. [Google Scholar] [CrossRef]
- Maréchal, M. Guided bone augmentation in edentulous areas. Ned. Tijdschr. Tandheelkd. 2002, 109, 439–443. [Google Scholar]
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.-L. The Peri-Implant Phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Hashimoto, F.; Ishibashi, K. Time-Dependent Changes of Collagen Crosslinks in the Socket after Tooth Extraction in Rabbits. J. Dent. Res. 1988, 67, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New Attachment Formation as the Result of Controlled Tissue Regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Ruskin, J.; Higginbottom, F.; Hardwick, R.; Dahlin, C.; Schenk, R.K. Osseointegration of Titanium Implants in Bone Regenerated in Membrane-Protected Defects: A Histologic Study in the Canine Mandible. Int. J. Oral Maxillofac. Implant. 1995, 10, 666–681. [Google Scholar] [CrossRef]
- Kuroshima, S.; Al-Salihi, Z.; Yamashita, J. Parathyroid Hormone Related to Bone Regeneration in Grafted and Nongrafted Tooth Extraction Sockets in Rats. Implant. Dent. 2013, 22, 71–76. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Jung, R.E.; Sanz-Martin, I.; Unger, S.; Cantalapiedra, A.; Hämmerle, C.H.F. Guided Bone Regeneration with Particulate vs. Block Xenogenic Bone Substitutes: A Pilot Cone Beam Computed Tomographic Investigation. Clin. Oral Implant. Res. 2017, 28, e262–e270. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; de Ruvo, E.; Campanelli, M.; Palermo, A.; Fabbro, M.D.; Blasio, M.D.; Inchingolo, A.D.; Dipalma, G. Guided Bone Regeneration: CGF and PRF Combined With Various Types of Scaffolds-A Systematic Review. Int. J. Dent. 2024, 2024, 4990295. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Di Pede, C.; Garibaldi, M.; Ciocia, A.M.; et al. Treatment of Class III Malocclusion and Anterior Crossbite with Aligners: A Case Report. Medicina 2022, 58, 603. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Hard-Tissue Alterations Following Immediate Implant Placement in Extraction Sites. J. Clin. Periodontol. 2004, 31, 820–828. [Google Scholar] [CrossRef]
- Claflin, R.S. Healing of Disturbed and Undisturbed Extraction Wounds. J. Am. Dent. Assoc. 1922 1936, 23, 945–959. [Google Scholar] [CrossRef]
- Beck, T.M.; Mealey, B.L. Histologic Analysis of Healing after Tooth Extraction with Ridge Preservation Using Mineralized Human Bone Allograft. J. Periodontol. 2010, 81, 1765–1772. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Welander, M.; Lang, N.P.; Lindhe, J. Morphogenesis of the Peri-Implant Mucosa: An Experimental Study in Dogs. Clin. Oral Implant. Res. 2007, 18, 1–8. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Stumbras, A.; Goyushov, S.; Duruel, O.; Tözüm, T.F. Morphological Classification of Extraction Sockets and Clinical Decision Tree for Socket Preservation/Augmentation after Tooth Extraction: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e3. [Google Scholar] [CrossRef]
