Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Sample Size Calculation
2.2. Estrous Cycle
2.3. Ovariectomy
2.4. Experimental Groups
2.5. Sonification and Functionalization with Genistein
2.6. Peri-Implant Bone Defects and Implant Placement
2.7. Euthanasia
2.8. Biomechanical Test (Removal Torque)
2.9. Micro-CT
2.10. Molecular Analysis (RT-PCR)
2.11. Statistical Analysis
3. Results
3.1. Removal Torque Values
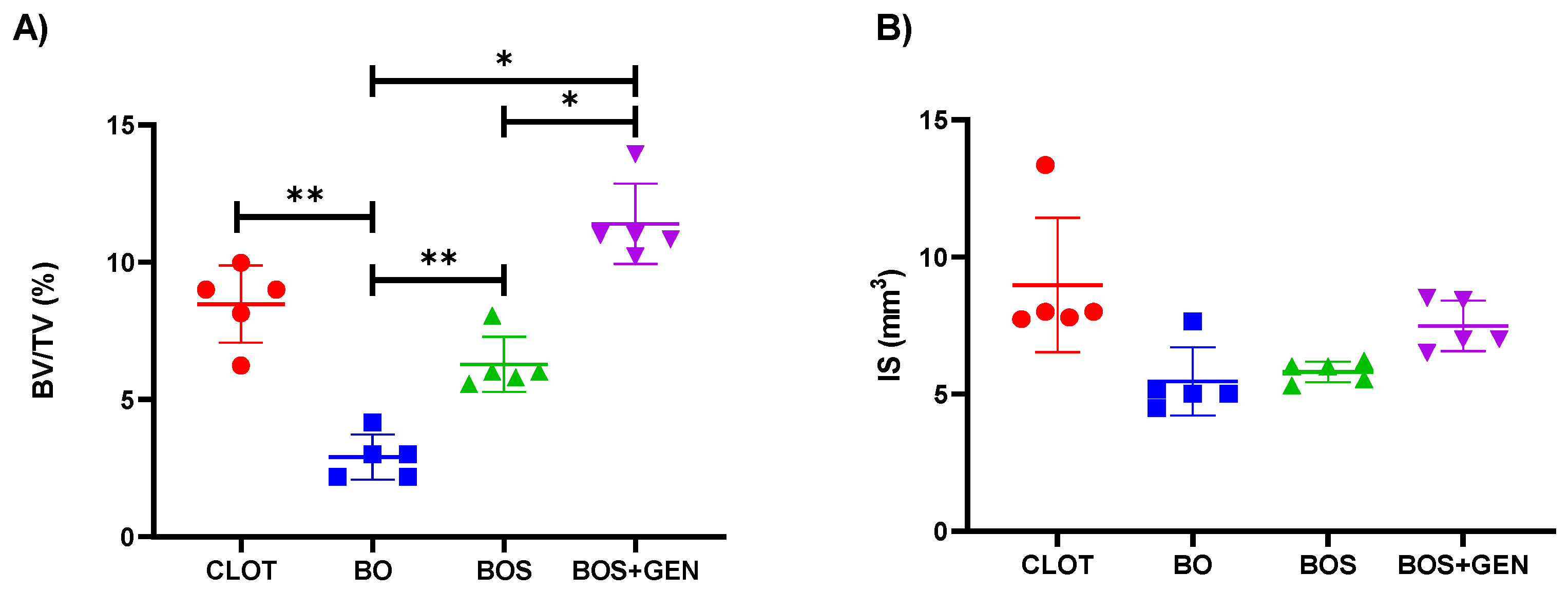
3.2. Micro-CT
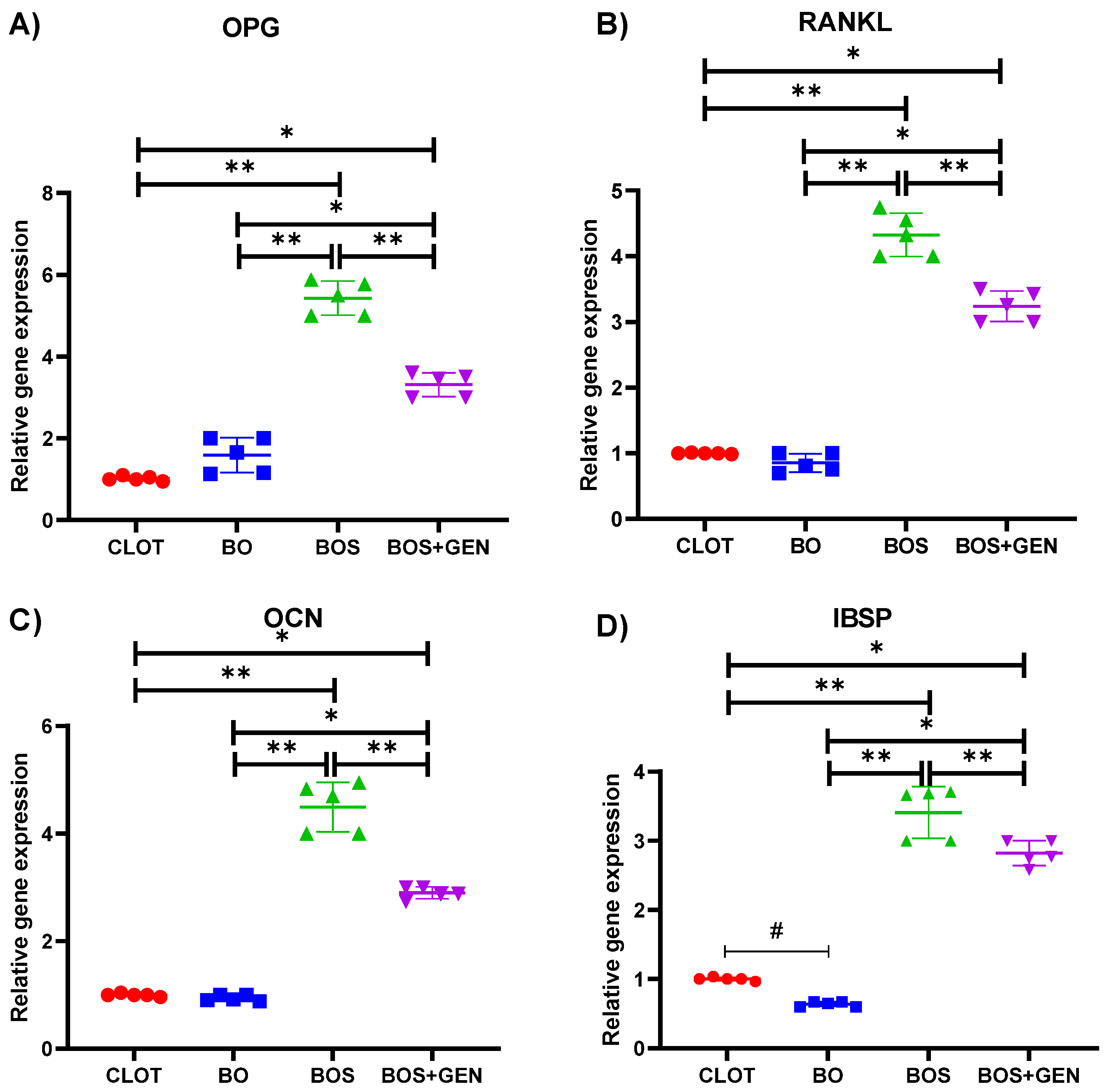
3.3. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huidrom, S.; Beg, M.A.; Masood, T. Post-menopausal Osteoporosis and Probiotics. Curr. Drug Targets 2021, 22, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: The role and mechanisms of estrogen. J. Endocrinol. 2023, 259, e230116. [Google Scholar] [CrossRef]
- Armas, L.A.G.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Zhang, D.; Ye, D.; Zhou, Y.; Qin, J.; Zhang, Y. The prevalence and treatment rate trends of osteoporosis in postmenopausal women. PLoS ONE 2023, 18, e0290289. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Peng, P.; Huang, X.T.; Qiu, Z.H.; Liu, B.C.; Yang, B.C.; Yang, T.L.; Yang, B.; Guo, Y. Isolation and culture of human primary osteoblasts: Comparing the effects of differences in method details on osteoblast characteristics. Genes Dis. 2023, 11, 546–549. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Oliveira, A.S.; Faé, D.S.; Oliveira, H.F.F.E.; Rosa, C.D.D.R.D.; Bento, V.A.A.; Verri, F.R.; Pellizzer, E.P. Do dental implants placed in patients with osteoporosis have higher risks of failure and marginal bone loss compared to those in healthy patients? A systematic review with meta-analysis. Clin. Oral Investig. 2023, 27, 2483–2493. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Micheletti, C.; Frigério, P.B.; Batista, F.R.S.; Monteiro, N.G.; Júnior-Bim, O.; Lisboa-Filho, P.N.; Grandfield, K.; Okamoto, R. PTH 1-34-functionalized bioactive glass improves peri-implant bone repair in orchiectomized rats: Microscale and ultrastructural evaluation. Biomater. Adv. 2022, 134, 112688. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Beil, F.T.; Barvencik, F.; Gebauer, M.; Seitz, S.; Rueger, J.M.; Ignatius, A.; Pogoda, P.; Schinke, T.; Amling, M. Effects of estrogen on fracture healing in mice. J. Trauma. 2010, 69, 1259–1265. [Google Scholar] [CrossRef]
