Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models
Abstract
1. Introduction
2. Materials and Methods
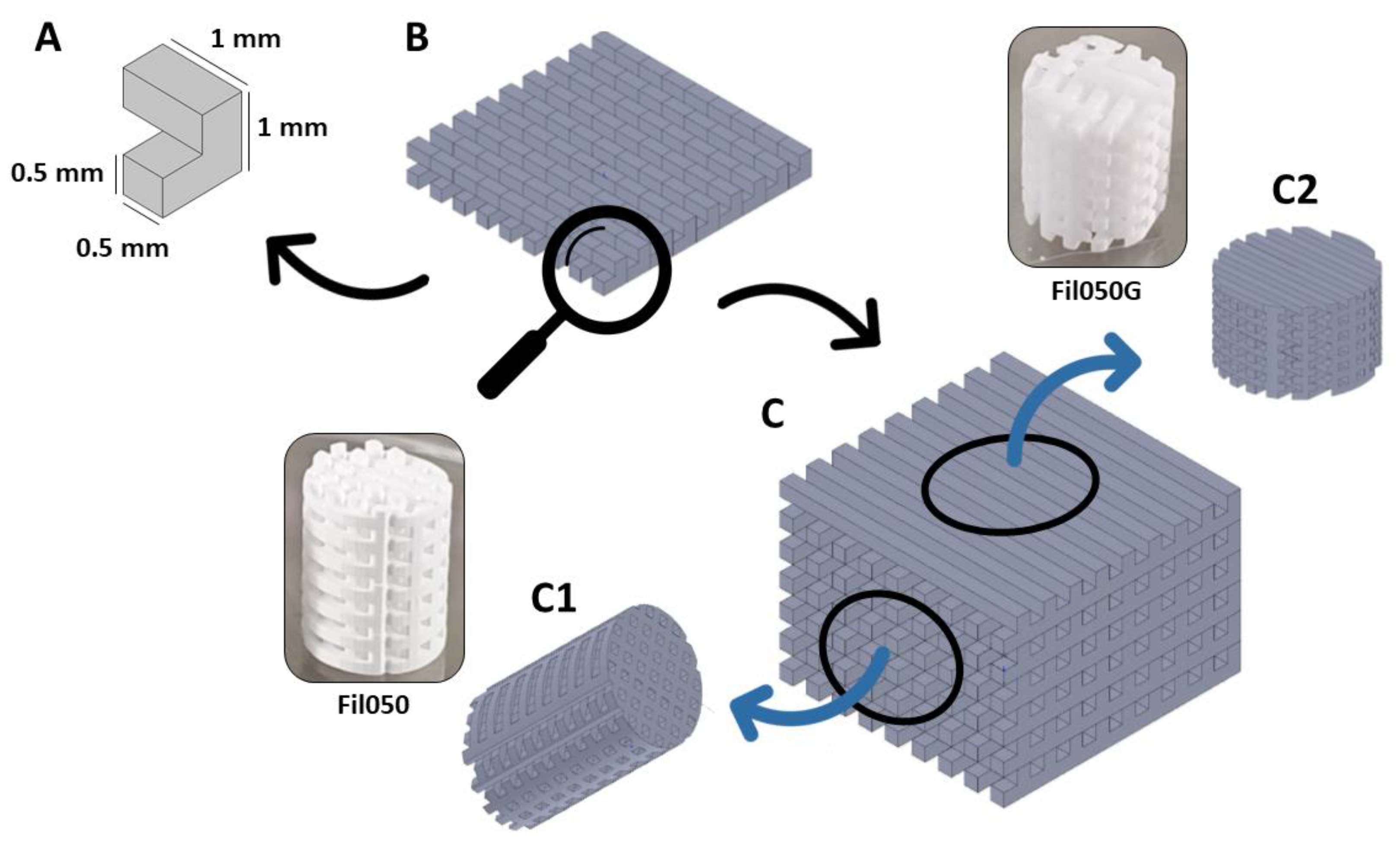
2.1. Scaffold Production
2.2. Scaffold Implantation
2.2.1. Osteoconduction
2.2.2. Bone Augmentation
2.3. Histomorphometry
2.4. Statistical Analysis
3. Results
3.1. Implantation of TCP-Based Scaffolds with TPMS Microarchitecture
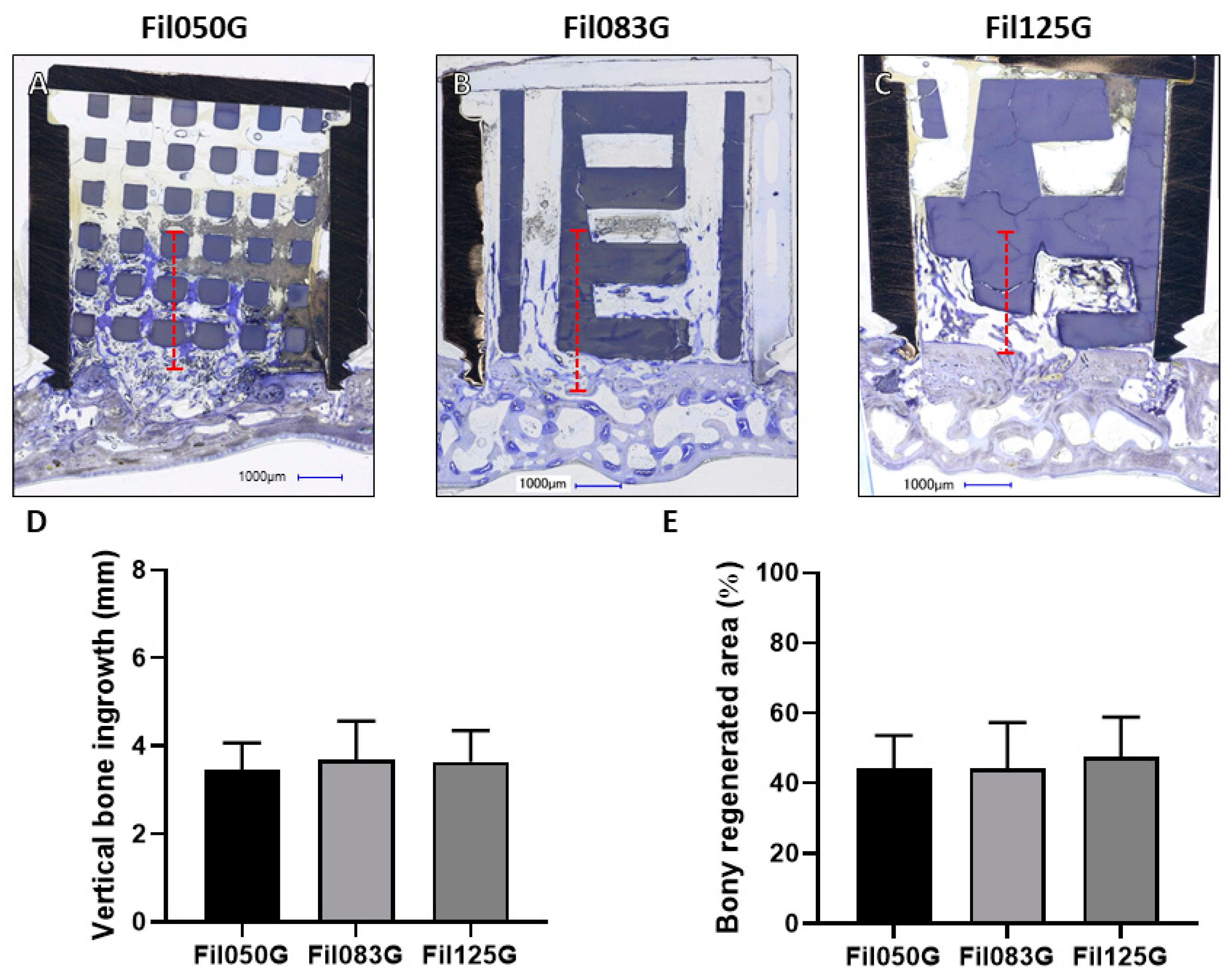
3.1.1. Performance of Filament-Based Microarchitectures in Bone Augmentation
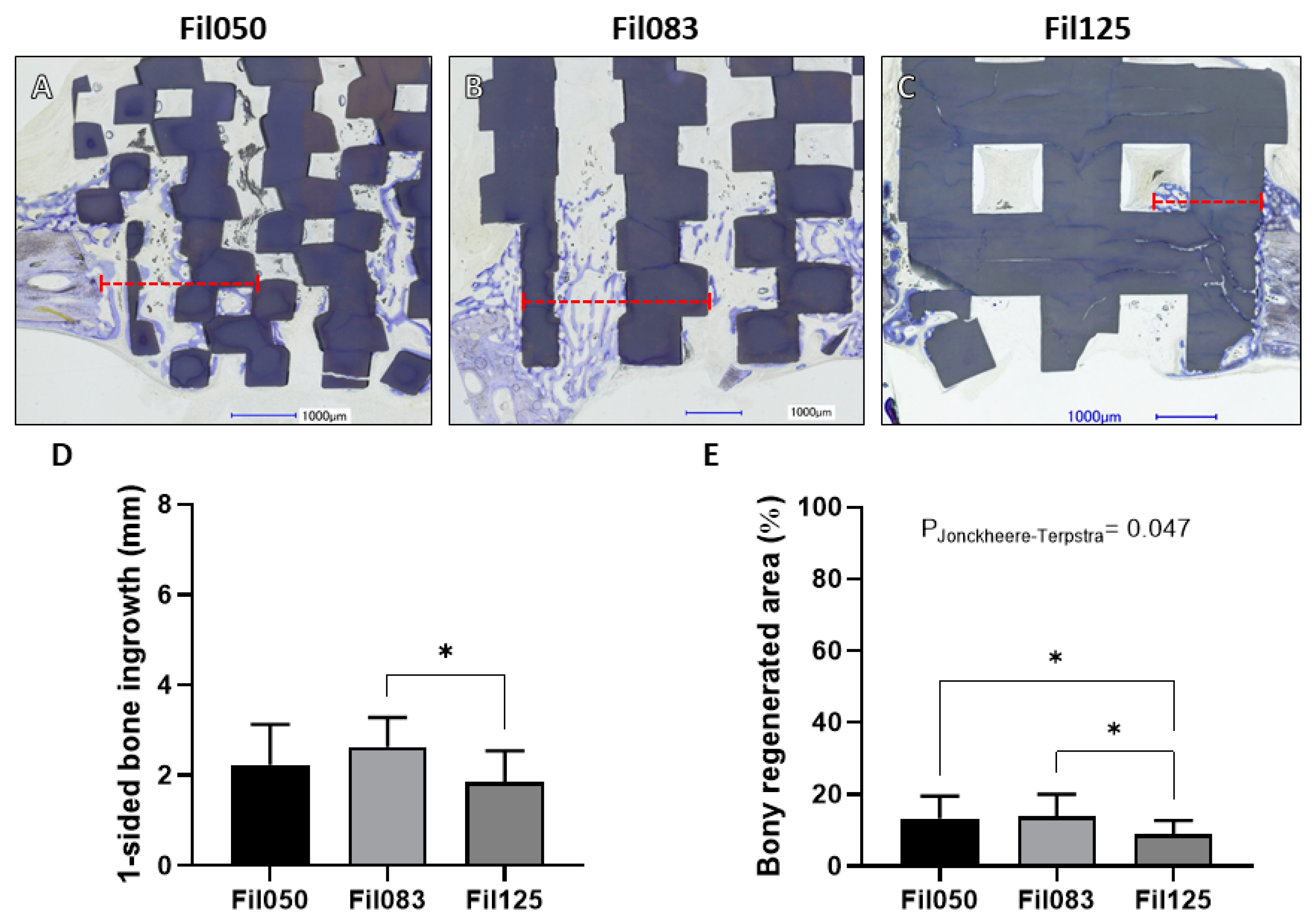
3.1.2. Performance of Filament-Based Microarchitectures in Osteoconduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive Manufacturing of Bone Scaffolds. Int. J. Bioprint 2019, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive Manufacturing Techniques for the Production of Tissue Engineering Constructs. J. Tissue Eng. Regen. Med. 2012, 9, 174–190. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, J.; Hussain, M.; Wang, M. Additive Manufacturing in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2022, 8, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Maevskaia, E.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Influence of Scaffold Microarchitecture on Angiogenesis and Regulation of Cell Differentiation During the Early Phase of Bone Healing: A Transcriptomics and Histological Analysis. Int. J. Mol. Sci. 2023, 24, 6000. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3d Bioprinting of Tissues/Organs for Regenerative Medicine and in-Vitro Models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Soile, S.; Angelopoulos, C.; Theodoridis, K.; Arampatzis, A.S.; Assimopoulou, A.N.; Christofilos, D.; Kapourani, A.; Pantazos, I.; et al. The Role of Cone Beam Computed Tomography in Periodontology: From 3d Models of Periodontal Defects to 3d-Printed Scaffolds. J. Pers. Med. 2024, 14, 207. [Google Scholar] [CrossRef]
