Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid–Polynucleotide Gel (Regenfast)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Study Design
2.3. Experimental Animals and Sample Size
2.4. Randomization and Allocation Concealment
2.5. Biomaterials
2.6. Anesthetic Procedures
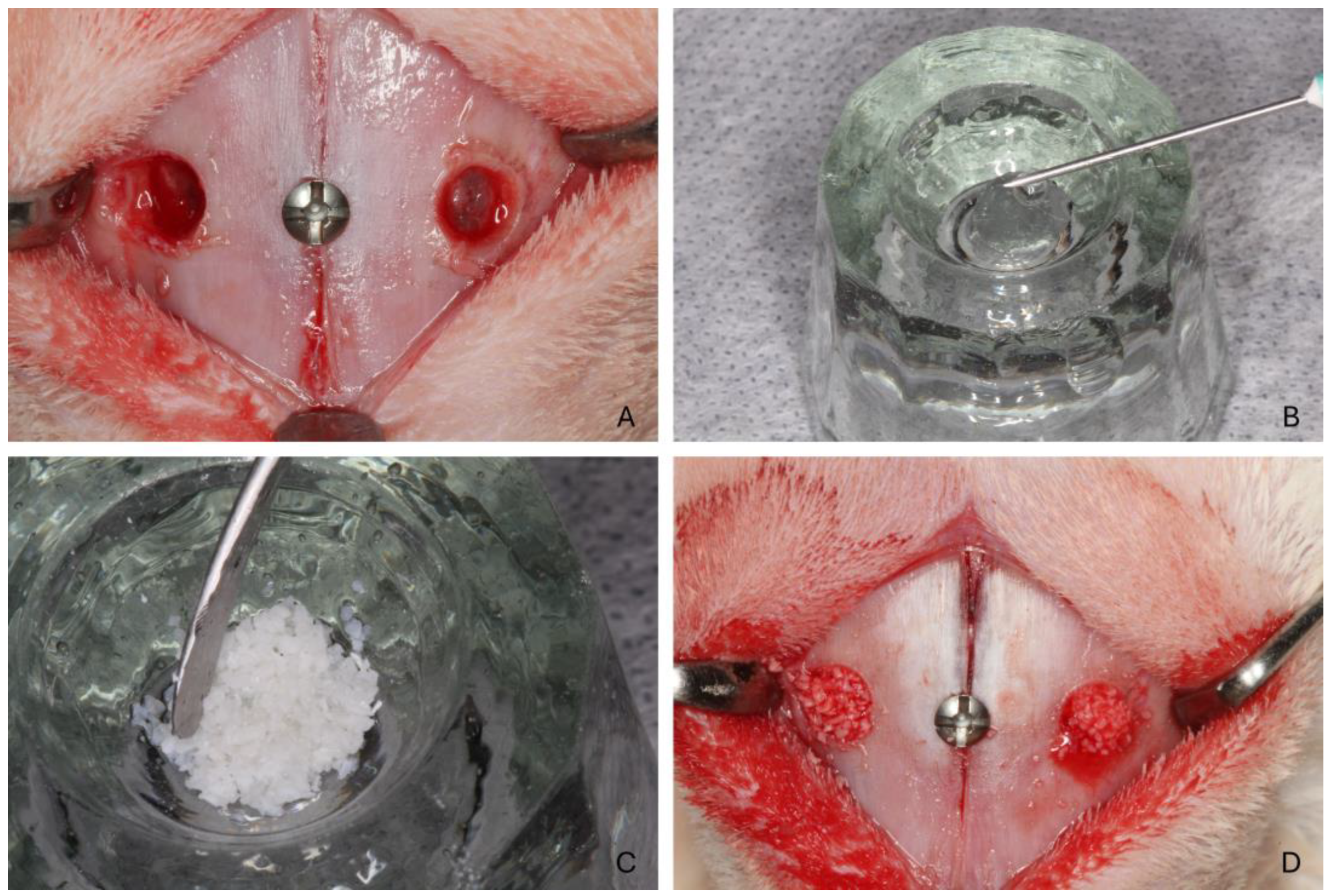
2.7. Surgical Procedure
2.8. Animal Maintenance
2.9. Euthanasia
2.10. Histological Processing
2.11. Histomorphometric Evaluation
2.12. Data Analysis
3. Results
3.1. Clinical Outcomes
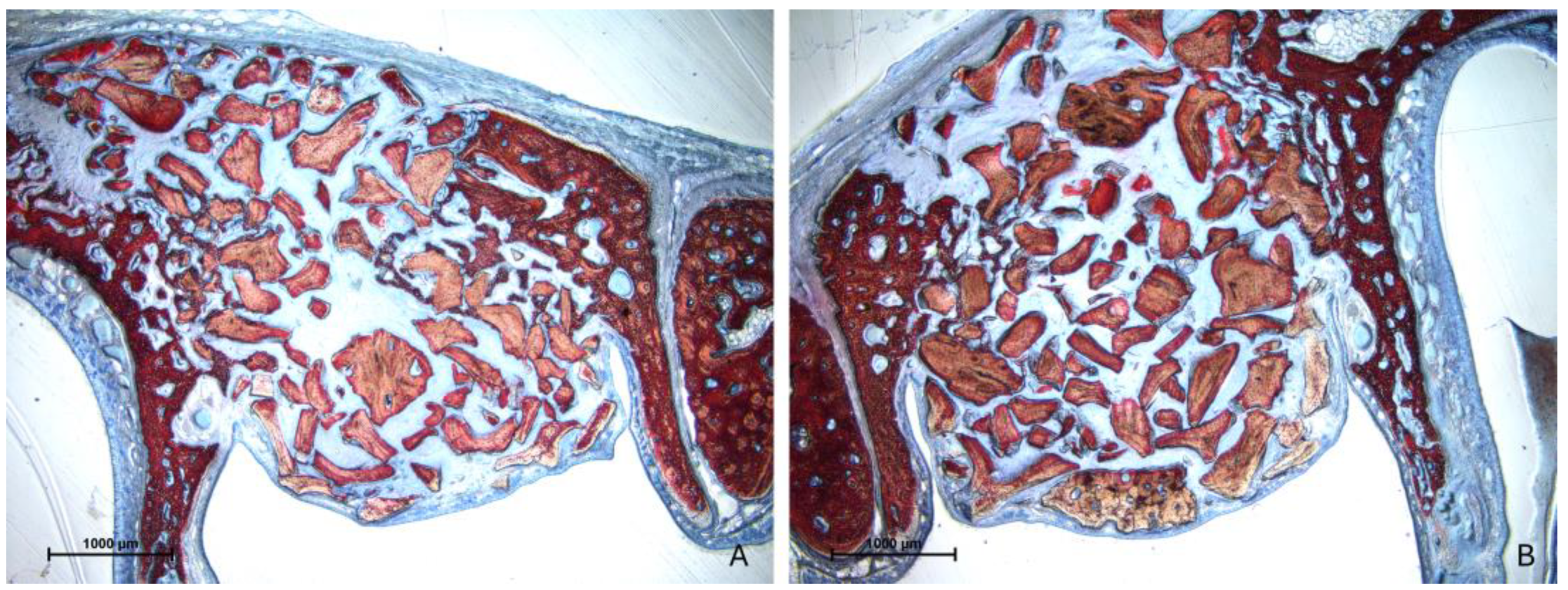
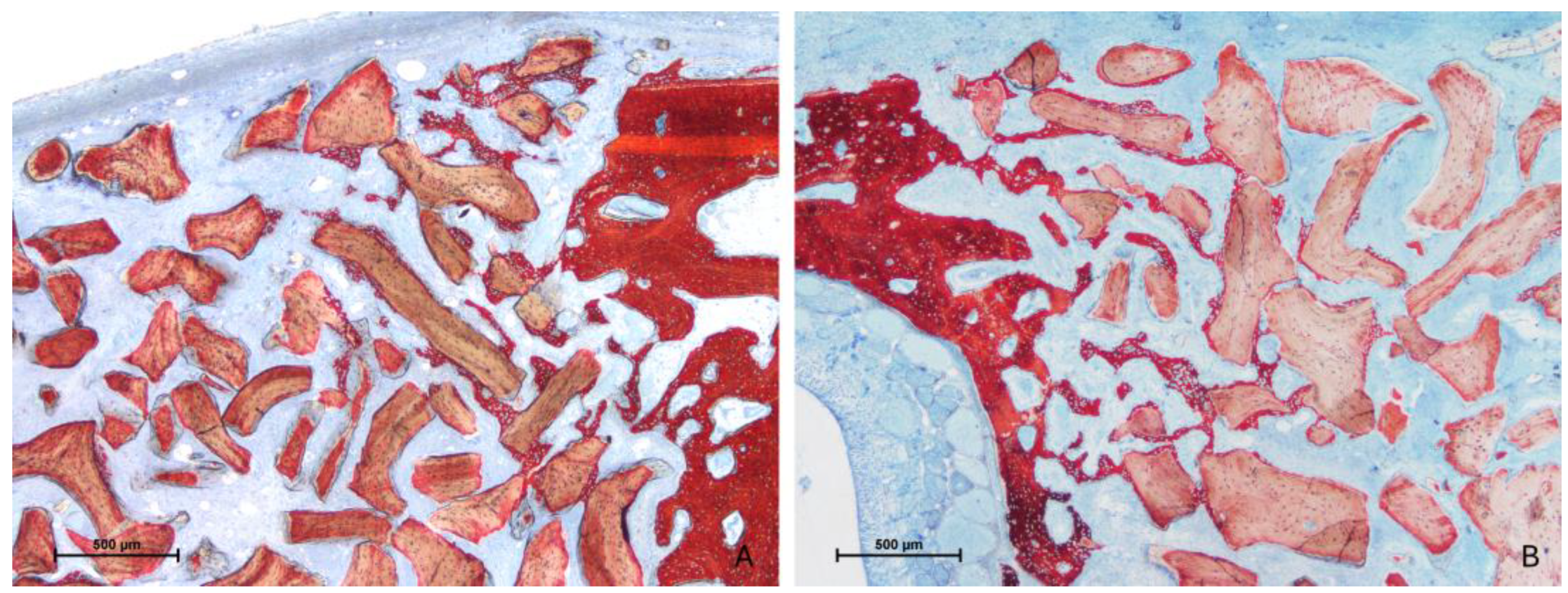
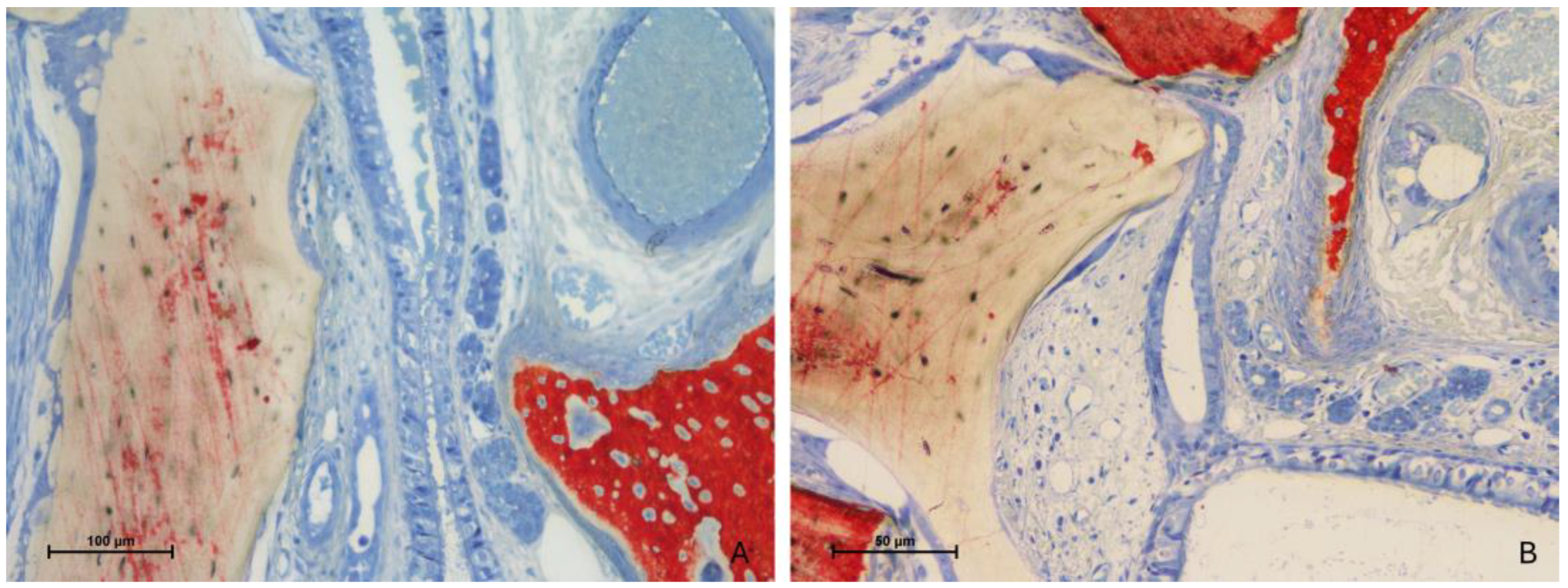
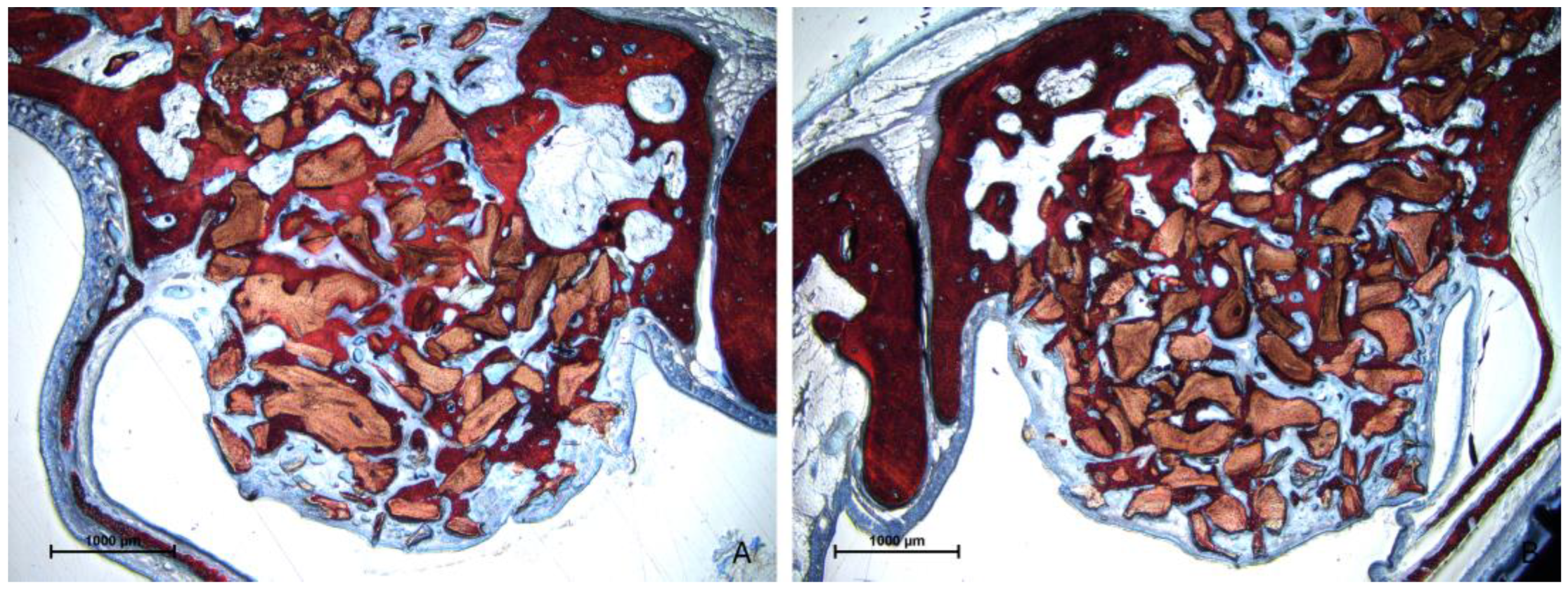
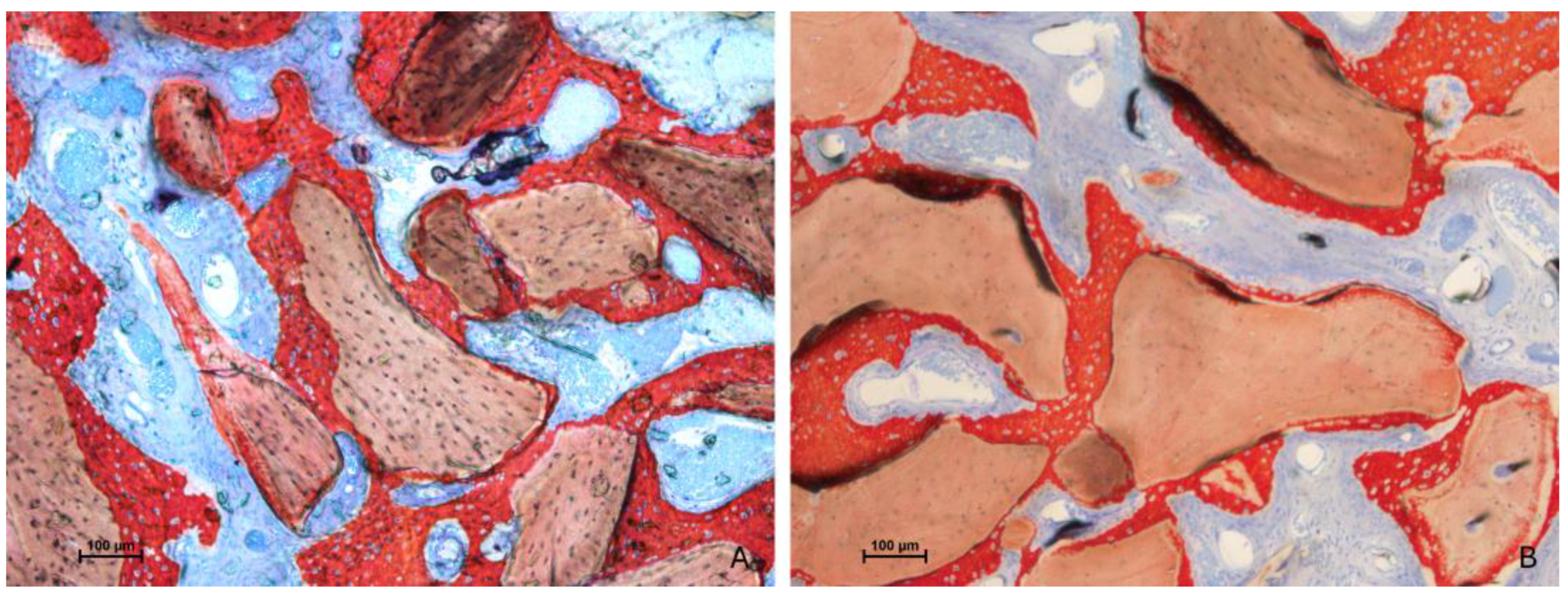
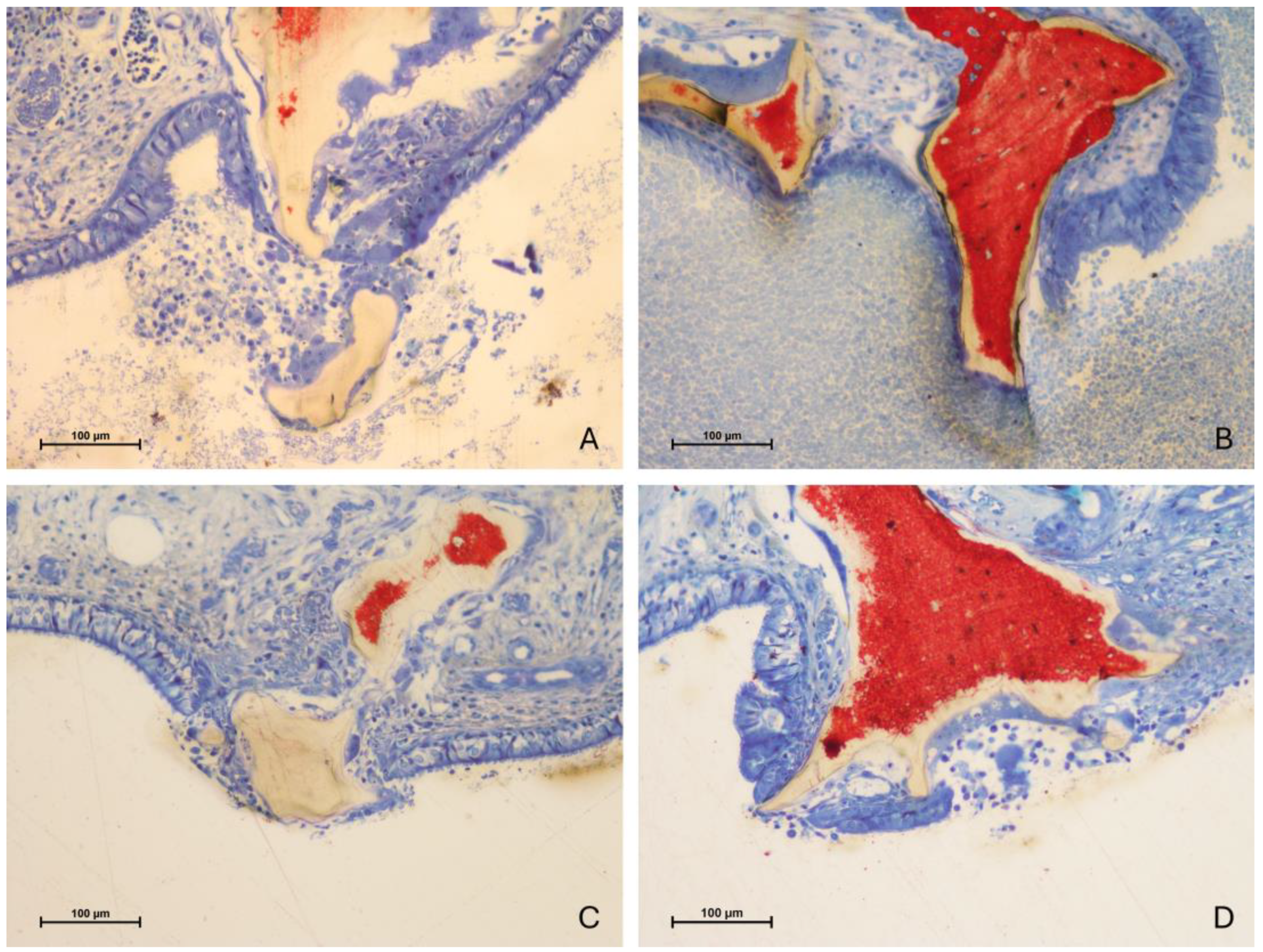
3.2. Descriptive Histological Evaluation
3.3. Histomorphometric Assessments
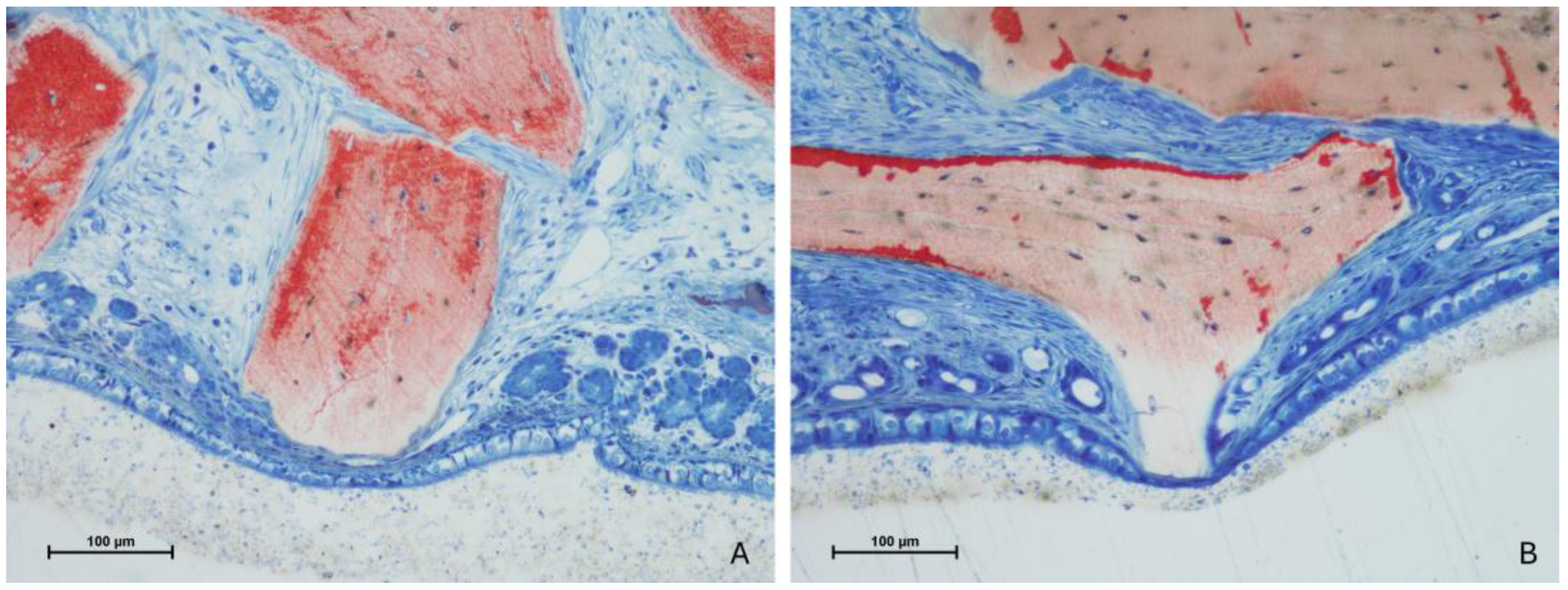
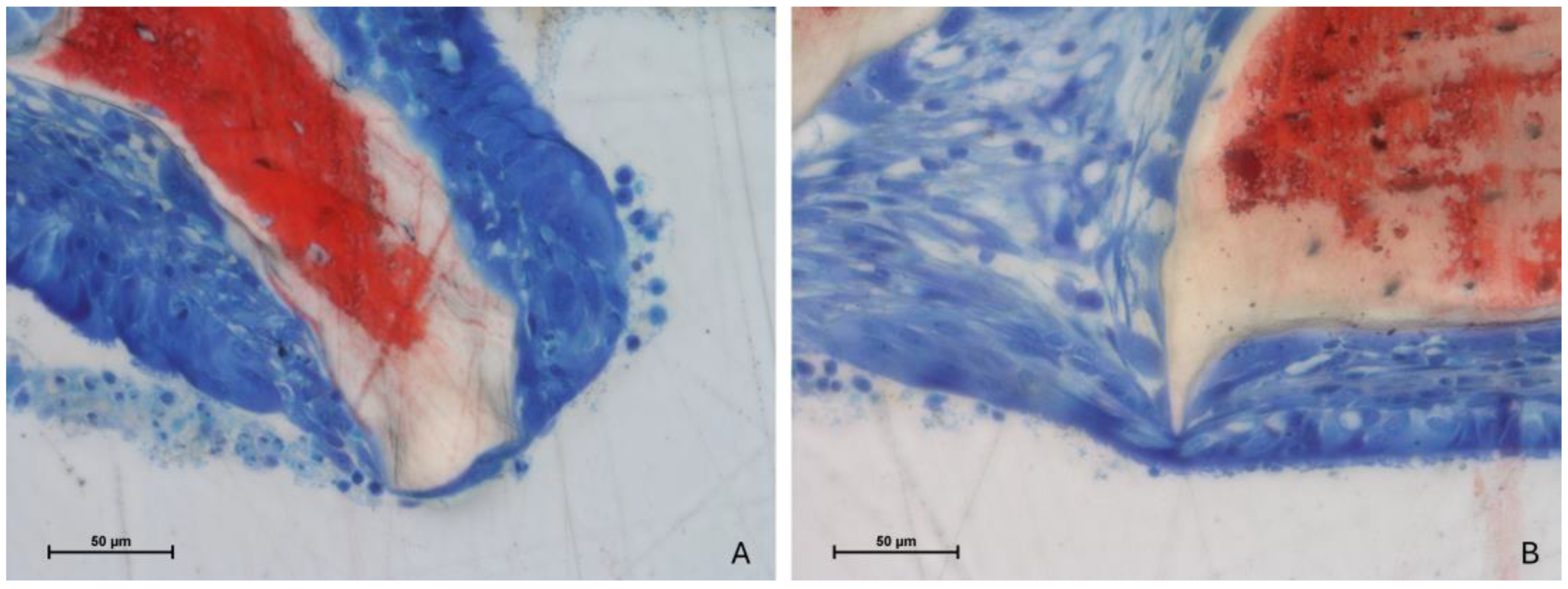
3.4. Mucosa Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Dula, K.; Hess, D.; Hirt, H.P.; Belser, U.C. Localized ridge augmentation with autografts and barrier membranes. Periodontol. 2000 1999, 19, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Cricchio, G.; Hallman, M.; Jungner, M.; Rasmusson, L.; Sennerby, L. Sinus floor elevation procedures to enable implant placement and integration: Techniques, biological aspects and clinical outcomes. Periodontol. 2000 2017, 73, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Grafting of deproteinized bone particles inhibits bone resorption after maxillary sinus floor elevation. Clin. Oral Implant. Res. 2004, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Schou, S.; Svendsen, P.A.; Forman, J.L.; Gundersen, H.J.; Terheyden, H.; Holmstrup, P. Volumetric changes of the graft after maxillary sinus floor augmentation with Bio-Oss and autogenous bone in different ratios: A radiographic study in minipigs. Clin. Oral Implant. Res. 2012, 23, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Levin, L.; Guidetti, F.; Sbordone, C.; Glikman, A.; Schwartz-Arad, D. Apical and marginal bone alterations around implants in maxillary