- Cheung, W.H.; Miclau, T.; Chow, S.K.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, Y.; Hashimi, S.; Hamlet, S.M.; Ivanovski, S. The effects of implant topography on osseointegration under estrogen deficiency induced osteoporotic conditions: Histomorphometric, transcriptional and ultrastructural analysis. Acta Biomater. 2016, 42, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Glösel, B.; Kuchler, U.; Watzek, G.; Gruber, R. Review of dental implant rat research models simulating osteoporosis or diabetes. Int. J. Oral Maxillofac. Implant. 2010, 25, 516–524. [Google Scholar]
- Mardas, N.; Busetti, J.; de Figueiredo, J.A.; Mezzomo, L.A.; Scarparo, R.K.; Donos, N. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin. Oral Implant. Res. 2017, 28, 362–371. [Google Scholar] [CrossRef]
- Mulinari-Santos, G.; Dos Santos, J.S.; Kitagawa, I.L.; de Souza Batista, F.R.; Botacin, P.R.; Antoniali, C.; Lisboa-Filho, P.N.; Okamoto, R. Estrogen Deficiency Impairs Osseointegration in Hypertensive Rats Even Treated with Alendronate Coated on the Implant Surface. J. Funct. Biomater. 2023, 14, 471. [Google Scholar] [CrossRef]
- Zhuang, G.; Mao, J.; Yang, G.; Wang, H. Influence of different incision designs on bone increment of guided bone regeneration (Bio-Gide® collagen membrane + Bio-Oss® bone powder) during the same period of maxillary anterior tooth implantation. Bioengineered 2021, 12, 2155–2163. [Google Scholar] [CrossRef]
- Van Houdt, C.I.A.; Ulrich, D.J.O.; Jansen, J.A.; Van Den Beucken, J.J.J.P. The performance of CPC/PLGA and Bio-Oss® for bone regeneration in healthy and osteoporotic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 131–142. [Google Scholar] [CrossRef]
- Keil, C.; Gollmer, B.; Zeidler-Rentzsch, I.; Gredes, T.; Heinemann, F. Histological evaluation of extraction sites grafted with Bio-Oss® Collagen: Randomized controlled trial. Ann. Anat. 2021, 237, 151722. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Schuder, K.; Hof, M.; Heuberer, S.; Seemann, R.; Dvorak, G. Does osteoporosis influence the marginal peri-implant bone level in female patients? A cross-sectional study in a matched collective. Clin. Implant. Dent. Relat. Res. 2017, 19, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual role in women’s health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Holubiec, M.; Zalewski, P. Genistein–Opportunities related to an exciting molecule of natural origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Borrás, C.; Viña, J. The multimodal action of genistein in Alzheimer’s and other age-related diseases. Free Radic. Biol. Med. 2022, 183, 127–137. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Wu, G.J.; Cherng, Y.G.; Chen, J.T.; Chang, C.C.; Liu, S.H.; Chen, R.M. Genistein triggers translocation of estrogen receptor-alpha in mitochondria to induce expressions of ATP synthesis-associated genes and improves energy production and osteoblast maturation. Am. J. Chin. Med. 2021, 42, 901–923. [Google Scholar] [CrossRef]
- Kauffmann, P.; Rau, A.; Seidlová-Wuttke, D.; Jarry, H.; Schminke, B.; Matthes, S.; Wiese, K.G. Effect of dihydrotestosterone, 17-β-estrogen, genistein and equol on remodeling and morphology of bone in osteoporotic male rats during bone healing. Bone Rep. 2020, 13, 100300. [Google Scholar] [CrossRef]
- Kolios, L.; Sehmisch, S.; Daub, F.; Rack, T.; Tezval, M.; Stuermer, K.M.; Stuermer, E.K. Equol but not genistein improves early metaphyseal fracture healing in osteoporotic rats. Planta Med. 2009, 75, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Inpan, R.; Takuathung, M.N.; Sakuludomkan, W.; Dukaew, N.; Teekachunhatean, S.; Koonrungsesomboon, N. Isoflavone intervention and its impact on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2024, 35, 413–430. [Google Scholar] [CrossRef]
- Zou, Z.; Lin, K.; Chen, L.; Chang, J. Ultrafast synthesis and characterization of carbonated hydroxyapatite nanopowders via sonochemistry-assisted microwave process. Ultrason. Sonochem 2012, 19, 1174–1179. [Google Scholar] [CrossRef]
- Arruda, L.B.; Orlandi, M.O.; Lisboa-Filho, P.N. Morphological modifications and surface amorphization in ZnO sonochemically treated nanoparticles. Ultrason. Sonochem 2013, 20, 799–804. [Google Scholar] [CrossRef]
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. B 2013, 10, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Ferreira, P.H.S.; Lisboa-Filho, P.N.; Silva, A.C.; Bim-Júnior, O.; Batista, F.R.S.; Ervolino-Silva, A.C.; Garcia-Junior, I.R.; Okamoto, R. Sonochemical time standardization for bioactive materials used in peri-implant defects filling. Ultrason. Sonochem. 2019, 56, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Long, J.A.; Evans, H.M. The Oestrus Cycle in the Rat and Its Related Phenomena, 1st ed.; University of California Press: Berkeley, CA, USA, 1922. [Google Scholar]
