Abstract
Osteosynthesis in fracture treatment typically uses hardware that remains in the patient’s body, which brings a permanent risk of negative side effects such as foreign body reactions or chronic inflammation. Bioabsorbable materials, however, can degrade and slowly be replaced by autologous bone tissue. A suitable material is requested to offer great biocompatibility alongside excellent mechanical properties and a reasonable corrosion rate. Zinc–silver alloys provide these characteristics, which makes them a promising candidate for research. This study investigated the aptitude as a bioabsorbable implant of a novel zinc–silver alloy containing 3.3 wt% silver (ZnAg3). Here, the tensile strength as well as the corrosion rate in PBS solution (phosphate buffered solution) of ZnAg3 were assessed. Furthermore, shear tests, including fatigue and quasi-static testing, were conducted with ZnAg3 and magnesium pins (MAGNEZIX®, Syntellix AG, Hannover, Germany), which are already in clinical use. The detected corrosion rate of 0.10 mm/year for ZnAg3 was within the proposed range for bioabsorbable implants. With a tensile strength of 237.5 ± 2.12 MPa and a shear strength of 144.8 ± 13.2 N, ZnAg3 satisfied the mechanical requirements for bioabsorbable implants. The fatigue testing did not show any significant difference between ZnAg3 and magnesium pins, whereas both materials withstood the cyclic loading. Thus, the results support the assumption that ZnAg3 is qualified for further investigation.
1. Introduction
In orthopedics and trauma surgery, many fractures are treated surgically by using hardware such as screws and nails. To ensure a sufficient healing process, the implanted material should offer the needed stability as well as a distinct biocompatibility. Nonetheless, many materials remain inside the body since they do not degrade, which can lead to serious foreign body reactions and inflammation, and consequently might require implant removal [1,2,3,4,5,6]. By developing bioabsorbable materials, this issue is addressed in the scientific community. Here, the human body can regain a fully healed bone structure consisting of autologous tissue after a fracture by absorbing the implanted material.
Currently, only polymer-based and magnesium-based materials are in clinical use [4,7]. Although materials such as polyglycolic acids and polylactic acids might show mostly satisfying results in special fields such as the treatment of pediatric maxillofacial fractures, several limitations have occurred, such as poor tensile strength and foreign body reactions with local swelling, sterile abscess, and fistula formation [8,9,10]. Since magnesium and its alloys reach the targeted tensile strength for bioabsorbable materials of 200 to 300 MPa, they have been attracting attention in this research field [11,12,13]. However, the high corrosion rate of magnesium at 0.2–2 mm/year and the formation of hydrogen gas pockets inside the tissue are remaining concerns in clinical use [14,15,16]. A corrosion rate that is too high can lead to premature degradation of the implant material and thus does not guarantee sufficient mechanical stability for the bone during the healing process. Furthermore, the degradation of magnesium leads to an increase in pH value within the implant environment, which has negative effects on the surrounding tissue [4]. This can disrupt the healing process and thus delay bone reconstruction [17].
Zinc alloys provide distinguished biocompatibility and meet the requested mechanical parameters, which is why they came to the attention of researchers [18,19,20,21,22,23]. Zinc is one of the trace elements of the human body and is involved in many vital processes [24]. Among other things, zinc is known to have crucial effects on bone formation by activating aminoacyl-tRNA synthetase in osteoblastic cells and thus increasing bone formation [25]. It is further known that zinc disrupts bone resorption by interfering with osteoclast-like cell formations [24,25]. Zinc also affects mesenchymal stem cells by increasing their growth rate and influencing their cell survival positively [26]. Furthermore, Ma et al. [27] showed that zinc supports cell migration and cell proliferation in smooth muscle cells. However, pure zinc does not offer the necessary mechanical stability, which is why it has to be alloyed with other metals such as silver to reach the benchmarks of the proposed mechanical properties for bioabsorbable materials [18,28]. By alloying zinc with silver, the ultimate tensile strength (UTS) can be increased up to 287 MPa, compared to 100–150 MPa for pure zinc [29,30].
Silver has been intensively researched as an implant material and has shown excellent biocompatibility [31,32]. It is proven that silver supports bone regeneration in the cancellous bone and, therefore, is a promising choice as an implant material [31]. Here, Zhang et al. [33] also showed that silver improves the healing process after fracture by introducing silver nanoparticles. Here, silver would increase the mesenchymal stem cell proliferation in the mouse model. Moreover, silver exhibits antibacterial properties by preventing the adhesion of bacteria to the surface of the material [34]. This material property is intensively researched in several medical fields to prevent bacterial infections after implantation [35,36,37].