sinus augmentation grafted with autogenous bone or bovine bone material and simultaneous or delayed dental implant positioning. Clin. Oral Implant. Res. 2011, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: A systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Manfro, R.; Fonseca, F.S.; Bortoluzzi, M.C.; Sendyk, W.R. Comparative, Histological and Histomorphometric Analysis of Three Anorganic Bovine Xenogenous Bone Substitutes: Bio-Oss, Bone-Fill and Gen-Ox Anorganic. J. Maxillofac. Oral Surg. 2014, 13, 464–470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, J.-W.; Sohn, D.-S.; Heo, J.-U.; Kim, J.S. Comparison of two kinds of bovine bone in maxillary sinus augmentation: A histomorphometric study. Implant Dent. 2015, 24, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mordenfeld, A.; Lindgren, C.; Hallman, M. Sinus Floor Augmentation Using Straumann® BoneCeramic™ and Bio-Oss® in a Split Mouth Design and Later Placement of Implants: A 5-Year Report from a Longitudinal Study. Clin. Implant Dent. Relat. Res. 2016, 18, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Yamada, Y.; Canullo, L.; Xavier, S.P.; Baba, S. Bioactivation of Bovine Bone Matrix Using Argon Plasma: An Experimental Study for Sinus Augmentation in Rabbits. Int. J. Oral Maxillofac. Implant. 2020, 35, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral Implant. Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Aneiros-Fernández, J.; Camara, M.; Mesa, F.; Wallace, S.; O’Valle, F. Morphological evidences of Bio-Oss® colonization by CD44-positive cells. Clin. Oral Implant. Res. 2014, 25, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colangelo, M.T.; Govoni, P.; Belletti, S.; Squadrito, F.; Guizzardi, S.; Galli, C. Polynucleotide biogel enhances tissue repair, matrix deposition and organization. J. Biol. Regul. Homeost. Agents 2021, 35, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Rønnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Müller, H.-D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal Res. 2019, 54, 33–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslan, M.; Simsek, G.; Dayi, E. The effect of hyaluronic acid-supplemented bone graft in bone healing: Experimental study in rabbits. J. Biomater. Appl. 2006, 20, 209–220. [Google Scholar] [CrossRef] [PubMed]