- Charbonnier, B.; Hadida, M.; Marchat, D. Additive Manufacturing Pertaining to Bone: Hopes, Reality and Future Challenges for Clinical Applications. Acta Biomater. 2020, 121, 1–28. [Google Scholar] [CrossRef]
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Wu, J.; Zhang, X.; Su, R.; Ma, L.; Sun, Q.; He, R. Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 1164–1189. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of Pore Size and Morphology in Musculo-Skeletal Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Deering, J.; Grandfield, K. Current Interpretations on the in Vivo Response of Bone to Additively Manufactured Metallic Porous Scaffolds: A Review. Biomater. Biosyst. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N.; Lane, J.M. Current Understanding of Osteoconduction in Bone Regeneration. Clin. Orthop. Relat. Res. 1998, 355, S267–S273. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3d Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.W.; Park, J.Y.; Lee, D.N.; Jin, X.; Cha, J.K.; Paik, J.W.; Choi, S.H. Three-Dimensionally Printed Biphasic Calcium Phosphate Blocks with Different Pore Diameters for Regeneration in Rabbit Calvarial Defects. Biomater. Res. 2022, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Seehanam, S.; Khrueaduangkham, S.; Sinthuvanich, C.; Sae-Ueng, U.; Srimaneepong, V.; Promoppatum, P. Evaluating the Effect of Pore Size for 3d-Printed Bone Scaffolds. Heliyon 2024, 10, e26005. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, Y.; Huang, Z.; Wu, T.; Xu, W.; Wu, W.; Xu, Z. Advances in Filament Structure of 3d Bioprinted Biodegradable Bone Repair Scaffolds. Int. J. Bioprint 2021, 7, 426. [Google Scholar] [CrossRef]
- Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Osteoconductivity of Bone Substitutes with Filament-Based Microarchitectures: Influence of Directionality, Filament Dimension, and Distance. Int. J. Bioprint 2022, 9, 626. [Google Scholar] [CrossRef]
- Ghayor, C.; Chen, T.H.; Bhattacharya, I.; Ozcan, M.; Weber, F.E. Microporosities in 3d-Printed Tricalcium-Phosphate-Based Bone Substitutes Enhance Osteoconduction and Affect Osteoclastic Resorption. Int. J. Mol. Sci. 2020, 21, 9270. [Google Scholar] [CrossRef]
- Benito-Garzon, L.; Guadilla, Y.; Diaz-Guemes, I.; Valdivia-Gandur, I.; Manzanares, M.C.; de Castro, A.G.; Padilla, S. Nanostructured Zn-Substituted Monetite Based Material Induces Higher Bone Regeneration Than Anorganic Bovine Bone and Beta-Tricalcium Phosphate in Vertical Augmentation Model in Rabbit Calvaria. Nanomaterials 2021, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.E.; Ku, H.C.; Dluzen, D.F.; Xing, C.; Zhou, Z. A Nonparametric Alternative to the Cochran-Armitage Trend Test in Genetic Case-Control Association Studies: The Jonckheere-Terpstra Trend Test. PLoS ONE 2023, 18, e0280809. [Google Scholar] [CrossRef] [PubMed]
- Tom, T.; Sreenilayam, S.P.; Brabazon, D.; Jose, J.P.; Joseph, B.; Madanan, K.; Thomas, S. Additive Manufacturing in the Biomedical Field-Recent Research Developments. Results Eng. 2022, 16, 100661. [Google Scholar] [CrossRef]
- Zeng, X.; Meng, Z.; He, J.; Mao, M.; Li, X.; Chen, P.; Fan, J.; Li, D. Embedded Bioprinting for Designer 3d Tissue Constructs with Complex Structural Organization. Acta Biomater. 2021, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef]