Studies showed that the silver content of zinc–silver alloys correlates with the enhancement of the mechanical properties and, furthermore, alters the corrosion rate as well as the biocompatibility [30,38]. Zinc–silver alloys with an Ag percentage of, e.g., 6 wt% showed a UTS of 211 MPa, a yield strength (YS) of 213 MPa, and an elongation at fracture of 25% compared to pure zinc with a UTS of 115 MPa, a YS of 110 MPa, and an elongation at fracture of 9.7% [38]. In addition, the grain size has an enormous impact on the mechanical properties. Metals with smaller grains can have greater ductility, which is important for ensuring adequate osteosynthesis [39]. A moderate elongation at fracture is one key to a successful implant material. Hence, for orthopedic internal fixation devices, an elongation at fracture of 20–40% is recommended [40].
The degradation rate of zinc with a standard electrode potential of −0.76 V is lower and more beneficial for bone healing than that of magnesium with a standard electrode potential of −2.38 V [41,42]. The corrosion rate of zinc–silver alloys increases the higher the Ag content is and lies approximately between 0.05 and 0.15 mm/year [38]. This is within the suggested range of 0.04–0.14 mm/year for bioabsorbable implants in orthopedics and trauma surgery [18]. The degradation of zinc also does not lead to the formation of hydrogen gas pockets, as is the case for magnesium [14,43,44,45]. During the degradation process, the material slowly becomes absorbed by the patient’s body. Here, Vojtěch et al. [19] could display that the amount of zinc exposed to the metabolism is harmless and does not cause any negative effects since it is far below the tolerable daily dose of zinc with a limited maximum intake of 40 mg/day [19,46].
In this study, the mechanical properties and corrosion rate of ZnAg3 were investigated. The objective of this study was the qualification of this novel alloy with regard to its mechanical properties in order to validate its suitability for clinical use. The alloy examined has not yet been described in the literature with regard to its mechanical properties and may represent a novel material for osteosynthesis. The zinc–silver alloy contained 3.3 wt% Ag and showed promising biocompatibility on human osteoblasts in previous investigations, which is why this alloy was selected for further investigations [47]. More details about the tested alloy can be found in a previously published paper characterizing the alloy [47]. The alloy in this study was not coated and did not receive any surface modifications. To assess its aptitude as a bioabsorbable implant for osteosynthesis, mechanical measurements were conducted and included tensile tests and shear tests. For the shear tests, the ZnAg3 pins manufactured by Limedion and Quadralux (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany) were compared to magnesium pins (MAGNEZIX®, Syntellix AG, Hannover, Germany), which are already in clinical use. Here, the samples were subjected to typical loads after hallux-valgus surgery to examine the fatigue strength [48]. The quasi-static strength was assessed by loading the samples to the point of failure. Moreover, corrosion measurements were conducted to confirm the supposed degradation rate within the proposed range for bioabsorbable implants.
2. Materials and Methods
2.1. Tensile Testing
The tensile tests were conducted by the State Materials Testing Institute Darmstadt, Germany, where dog bone-shaped specimens of ZnAg3 manufactured by Limedion and Quadralux (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany) were tested according to ISO standard 6892-1:2020-06 [49]. The samples measured 4 mm in diameter and 20 mm in length. Measurements were conducted at room temperature with a class 1 universal testing machine (Hegewald & Peschke Inspect 250, Hegewald & Peschke Mess- und Prüftechnik GmbH, Nossen, Germany). Tensile strength and yield strength were investigated for ZnAg3. Elongation at fracture for ZnAg3 was determined according to ISO standard 6892-1:2020-06 Appendix I (Determination of elongation at fracture at subdivision of the initial measuring length) [49].
2.2. Shear Testing
To assess the mechanical performance, shear tests were performed with ZnAg3 pins manufactured by Limedion and Quadralux (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany) with a diameter of 2 mm and a length of 30 mm. For comparison, MAGNEZIX® pins (Serial No. 1127.030, Syntellix AG, Hannover, Germany) with the same diameter and length were also tested. To simulate physiological conditions, the pins were inserted into 20 PCF Cellular Rigid Polyurethane Foam (1522-12, Sawbones USA, Pacific Research Laboratories Inc., Vashon, WA, USA) as a functional bone substitute material. After predrilling the blocks with a standing drill (BF M3, Arnz Flott GmbH, Remscheid, Germany) at 650 rpm, a pin was inserted using an impactor (Impactor 6127.010, Syntellix AG, Hannover, Germany). Two blocks were joined together with a pin and deflected against each other during the shear tests.
Quasi-static strength and fatigue strength were evaluated by performing generic loading scenarios comparable to general loading conditions after hallux-valgus surgery. All mechanical measurements were conducted with a servo-hydraulic testing machine (Amsler HC 10, Zwick-Roell GmbH, Ulm, Germany) equipped with a 10 kN load cell as well as an eligible software (TestXpert R V1.4.2, Zwick-Roell GmbH, Ulm, Germany).