- de Brito Bezerra, B.; Braz~ao, M.A.M.; de Campos, M.L.G.; Casati, M.Z.; Sallum, E.A.; Sallum, A.W. Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects. Clin. Oral Implant. Res. 2012, 23, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Arpağ, O.F.; Damlar, I.; Altan, A.; Tatli, U.; Günay, A. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J. Appl. Oral Sci. 2018, 26, e20170004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.J.; Song, H.Y.; Ben Amara, H.; Kyung-Rim, K.; Koo, K.T. Hyaluronic Acid Improves Bone Formation in Extraction Sockets with Chronic Pathology: A Pilot Study in Dogs. J. Periodontol. 2016, 87, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.M.; Hammad, M.M.; Abdelhadi, I.N.; Khalifeh, M.S. Effects of topically applied agents on intra-oral wound healing in a rat model: A clinical and histomorphometric study. Int. J. Dent. Hyg. 2011, 9, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 52, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Nakamura, T.; Kawakami, Y.; Imafuji, T.; Shinohara, Y.; Noguchi, K.; Sculean, A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J. Clin. Periodontol. 2021, 48, 570–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gocmen, G.; Gonul, O.; Oktay, N.S.; Yarat, A.; Goker, K. The antioxidant and anti-inflammatory efficiency of hyaluronic acid after third molar extraction. J. Craniomaxillofac. Surg. 2015, 43, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, C.E.P.; Castro, M.A.A.; de Noronha, M.S.; Martins-Junior, P.A.; Mendes, R.M.; Caliari, M.V.; Mesquita, R.A.; Ferreira, A.J. Hyaluronic acid accelerates bone repair in human dental sockets: A randomized triple-blind clinical trial. Braz. Oral Res. 2018, 32, e84. [Google Scholar] [CrossRef] [PubMed]