- Rich, A. Most Cited: Number 4. The Healing of Extraction Wounds: An Experimental Study Based on Microscopic and Radiographic Investigations. N. Z. Dent. J. 2009, 105, 28. [Google Scholar]
- Chen, H.; Fu, T.; Ma, Y.; Wu, X.; Li, X.; Li, X.; Shen, J.; Wang, H. Intermittent Administration of Parathyroid Hormone Ameliorated Alveolar Bone Loss in Experimental Periodontitis in Streptozotocin-Induced Diabetic Rats. Arch. Oral Biol. 2017, 83, 76–84. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. Interventions for Replacing Missing Teeth: Horizontal and Vertical Bone Augmentation Techniques for Dental Implant Treatment. Cochrane Database Syst. Rev. 2009, 2009, CD003607. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J. Is There Clinical Evidence to Support Alveolar Ridge Preservation over Extraction Alone? A Review of Recent Literature and Case Reports of Late Graft Failure. Br. Dent. J. 2022, 233, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Tabatabaei, S.; Baghaei, K.; Fathi, A.; Atash, R. Long-Term Clinical Outcomes of Single Crowns or Short Fixed Partial Dentures Supported by Short (≤6 Mm) Dental Implants: A Systematic Review. Eur. J. Dent. 2024, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Barry, O.; de Jong, L.; Wahl, G. Long-Term Effects of Vertical Bone Augmentation: A Systematic Review. J. Appl. Oral Sci. 2016, 24, 3–17. [Google Scholar] [CrossRef]
- Simion, M.; Fontana, F.; Rasperini, G.; Maiorana, C. Long-Term Evaluation of Osseointegrated Implants Placed in Sites Augmented with Sinus Floor Elevation Associated with Vertical Ridge Augmentation: A Retrospective Study of 38 Consecutive Implants with 1- to 7-Year Follow-Up. Int. J. Periodontics Restor. Dent. 2004, 24, 208–221. [Google Scholar]
- Xu, H.; Shimizu, Y.; Ooya, K. Histomorphometric Study of the Stability of Newly Formed Bone after Elevation of the Floor of the Maxillary Sinus. Br. J. Oral Maxillofac. Surg. 2005, 43, 493–499. [Google Scholar] [CrossRef]
- Parvini, P.; Schliephake, C.; Al-Maawi, S.; Schwarz, K.; Sader, R.; Ghanaati, S.; Schwarz, F. Histomorphometrical Assessment of Vertical Alveolar Ridge Augmentation Using Extracted Tooth Roots in the Canine. Clin. Oral Investig. 2020, 24, 317–323. [Google Scholar] [CrossRef]
- Urban, I.A.; Lozada, J.L.; Jovanovic, S.A.; Nagy, K. Horizontal Guided Bone Regeneration in the Posterior Maxilla Using Recombinant Human Platelet-Derived Growth Factor: A Case Report. Int. J. Periodontics Restor. Dent. 2013, 33, 421–425. [Google Scholar] [CrossRef]
- Salata, L.A.; Hatton, P.V.; Devlin, A.J.; Craig, G.T.; Brook, I.M. In Vitro and in Vivo Evaluation of E-PTFE and Alkali-Cellulose Membranes for Guided Bone Regeneration. Clin. Oral Implant. Res. 2001, 12, 62–68. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Initial Case Report of an Extracted Tooth Root Used for Lateral Alveolar Ridge Augmentation. J. Clin. Periodontol. 2016, 43, 985–989. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.M.; Lauria, P.; Marotti, P.; Chieppa, S.; Venere, D.D.; Palermo, A.; Corsalini, M.; Inchingolo, F.; Inchingolo, A.D. Unilateral Agenesis of the Upper Permanent Lateral Incisors in Growing Patients: Gap Closure or Gap Opening? A Systematic Review. Int. Dent. J. 2025, 75, 100815. [Google Scholar] [CrossRef]