2.2.1. Fatigue Testing
A force-controlled, sinusoidal dynamic loading at 3 Hz for 250,000 cycles was applied to the pins to simulate the average walking load after hallux-valgus surgery [48]. The fatigue testing was conducted with a preload of 18 N and was cyclically loaded with a maximum force of 20 N or 30 N, and a minimum force of 6 N, which corresponds to a loading period of approximately 6 weeks assuming 5000–7000 cycles per day [48]. The recording was made with 1000 values per second for the applied force (N) and deformation (mm). A displacement of >2 mm was defined as pin failure [50,51].
2.2.2. Quasi-Static Testing
The pins were loaded to the point of failure, defined as a deformation of >2 mm, in displacement-controlled static tests at a rate of 5 mm/min. The recording was made with 1000 values per second for the applied force (N) and displacement (mm). Thus, the ultimate load (Fmax) could be determined.
2.3. Electrochemical Testing
To determine the corrosion rate, test specimens (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany) with a diameter of 6 mm, a height of 1 mm, and an exposed surface area of 0.2827 cm2 were secured watertight in a sample holder made of FC53 polyol and FC52/23 isocyanate (Rencast®, OBO-Werke GmbH, Stadthagen, Germany). The samples were then placed in a corrosion measuring cell (KMZ5, Sensortechnik Meinsberg, Xylem Analytics Germany Sales GmbH & Co. KG, Waldheim, Germany), which was filled with PBS solution (8 g/L NaCl, 0.2 g/L KCl, 1.44 g/L Na2HPO4, 0.245 g/L KH2PO4) and equipped with a potentiostat/galvanostat PS2000, an Ag/AgCl reference electrode SE11, and a counter electrode made of a 4 cm2 Pt foil. The measurements were conducted at a pH of 7.4 ± 0.2 and a temperature of 37 ± 2 °C.
At the beginning of the measurements, an open-circuit voltage was established for 30 min to ensure system stability. The corrosion measurements were then carried out at ± 50 mV around the resting potential at a scan rate of 0.25 mV/s. To define the corrosion current and corrosion potential, the linear sections of the logarithmic current–potential curve were extended with Tafel fittings in Origin 2022 Professional SR1 (Origin Lab, Northampton, MA, USA), and the intersection point was determined. The corrosion rate was calculated using Faraday’s law according to the equation below [52]:
Here, M is atomic mass, n is the number of electrons involved in the reaction, is density, and F is 96,485 C/mol.
2.4. Statistics
All calculations and designs of diagrams were executed with Origin 2022 Professional SR1 (OriginLab, Northampton, MA, USA). The conducted measurements are presented by their means and standard deviations. The level of significance was set at p < 0.05.
3. Results
3.1. Tensile Testing
The ultimate tensile strength and the yield strength of ZnAg3 could be determined at 237.5 ± 2.12 MPa and 168 ± 1.41 MPa, respectively. The elongation at fracture was found to be 38.5 ± 1.41%.
3.2. Shear Testing
All specimens could be implanted into the artificial bones safely and without distortion.
3.2.1. Fatigue Testing
No pin failure with a displacement of >2 mm occurred in all conducted runs. All specimens revealed microscopic relative movements with an amplitude of 5–30 µm, which were similar for both MAGNEZIX® pins and ZnAg3 pins. Over the course of 250,000 cycles, the overall migration did not exceed 100 µm (Figure 1).
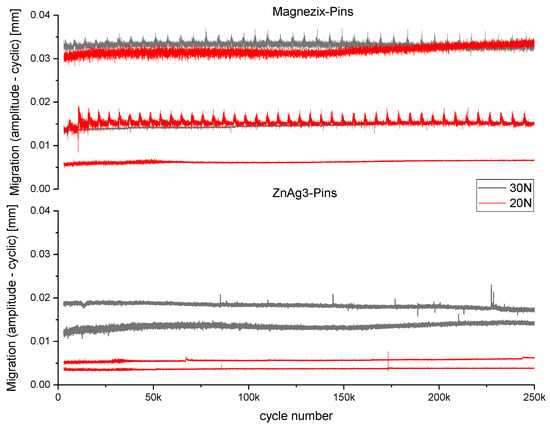
Figure 1.
Cyclic loading curve showing the migration (mm) over 250,000 cycles for ZnAg3 pins (bottom) and MAGNEZIX® pins (top) at 30 N (gray) and 20 N (red).