- Afat, İ.M.; Akdoğan, E.T.; Gönül, O. Effects of Leukocyte- and Platelet-Rich Fibrin Alone and Combined with Hyaluronic Acid on Pain, Edema, and Trismus After Surgical Extraction of Impacted Mandibular Third Molars. J. Oral Maxillofac. Surg. 2018, 76, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Afat, I.M.; Akdoğan, E.T.; Gönül, O. Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: A prospective clinical study. J. Cranio-Maxillofac. Surg. 2019, 47, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Renatus, A.; Heinicke, M.; Pfister, W.; Stratul, S.I.; Jentsch, H. Hyaluronic Acid as an adjunct after scaling and root planing: A prospective randomized clinical trial. J. Periodontol. 2013, 84, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.J.; Shah, S.A.; Vijayakar, H.N.; Rodrigues, S.V.; Mitra, D.K.; Shah, R.A. To compare the effect of the local delivery of hyaluronan as an adjunct to scaling and root planing versus scaling and root planing alone in the treatment of chronic periodontitis. J. Indian Soc. Periodontol. 2016, 20, 549–556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Shammari, N.M.; Shafshak, S.M.; Ali, M.S. Effect of 0.8% Hyaluronic Acid in Conventional Treatment of Moderate to Severe Chronic Periodontitis. J. Contemp. Dent. Pract. 2018, 19, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef] [PubMed]
- Brignardello-Petersen, R. Hyaluronic acid may result in benefits in clinical attachment levels and probing depth in patients undergoing periodontal treatment in the short term. J. Am. Dent. Assoc. 2020, 151, e10. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar] [CrossRef] [PubMed]
- de Santana, R.B.; de Santana, C.M. Human intrabony defect regeneration with rhFGF-2 and hyaluronic acid—A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Polepalle, T.; Kumar, R.; Srinivas, M.; Pai, J.; Suragimath, G.; Prasad, K. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Millers Class I recession: A clinical study. J. Indian Soc. Periodontol. 