- Lee, J.B.; Maeng, W.Y.; Koh, Y.H.; Kim, H.E. Porous Calcium Phosphate Ceramic Scaffolds with Tailored Pore Orientations and Mechanical Properties Using Lithography-Based Ceramic 3d Printing Technique. Materials 2018, 11, 1711. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone Defect Animal Models for Testing Efficacy of Bone Substitute Biomaterials. J. Orthop. Translat 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Tsetsenekou, E.; Papadopoulos, T.; Kalyvas, D.; Papaioannou, N.; Tangl, S.; Watzek, G. The Influence of Alendronate on Osseointegration of Nanotreated Dental Implants in New Zealand Rabbits. Clin. Oral. Implants Res. 2011, 23, 659–666. [Google Scholar] [CrossRef]
- Gao, H.; Huang, J.; Wei, Q.; He, C. Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering 2023, 10, 201. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of Animal Models in Biomedical Research: A Review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Scherrer, S.; Durual, S. Large Bone Vertical Augmentation Using a Three-Dimensional Printed Tcp/Ha Bone Graft: A Pilot Study in Dog Mandible. Clin. Implant. Dent. Relat. Res. 2016, 18, 1183–1192. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3d Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3d Printed Tcp/Ha Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation. Clin. Oral. Implants Res. 2014, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels. Materials 2023, 16, 7518. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of Macropore Size in Carbonate Apatite Honeycomb Scaffolds on Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Weber, F.E. The Optimal Microarchitecture of 3d-Printed Β-Tcp Bone Substitutes for Vertical Bone Augmentation Differs from That for Osteoconduction. Mater. Des. 2021, 204, 109650. [Google Scholar] [CrossRef]