3.2.2. Quasi-Static Testing
The measurements of both pins showed very similar results, with an ultimate load of 144.8 ± 13.2 N for ZnAg3 pins and 141.2 ± 12.3 N for MAGNEZIX® pins (Figure 2). No significant difference could be observed (p = 0.71484) between the ZnAg3 pins and MAGNEZIX® pins.
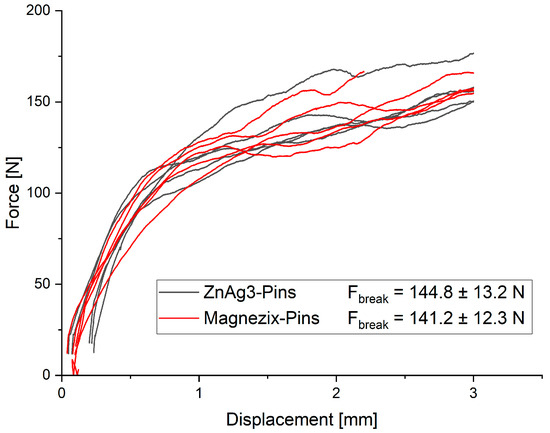
Figure 2.
Force–displacement curve for ZnAg3 pins and MAGNEZIX® pins no significant difference (p = 0.71484).
3.3. Electrochemical Testing
The established OCP was at −1013 ± 28.5 mV. With a corrosion potential (Ucorr) of −1001 ± 5 mV and a corrosion current (Icorr) of 1.82 ± 0.49 µA, the calculated corrosion rate was 0.10 mm/year for ZnAg3 (Figure 3).
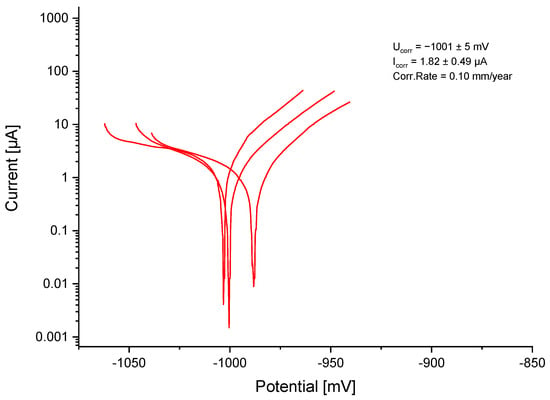
Figure 3.
Logarithmic current–potential curve for ZnAg3.
4. Discussion
4.1. Mechanical Measurements
The results of the tensile testing could display that ZnAg3 showed promising mechanical properties as a bioabsorbable material with an ultimate tensile strength of 237.5 ± 2.12 MPa, a yield strength of 168 ± 1.41 MPa, and an elongation at fracture of 38.5 ± 1.41%. The difficulty in finding suitable materials for bioabsorbable implants is that sufficient strength must be accompanied by high ductility [30,53]. Many materials could not offer both properties. Among others, Mg alloys showed great tensile strength but were poorly ductile, which can cause long-term failure [54,55,56,57]. The tested alloy offered the required ductility with an elongation at fracture of >30% as well as an adequate tensile strength of >200 MPa. In comparison, the MAGNEZIX® pins provide a high tensile strength of >290 MPa and yield strength of >260 MPa, but they do not retain enough ductility with an elongation at fracture of >8% [58].
A significant enhancement in tensile strength while sustaining high elongation at fracture is most likely facilitated by the countervailing effects of precipitation hardening and favorable texture orientation during extrusion [30]. Therefore, the extrusion process and the associated effects on grain size have an impact on the material properties. In this study, ZnAg3 manufactured by Limedion and Quadralux (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany) showed a grain size of 5–10 µm after hot extrusion at 250–300 °C with an extrusion rate of 25:1 [47]. In comparison, Sikora-Jasinska et al. [30] reported a UTS of 203 MPa, a YS of 157 MPa, and an elongation at fracture of approximately 34% for ZnAg2.5 with a grain size of 16 µm after hot extrusion at 250 °C with an extrusion rate of 14:1. Furthermore, Yang et al. [59] reported a UTS of 234 MPa, a YS of 188 MPa, and an elongation at fracture of 36% for ZnAg2 with a grain size of <10 µm after hot extrusion at 260 °C with an extrusion rate of 36:1.