2014, 18, 746–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.P.; Kim, H.J.; Yu, S.J.; Kim, B.O. Six Month Clinical Evaluation of Interdental Papilla Reconstruction with Injectable Hyaluronic Acid Gel Using an Image Analysis System. J. Esthet. Restor. Dent. 2016, 28, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Shu, R.; Li, C. Efficacy Evaluation of Hyaluronic Acid Gel for the Restoration of Gingival Interdental Papilla Defects. J. Oral Maxillofac. Surg. 2019, 77, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Turgut Çankaya, Z.; Gürbüz, S.; Bakirarar, B.; Ünsal, B.; Kurtiş, B. Evaluation of the effect of the application of hyaluronic acid following laser-assisted frenectomy: An examiner-blind, randomized, controlled clinical study. Quintessence Int. 2020, 51, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Ficho, A.C.; de Souza Faloni, A.P.; Pennisi, P.R.C.; Borges, L.G.F.; de Macedo Bernadino, Í.; Paranhos, L.R.; Queiroz, T.P.; Santos, P.L. Is interdental papilla filling using hyaluronic acid a stable approach to treat black triangles? A systematic review. J. Esthet. Restor. Dent. 2021, 33, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, S.; Özener, H.Ö.; Doğan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 2018, 89, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Turgut Çankaya, Z.; Tamam, E. An examination of the 2-year results obtained from hyaluronic acid filler injection for interdental papilla losses. Quintessence Int. 2020, 51, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Rohde, L.E.; Clausell, N.; Ribeiro, J.P.; Goldraich, L.; Netto, R.; William Dec, G.; DiSalvo, T.G.; Polanczyk, C.A. Health outcomes in decompensated congestive heart failure: A comparison of tertiary hospitals in Brazil and United States. Int. J. Cardiol. 2005, 102, 71–77. [Google Scholar] [CrossRef] [PubMed]
- William, D.; Plummer, W.D. Power and sample size calculations: A review and computer program. Control. Clin. Trials 1990, 11, 116–128, ISSN 0197-2456. [Google Scholar] [CrossRef]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Kandler, B.; Fuerst, G.; Fisher, M.B.; Watzek, G. Porcine sinus mucosa holds cells that respond to bone morphogenetic protein (BMP)-6 and BMP-7 with increased osteogenic differentiation in vitro. Clin. Oral Implant. Res. 2004, 15, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Kizhner, T.; Ben David, D.; Riminucci, M.; Bianco, P.; Livne, E. The Schneiderian membrane contains osteoprogenitor cells: In vivo and in vitro study. Calcif. Tissue Int. 2009, 84, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Ben-David, D.; Lotan, R.; Riminucci, M.; Livne, E.; Bianco, P. The innate osteogenic potential of the maxillary sinus (Schneiderian) membrane: An ectopic tissue transplant model simulating sinus lifting. Int. J. Oral Maxillofac. Surg. 2010, 39, 793–801. [Google Scholar] [CrossRef]
- Srouji, S.; Ben-David, D.; Funari, A.; Riminucci, M.; Bianco, P. Evaluation of the osteoconductive potential of bone substitutes embedded with schneiderian membrane- or maxillary bone marrow-derived osteoprogenitor cells. Clin. Oral Implant. Res. 2013, 24, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Falah, M.; Srouji, S. Raised Schneiderian membrane compared with peeled bony walls in the formation of bone. Br. J. Oral Maxillofac. Surg. 2016, 54, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Botticelli, D.; Rangel, I.G., Jr.; de Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant. Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef]
- Lim, S.T.; Kusano, K.; Taniyama, T.; Sakuma, S.; Nakajima, Y.; Xavier, S.P.; Baba, S. Contribution to Bone Formation of the Schneiderian Membrane after Sinus Augmentation: A Histological Study in Rabbits. Materials 2022, 15, 8077. [Google Scholar] [CrossRef]
- Miki, M.; Botticelli, D.; Silva, E.R.; Xavier, S.P.; Baba, S. Incidence of Sinus Mucosa Perforations During Healing After Sinus Elevation Using Deproteinized Bovine Bone Mineral as Grafting Material: A Histologic Evaluation in a Rabbit Model. Int. J. Oral Maxillofac. Implant 2021, 36, 660–668. [Google Scholar] [CrossRef]
- Kato, S.; Botticelli, D.; De Santis, E.; Kanayama, M.; Ferreira, S.; Rangel Garcia, I., Jr. Sinus mucosa thinning and perforation after sinus augmentation. A histological study in rabbits. Oral Maxillofac. Surg. 2021, 25, 477–485. [Google Scholar] [CrossRef]