- Stevens, A.; Zuliani, T.; Olejnik, C.; LeRoy, H.; Obriot, H.; Kerr-Conte, J.; Formstecher, P.; Bailliez, Y.; Polakowska, R.R. Human Dental Pulp Stem Cells Differentiate into Neural Crest-Derived Melanocytes and Have Label-Retaining and Sphere-Forming Abilities. Stem Cells Dev. 2008, 17, 1175–1184. [Google Scholar] [CrossRef]
- Morelli, T.; Neiva, R.; Wang, H.-L. Human Histology of Allogeneic Block Grafts for Alveolar Ridge Augmentation: Case Report. Int. J. Periodontics Restor. Dent. 2009, 29, 649–656. [Google Scholar]
- Nkenke, E.; Hahn, M.; Weinzierl, K.; Radespiel-Tröger, M.; Neukam, F.W.; Engelke, K. Implant Stability and Histomorphometry: A Correlation Study in Human Cadavers Using Stepped Cylinder Implants. Clin. Oral Implant. Res. 2003, 14, 601–609. [Google Scholar] [CrossRef]
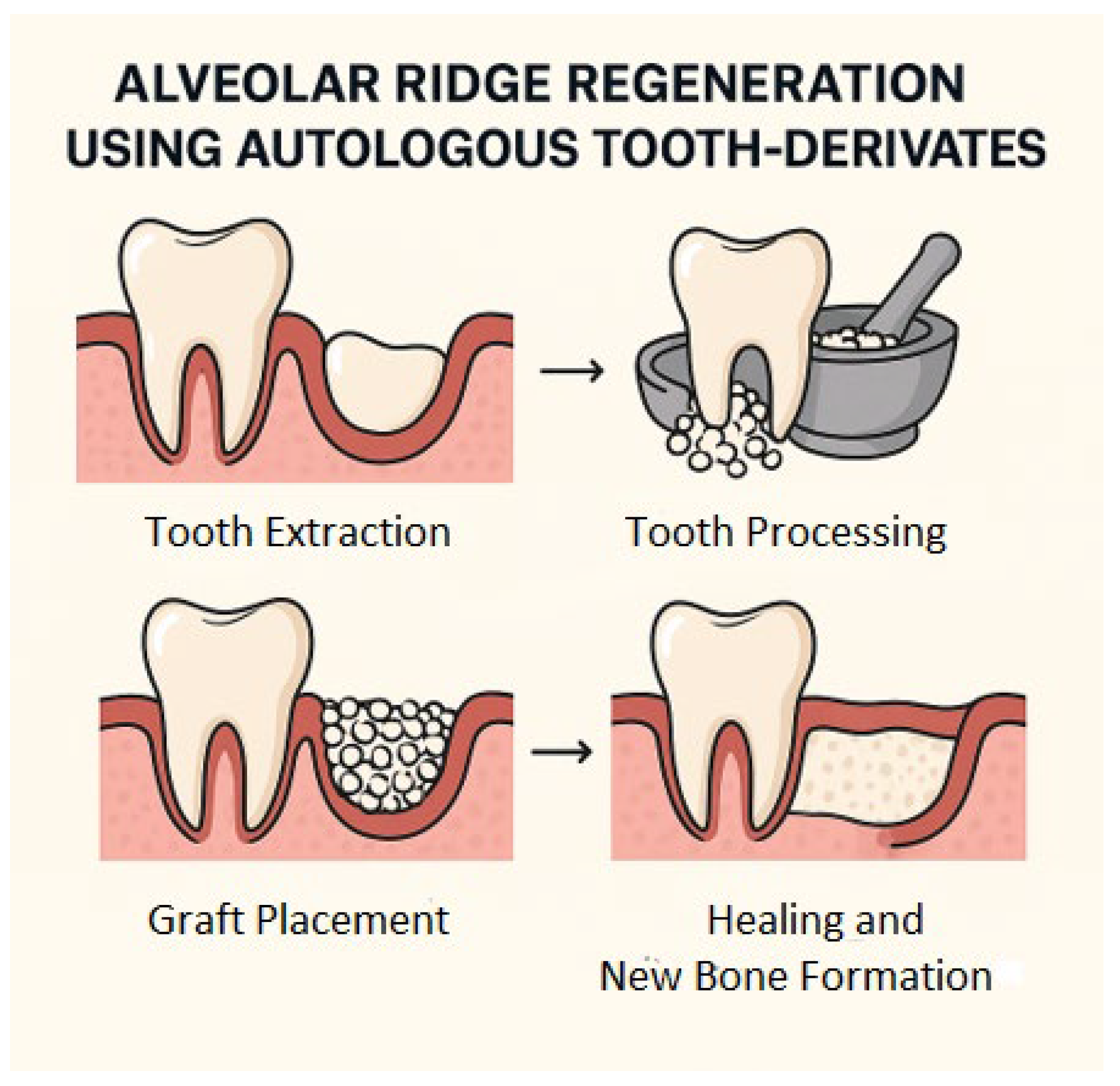

| Population: Patients undergoing alveolar bone preservation or regeneration (post-extraction or implant preparation) |
| Intervention: Use of autologous biomaterials derived from teeth (demineralised dentine, mineralised dentine, dentine blocks) |
Comparison:
|
Outcome:
|
| Study design: Systematic review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.M.; Marinelli, G.; Inchingolo, F.; Giorgio, R.V.; Colonna, V.; Pennacchio, B.F.P.; Del Fabbro, M.; Tartaglia, G.; Palermo, A.; Inchingolo, A.D.; et al. Autologous Tooth-Derived Biomaterials in Alveolar Bone Regeneration: A Systematic Review of Clinical Outcomes and Histological Evidence. J. Funct. Biomater. 2025, 16, 367. https://doi.org/10.3390/jfb16100367
Inchingolo AM, Marinelli G, Inchingolo F, Giorgio RV, Colonna V, Pennacchio BFP, Del Fabbro M, Tartaglia G, Palermo A, Inchingolo AD, et al. Autologous Tooth-Derived Biomaterials in Alveolar Bone Regeneration: A Systematic Review of Clinical Outcomes and Histological Evidence. Journal of Functional Biomaterials. 2025; 16(10):367. https://doi.org/10.3390/jfb16100367
Chicago/Turabian StyleInchingolo, Angelo Michele, Grazia Marinelli, Francesco Inchingolo, Roberto Vito Giorgio, Valeria Colonna, Benito Francesco Pio Pennacchio, Massimo Del Fabbro, Gianluca Tartaglia, Andrea Palermo, Alessio Danilo Inchingolo, and et al. 2025. "Autologous Tooth-Derived Biomaterials in Alveolar Bone Regeneration: A Systematic Review of Clinical Outcomes and Histological Evidence" Journal of Functional Biomaterials 16, no. 10: 367. https://doi.org/10.3390/jfb16100367
APA StyleInchingolo, A. M., Marinelli, G., Inchingolo, F., Giorgio, R. V., Colonna, V., Pennacchio, B. F. P., Del Fabbro, M., Tartaglia, G., Palermo, A., Inchingolo, A. D., & Dipalma, G. (2025). Autologous Tooth-Derived Biomaterials in Alveolar Bone Regeneration: A Systematic Review of Clinical Outcomes and Histological Evidence. Journal of Functional Biomaterials, 16(10), 367. https://doi.org/10.3390/jfb16100367