It is important to note that, e.g., Bowen et al. [60] proposed different mechanical requirements for bioabsorbable implants such as stents. Here, a tensile strength of >300 MPa, a yield strength of >200 MPa, and an elongation at fracture of >18% were suggested [60]. Although these values are mostly accepted by the research community, it is crucial to notice that they were assimilated from the parameters of permanent 316 L stainless steel (SS) stents [61,62]. A permanent implant is requested to last for a much longer period of time than a bioabsorbable implant. By degrading the implant material and rebuilding the bone structure, the body is able to increasingly take on mechanical stress. As a result, the suggested values may be appropriate for permanent implants but might not comply with the optimum for bioabsorbable implants. In addition, similar mechanical values are being aimed at for different bioabsorbable implant materials, regardless of their usage site. Consequently, identical mechanical requirements are applied to vascular stent devices and bone implant materials, which are not in accordance with the distinct requirements established by their respective applications [39]. Thus, it may be necessary to revise the proposed values in order to distinguish between permanent and bioresorbable implants and to modify the requirements for mechanical performance according to the particular application.
The yield strength indicates the stress that a material can withstand until permanent deformation occurs. As soon as this load is exceeded, the material does not return to its original shape. Most commonly, single-loading event scenarios are performed to determine the load to the point of plastic deformation. In this study, ZnAg3 showed a yield strength of 168 ± 1.41 MPa, which is in the range of results from previously conducted studies [30,38,59]. However, a single-loading event is usually not the reason for implant breakage in clinical use. Typically, cyclic events cause implant failure since repetitive loading especially afflicts weak points, such as the material close to the fracture location. It is known that the yield strength correlates with the number of cycles until failure or breakage of the material in cyclic loading scenarios. This reflects the importance of fatigue testing, which should be carried out as part of the mechanical testing.
To assess a reliable fatigue strength, the experiment should conform to the expected load scenarios. During operations in trauma surgery, two or more bone fragments are usually fixed together. In order to avoid the fragments from sliding against each other, the implant usually has to withstand in particular shear, bending, and torsion forces after surgery [63]. Hence, the testing of orthopedic implant materials should focus on their shear, bending, and torsion fatigue strengths [29]. For this reason, the tests conducted included quasi-static shear testing, mimicking the mechanical stress after hallux-valgus surgery.
The results of the shear testing could prove sufficient fatigue strength as well as adequate resistance during the quasi-static testing of ZnAg3. The tested alloy could withstand the expected load after hallux-valgus surgery with approximately 5000 to 7000 steps a day, with a maximum load of 30 N per step for a period of 4 to 6 weeks until the healing process is expected to be concluded [48,64]. Here, the performance of ZnAg3 pins was equal to the MAGNEZIX® pins, which corresponds to the data in previous studies [65]. Moreover, a shear strength of 144.8 ± 13.2 N for the ZnAg3 pins qualifies the tested alloy as a fixation device in orthopedics and trauma surgery since there was no significant difference from the MAGNEZIX® pins with a shear strength of 141.2 ± 12.3 N, which are already in clinical use.
The shear tests were conducted in air and thus did not simulate physiological conditions, since the material is surrounded by body fluid in clinical use. Here, degradation of the implant material would take place, and might ultimately affect the mechanical properties. Very few studies have investigated this degradation process and its effects on zinc alloys. For example, Li et al. [66] examined the tensile strength of pure zinc and zinc alloys containing 1 wt% of magnesium, calcium, and strontium, respectively, after 8 weeks of immersion in simulated body fluid (m-SBF). The results showed that zinc and its alloys are able to maintain their mechanical integrity. Törne et al. [67] could prove that pure zinc in simulated body fluid would continue to show high ductility and great resistance to stress corrosion cracking. According to these results, it is very likely that the tested alloy in this study maintains its mechanical properties in a similar manner. This implies that the outcomes achieved are highly likely to be applicable to actual conditions.
When performing shear tests, such as in this study, the used bone substitute material and its holding forces play an important role. The 20 PCF cellular rigid polyurethane foam used in this study resembled the average density of a metatarsal bone at 0.32 g/cm3 [68]. This ensures a high degree of comparability with physiological conditions. For instance, Seebeck et al. [69] utilized human tibiae in their research and achieved significantly higher values compared to studies that used synthetic bone [65]. However, since there is a high degree of variation in bone structure, bone density, and bone composition when using cadaver bones, synthetic bone should be preferred in experimental tests in the interest of reproducibility [70].
4.2. Electrochemical Measurements
The corrosion rate of the tested ZnAg3 alloy was, at 0.10 mm/year, in the expected range between 0.05 and 0.15 mm/year, according to previous studies [17,38]. This could also prove that ZnAg3 showed a much higher corrosion resistance than Mg alloys and thus would provide better long-term stability in clinical use [55,71]. Hagelstein et al. [38] described a corrosion rate of 0.14 mm/year for ZnAg3 with an Ag content of 3.0 wt% manufactured by Limedion and Quadralux (Limedion GmbH, Mannheim, Germany; Quadralux e.K., Mannheim, Germany), which is slightly higher than the measured corrosion rate in this study. This circumstance might be due to the fact that the grain size of the ZnAg3 in this study was determined to be smaller at 5–10 µm and the Ag content was higher at 3.3 wt%, as previous investigations could show [47]. Thus, it is apparent that the grain size in fact impacts the corrosion rate. A homogeneous material with a small grain size automatically offers a significantly smoother surface, which is likely to increase the corrosion resistance. Salahshoor et al. [72] showed that a decrease in the corrosion rate of up to 50% is possible by altering the grain size through different manufacturing techniques in Mg–Ca alloys.