- Nakajima, Y.; Botticelli, D.; De Rossi, E.F.; Ferreira Balan, V.; Pires Godoy, E.; Ricardo Silva, E.; Xavier, S.P. Schneiderian Membrane Collateral Damage Caused by Collagenated and Non-Collagenated Xenografts: A Histological Study in Rabbits. Dent. J. 2023, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Botticelli, D.; Ferri, M.; Delgado-Ruiz, R.; Ferreira Balan, V.; Porfirio Xavier, S. Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits. Dent. J. 2021, 9, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawakami, S.; Botticelli, D.; Nakajima, Y.; Sakuma, S.; Baba, S. Anatomical analyses for maxillary sinus floor augmentation with a lateral approach: A cone beam computed tomography study. Ann. Anat. 2019, 226, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Lang, N.P.; De Rossi, E.F.; Xavier, S.P. Mucosal adhesion phenomenon after maxillary sinus floor elevation: A preclinical study. Clin. Oral Implant. Res. 2023, 34, 967–978. [Google Scholar] [CrossRef] [PubMed]


| 2 Weeks | Bio-Oss Alone | Bio-Oss Regenfast | ||||
|---|---|---|---|---|---|---|
| New Bone | Xenograft | Soft Tissue | New Bone | Xenograft | Soft Tissue | |
| Sub-Schneiderian | 4.4 ± 2.8 | 45.7 ± 8.8 * | 49.9 ± 9.6 * | 4.6 ± 3.8 | 37.7 ± 3.8 * | 57.7 ± 4.6 * |
| Walls | 12.7 ± 8.4 | 46.9 ± 7.5 | 40.4 ± 7.6 | 17.6 ± 6.3 | 36.2 ± 8.7 | 46.3 ± 8.7 |
| Central | 7.4 ± 8.3 | 43.2 ± 7.6 | 49.4 ± 13.3 | 3.8 ± 3.7 | 41.0 ± 14.4 | 55.1 ± 12.7 |
| Sub-Window | 6.4 ± 5.2 | 40.4 ± 10.9 | 53.2 ± 13.2 | 6.4 ± 5.9 | 38.3 ± 10.1 | 55.3 ± 6.1 |
| Total | 7.7 ± 4.3 | 44.0 ± 6.2 | 48.2 ± 8.9 | 8.1 ± 3.8 | 38.3 ± 7.4 | 53.6 ± 5.6 |
| 10 Weeks | Bio-Oss Alone | Bio-Oss Regenfast | ||||
|---|---|---|---|---|---|---|
| New Bone | Xenograft | Soft Tissue | New Bone | Xenograft | Soft Tissue | |
| Sub-Schneiderian | 27.6 ± 27.6 | 34.2 ± 7.8 * | 38.2 ± 14.3 * | 25.3 ± 11.9 | 24.3 ± 10.8 * | 50.5 ± 16.0 * |
| Walls | 29.9 ± 10.0 | 29.4 ± 13.9 | 40.7 ± 22.5 | 27.6 ± 8.4 | 26.7 ± 11.4 | 45.7 ± 16.3 |
| Central | 27.9 ± 10.8 | 35.5 ± 7.5 * | 36.5 ± 12.8 | 28.0 ± 12.8 | 22.2 ± 14.7 * | 49.9 ± 23.8 |