- Dias, M.R.; Guedes, J.M.; Flanagan, C.L.; Hollister, S.J.; Fernandes, P.R. Optimization of Scaffold Design for Bone Tissue Engineering: A Computational and Experimental Study. Med. Eng. Phys. 2014, 36, 448–457. [Google Scholar] [CrossRef]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.H.; Yoo, W.Y.; Kim, Y.Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of Pore Size on the Osteoconductivity and Mechanical Properties of Calcium Phosphate Cement in a Rabbit Model. Artif. Organs 2016, 41, 199–204. [Google Scholar] [CrossRef]
- Ntousi, O.; Roumpi, M.; Siogkas, P.; Deligianni, D.; Fotiadis, D.I. Computational Fluid Dynamic Analysis of Customised 3d-Printed Bone Scaffolds with Different Architectures. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2023, 2023, 1–4. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, J.; Maevskaia, E.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. J. Funct. Biomater. 2024, 15, 174. https://doi.org/10.3390/jfb15070174
Guerrero J, Maevskaia E, Ghayor C, Bhattacharya I, Weber FE. Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. Journal of Functional Biomaterials. 2024; 15(7):174. https://doi.org/10.3390/jfb15070174
Chicago/Turabian StyleGuerrero, Julien, Ekaterina Maevskaia, Chafik Ghayor, Indranil Bhattacharya, and Franz E. Weber. 2024. "Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models" Journal of Functional Biomaterials 15, no. 7: 174. https://doi.org/10.3390/jfb15070174
APA StyleGuerrero, J., Maevskaia, E., Ghayor, C., Bhattacharya, I., & Weber, F. E. (2024). Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. Journal of Functional Biomaterials, 15(7), 174. https://doi.org/10.3390/jfb15070174