For bioabsorbable implants, the suggested values vary greatly and are highly dependent on the implantation site. Bowen et al. [28] proposed a required corrosion rate of 0.02 mm/year for biodegradable stent material. In comparison, e.g., Venezuela et al. [18] mentioned in their review a corrosion rate of approximately 0.04 mm/year to 0.14 mm/year for fixation devices such as screws or plates. With a degradation rate of 0.05–0.15 mm/year, the investigated ZnAg3 alloy is therefore within the range suggested by Venezuela et al. [18]. Overall, a bioabsorbable material in orthopedics and trauma surgery is requested to sustain its mechanical integrity for at least 3–6 months and subsequently dissolve completely within 1–2 years [59]. Yang et al. [59] could display that the degradation rate of pure zinc is too low, at approximately 0.015 mm/year. Moreover, the in vitro assessment was matched with in vivo observations regarding the volume loss. Here, pure zinc showed a volume loss of less than 10% after 8 weeks, which reflects an inadequately low corrosion rate.
A reasonable corrosion rate should be assured before considering a bioabsorbable material for clinical use. The measurements were conducted in an artificial environment, which does not fully represent physiological conditions. In order to be able to estimate the actual corrosion resistance of the tested alloy, referring to previous in vivo studies can be of avail. Yang et al. [59] introduced a ZnAg2 alloy into the rat model and could observe accelerated degradation compared to in vitro measurements. This finding seems reasonable since the implant material in vivo is subjected to the stress-shielding response of the organism after implantation [45]. That suggests that the corrosion behavior of ZnAg3 would similarly be reflected in living organisms, which would be highly compatible with bone formation at the measured corrosion rate, allowing sufficient osteointegration. Nonetheless, the performance of ZnAg3 as a bioabsorbable material needs to be confirmed with in vivo studies before considering clinical use.
5. Conclusions
The mechanical properties of ZnAg3 meet the requirements for bioabsorbable materials, as evidenced by the measurements conducted in this study. As requested for osteosynthetic implants, ZnAg3 offers a considerable tensile strength with high ductility as well as great shear strength, providing the needed mechanical stability during bone healing processes. An appropriate corrosion rate ensures adequate durability throughout the period of bone healing, thereby enabling the bone to form new bone material while still retaining sufficient stability. The results of this study are consistent with previous findings and therefore support the assumption that zinc–silver alloys are very suitable candidates for further investigations. Due to its beneficial mechanical properties and its distinguished biocompatibility, ZnAg3 represents a promising choice for in vivo studies.
6. Patents
The alloy, including its casting and extrusion process, is patented by the company Limedion. It includes the production of zinc with 90.0–99.5 wt% and silver with 0.05–10 wt%. The patent specification number is EP 3 250 247 B1.
Author Contributions
Conceptualization, M.S., S.Z. and M.R.; methodology, S.Z., M.R. and M.S.; software, S.Z. and M.R.; validation, M.R., S.Z. and M.S.; formal analysis, M.R., S.Z. and M.S.; investigation, S.Z. and M.R.; resources, M.S., R.B. and A.K.; data curation, M.R. and S.Z.; writing—original draft preparation, M.R.; writing—review and editing, M.S., A.K. and M.B.; visualization, S.Z. and M.R.; supervision, S.Z. and M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project (Z-BIORESORB) is funded by the Federal Ministry of Education and Research (BMBF) under the program Mat2MedTech. (funding code FZK 13XP5103A). The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and the University of Freiburg in the funding program Open Access Publishing.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Melanie Lynn Hart as a native speaker for proofreading and correcting grammar and spelling.
Conflicts of Interest
Adalbert Kovacs, Moritz Benner are employed by the company Limedion GmbH. Moritz Benner is also employed by the company Quadralux e.K. Roland Barkhoff is also employed by the company Quadralux e.K. The patent is from company Limedion GmbH.