| Sub-Window | 26.5 ± 14.2 | 31.8 ± 12.6 | 41.7 ± 23.9 | 28.3 ± 16.6 | 22.7 ± 9.4 | 49.0 ± 24.1 |
| Total | 28.0 ± 10.1 | 32.7 ± 7.6 * | 39.3 ± 16.8 | 27.3 ± 10.5 | 24.0 ± 9.6 * | 48.8 ± 18.0 |
| Pristine | Thinned Mucosae | Perforations | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Mean | Min | No | Dimension | Sinus | |||
| 2 weeks | Bio-Oss alone | 127 ± 33 | 11 | 25 ± 4 | 3 | 0 | NA | 0 |
| Bio-Oss Regenfast | 138 ± 54 | 11 | 24 ± 12 | 3 | 0 | NA | 0 | |
| 10 weeks | Bio-Oss alone | 172 ± 43 | 30 | 20 ± 4 | 3 | 5 | 297 ± 390 | 4 |
| Bio-Oss Regenfast | 130 ± 30 | 10 | 19 ± 8 | 5 | 11 | 158 ± 101 | 4 | |
| Pristine | Epithelial Cells in the Thinned Mucosa | |||
|---|---|---|---|---|
| Mean | Min | |||
| 2 weeks | Bio-Oss alone | 28 ± 4 | 16 ± 5 | 3 |
| Bio-Oss Regenfast | 33 ± 6 | 14 ± 7 | 3 | |
| 10 weeks | Bio-Oss alone | 38 ± 5 | 17 ± 3 | 3 |
| Bio-Oss Regenfast | 34 ± 4 | 17 ± 7 | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniwa, N.; Xavier, S.P.; Scombatti de Souza, S.L.; Silva, E.R.; Botticelli, D.; Morinaga, K.; Baba, S. Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid–Polynucleotide Gel (Regenfast). J. Funct. Biomater. 2024, 15, 361. https://doi.org/10.3390/jfb15120361
Maniwa N, Xavier SP, Scombatti de Souza SL, Silva ER, Botticelli D, Morinaga K, Baba S. Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid–Polynucleotide Gel (Regenfast). Journal of Functional Biomaterials. 2024; 15(12):361. https://doi.org/10.3390/jfb15120361
Chicago/Turabian StyleManiwa, Nozomi, Samuel Porfirio Xavier, Sergio Luis Scombatti de Souza, Erick Ricardo Silva, Daniele Botticelli, Kenzo Morinaga, and Shunsuke Baba. 2024. "Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid–Polynucleotide Gel (Regenfast)" Journal of Functional Biomaterials 15, no. 12: 361. https://doi.org/10.3390/jfb15120361
APA StyleManiwa, N., Xavier, S. P., Scombatti de Souza, S. L., Silva, E. R., Botticelli, D., Morinaga, K., & Baba, S. (2024). Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid–Polynucleotide Gel (Regenfast). Journal of Functional Biomaterials, 15(12), 361. https://doi.org/10.3390/jfb15120361