References
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal nanoparticles released from dental implant surfaces: Potential contribution to chronic inflammation and peri-implant bone loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.C.; Hofmann, K.J.; Velasco, B.T.; Kwon, J.Y. Removal of hardware after syndesmotic screw fixation: A systematic literature review. Foot Ankle Spec. 2016, 10, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.K.; Gamble, J.G.; Kha, S.T.; Hecht, G.G.; Vorhies, J.S.; Lucas, J.F. Indications for and risks associated with implant removal after pediatric trauma. J. Am. Acad. Orthop. Surgeons. Glob. Res. Rev. 2022, 6, e22. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Roossien, C.C.; van Bakelen, N.B.; Verkerke, G.J.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Comparison of the mechanical properties of biodegradable and titanium osteosynthesis systems used in oral and maxillofacial surgery. Sci. Rep. 2020, 10, 18143. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; de Bruijn, W.C. Foreign body reactions to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Kallela, I.; Iizuka, T.; Salo, A.; Lindqvist, C. Lag-screw fixation of anterior mandibular fractures using biodegradable polylactide screws: A preliminary report. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1999, 57, 113–118. [Google Scholar] [CrossRef]
- Turvey, T.A.; Proffit, W.P.; Phillips, C. Biodegradable fixation for craniomaxillofacial surgery: A 10-year experience involving 761 operations and 745 patients. Int. J. Oral Maxillofac. Surg. 2011, 40, 244–249. [Google Scholar] [CrossRef]
- Patel, N.R.; Gohil, P. A review on biomaterials: Scope, applications & human anatomy significance. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 91–101. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 2207–2214. [Google Scholar] [CrossRef]
- Song, G.-l.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Vojtěch, D. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Teh. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, E.; Shao, J.; Ge, S. Advances on biodegradable zinc-silver-based alloys for biomedical applications. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211062407. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.A.; Guillory, R.J., II; Flom, K.L.; Morath, L.M.; Kolesar, T.M.; Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Goldman, J. Analysis of vascular inflammation against bioresorbable zn–ag-based alloys. ACS Appl. Bio Mater. 2020, 3, 6779–6789. [Google Scholar] [CrossRef]
- Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A comprehensive review of the current research status of biodegradable zinc alloys and composites for biomedical applications. Materials 2023, 16, 4797. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M.H. Fabrication of biodegradable zn-al-mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Lin, S.; Yang, F.; Ling, M.; Fan, Y. Association between bone trace elements and osteoporosis in older adults: A cross-sectional study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x221125984. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Young, M.; Ma, J.; Zheng, Y.; Tang, L. Biological responses and mechanisms of human bone marrow mesenchymal stem cells to zn and mg biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, N.; Zhu, D. Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Sci. Rep. 2016, 6, 26661. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., 2nd; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable metals for cardiovascular stents: From clinical concerns to recent zn-alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed znag alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Zierold, A.A. Reaction of bone to various metals. Arch. Surg. 1924, 9, 365–412. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Müller, W.D.; Schade, R.; Liefeith, K.; Kainer, K.U.; Willumeit, R. Antibacterial biodegradable mg-ag alloys. Eur. Cell Mater. 2013, 25, 284–298, discussion 298. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, P.; Lui, V.C.H.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.K.; Wong, K.K.Y. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1949–1959. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical characteristics, in vitro degradation, cytotoxicity, and antibacterial evaluation of zn-4.0ag alloy as a biodegradable material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Kuehl, R.; Brunetto, P.S.; Woischnig, A.K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terracciano, L.; Fromm, K.M.; Khanna, N. Preventing implant-associated infections by silver coating. Antimicrob. Agents Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef]
- Jacquart, S.; Girod-Fullana, S.; Brouillet, F.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Grimoud, J.; Rey, C.; Roques, C.; Combes, C. Injectable bone cement containing carboxymethyl cellulose microparticles as a silver delivery system able to reduce implant-associated infection risk. Acta Biomater. 2022, 145, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Hagelstein, S.; Zankovic, S.; Kovacs, A.; Barkhoff, R.; Seidenstuecker, M. Mechanical analysis and corrosion analysis of zinc alloys for bioabsorbable implants for osteosynthesis. Materials 2022, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Märdian, S.; Seemann, R.; Schmidt-Bleek, K.; Heyland, M.; Duda, G. Biologie und biomechanik der frakturheilung und osteosynthese. Orthopädie Unfallchirurgie Up2date 2019, 14, 163–183. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an mg-zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Research. Part B Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef]
- Törne, K.B.; Ashraf, K.F.; Andreas, Ö.; Jonas, W. Zn–mg and zn–ag degradation mechanism under biologically relevant conditions. Surf. Innov. 2018, 6, 81–92. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Roesner, M.; Zankovic, S.; Kovacs, A.; Benner, M.; Barkhoff, R.; Seidenstuecker, M. Biocompatibility assessment of zinc alloys as a new potential material for bioabsorbable implants for osteosynthesis. Materials 2023, 16, 5224. [Google Scholar] [CrossRef]
- Acevedo, J.I.; Sammarco, V.J.; Boucher, H.R.; Parks, B.G.; Schon, L.C.; Myerson, M.S. Mechanical comparison of cyclic loading in five different first metatarsal shaft osteotomies. Foot Ankle Int. 2002, 23, 711–716. [Google Scholar] [CrossRef] [PubMed]
- DIN En ISO 6892-1:2020-06; Metallic Materials-Tensile Testing Part 1: Method of Test at Room Temperature (ISO 6892-1:2019). Beuth Verlag GmbH: Berlin, Germany, 2020.
- Kılıç, B.; Çalışkan, M.; Agar, A.; Uzun, B.; Ertem, F.; Gülabi, D.; Ertürk, C. Comparison of two different screw trajectories in the treatment of oblique scaphoid fractures: A mechanical study on composite bone models. Jt. Dis. Relat. Surg. 2021, 32, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Clyde, J.; Kosmopoulos, V.; Carpenter, B. A biomechanical investigation of a knotless tension band in medial malleolar fracture models in composite sawbones®. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2013, 52, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Faradaysches gesetz. Faust Metalltechnik Enzyklopädie; Engineering und Spezifikationsschreiben; Faust Metalltechnik GmbH: Laufach, Germany, 2022. [Google Scholar]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mostaed, E.; Cao, X.; Huang, G.; Fabrizi, A.; Bonollo, F.; Chi, C.; Vedani, M. Effects of texture and grain size on mechanical properties of az80 magnesium alloys at lower temperatures. Mater. Des. 2016, 89, 1–8. [Google Scholar] [CrossRef]
- Mostaed, E.; Vedani, M.; Hashempour, M.; Bestetti, M. Influence of ecap process on mechanical and corrosion properties of pure mg and zk60 magnesium alloy for biodegradable stent applications. Biomatter 2014, 4, e28283. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Ge, Q.; Vedani, M.; Botelho, P.; Zanella, C.; Deflorian, F. Investigation on the influence of grain size on strength, ductility, and corrosion properties in mg and mg-zn based alloys for biodegradable stents. Eur. Cells Mater. 2013, 26, 84. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable zn-mg alloy. J. Biomed. Mater. Research. Part B Appl. Biomater. 2015, 103, 1632–1640. [Google Scholar] [CrossRef]
- Syntellix, A.G. Innovation verstehen-die besonderen eigenschaft von magnezix®. In Syn Broschuere; Special Report DE Druck; Syntellix AG: Hannover, Germany, 2018. [Google Scholar]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron-manganese: New class of degradable metallic biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Suzuki, H.; Hata, Y.; Watanabe, F. Implant fracture under dynamic fatigue loading: Influence of embedded angle and depth of implant. Odontology 2016, 104, 357–362. [Google Scholar] [CrossRef]
- Trnka, H.J.; Parks, B.G.; Ivanic, G.; Chu, I.T.; Easley, M.E.; Schon, L.C.; Myerson, M.S. Six first metatarsal shaft osteotomies: Mechanical and immobilization comparisons. Clin. Orthop. Relat. Res. 2000, 381, 256–265. [Google Scholar] [CrossRef]
- Hagelstein, S.; Seidenstuecker, M.; Kovacs, A.; Barkhoff, R.; Zankovic, S. Fixation performance of bioabsorbable zn-6ag pins for osteosynthesis. Materials 2022, 15, 3280. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of biodegradable zn-1x binary alloys with nutrient alloying elements mg, ca and sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Törne, K.; Örnberg, A.; Weissenrieder, J. Influence of strain on the corrosion of magnesium alloys and zinc in physiological environments. Acta Biomater. 2017, 48, 541–550. [Google Scholar] [CrossRef]
- Sindel, M. Bone mineral density of the metatarsal bones and the first ray in male sportsmen. Anat. (Int. J. Exp. Clin. Anat.) 2010, 4, 39–44. [Google Scholar] [CrossRef][Green Version]
- Seebeck, J.; Goldhahn, J.; Städele, H.; Messmer, P.; Morlock, M.M.; Schneider, E. Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2004, 22, 1237–1242. [Google Scholar] [CrossRef]
- Chapman, J.R.; Harrington, R.M.; Lee, K.M.; Anderson, P.A.; Tencer, A.F.; Kowalski, D. Factors affecting the pullout strength of cancellous bone screws. J. Biomech. Eng. 1996, 118, 391–398. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Zhou, W.; Xiong, P.; Wang, P.; Yuan, W.; Zheng, Y.; Cheng, Y. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (mg/zn/fe) for the application of tracheobronchial stenosis. Bioact. Mater. 2019, 4, 114–119. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (mgca) alloys, processing, and corrosion performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).