- Maeng, L.Y.; Cover, K.K.; Landau, A.J.; Milad, M.R.; Lebron-Milad, K. Protocol for studying extinction of conditioned fear in naturally cycling female rats. J. Vis. Exp. 2015, 96, 52202. [Google Scholar]
- Mulinari-Santos, G.; de Souza Batista, F.R.; Kirchweger, F.; Tangl, S.; Gruber, R.; Okamoto, R. Losartan reverses impaired osseointegration in spontaneously hypertensive rats. Clin. Oral Implant. Res. 2018, 29, 1126–1134. [Google Scholar] [CrossRef]
- Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Batista, F.R.S.; Momesso, G.A.C.; Faverani, L.P.; Okamoto, R. Bone repair with raloxifene and bioglass nanoceramic composite in animal experiment. Connect. Tissue Res. 2018, 58, 97–100. [Google Scholar] [CrossRef]
- Inoue, B.K.N.; Paludetto, L.V.; Monteiro, N.G.; Batista, F.R.S.; Kitagawa, I.L.; da Silva, R.S.; Antoniali, C.; Lisboa Filho, P.N.; Okamoto, R. Synergic Action of Systemic Risedronate and Local Rutherpy in Peri-implantar Repair of Ovariectomized Rats: Biomechanical and Molecular Analysis. Int. J. Mol. Sci. 2023, 24, 16153. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 27, 1468–1486. [Google Scholar] [CrossRef]
- Dhayanithi, J.; Rajasekar, A. Comparison of Alveolar Bone Level around Osseointegrated Dental Implants among Premenopausal and Postmenopausal Women: A 2-Year Study. J. Long. Term. Eff. Med. Implant. 2024, 34, 89–92. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of osteocalcin in bone, pancreas, testis, and muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.B.; Tan, M.H.; Kolli, T.N.; Zachariadou, C.; Farah, F.; Mohamed, F.F.; Chu, E.Y.; Foster, B.L. Bone sialoprotein is critical for alveolar bone healing in mice. J. Dent. Res. 2023, 102, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, C.; DiCecco, L.A.; Deering, J.; Chen, W.; Ervolino da Silva, A.C.; Shah, F.A.; Palmquist, A.; Okamoto, R.; Grandfield, K. Mesoscale characterization of osseointegration around an additively manufactured genistein-coated implant. Sci. Rep. 2024, 14, 15339. [Google Scholar] [CrossRef]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteo-blastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Low, S.S.; Yew, M.; Lim, C.N.; Chai, W.S.; Low, L.E.; Manickam, S.; Tey, B.T.; Show, P.L. Sonoproduction of nanobiomaterials—A critical review. Ultrason. Sonochem. 2022, 82, 105887. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kekatpure, A.L. Postmenopausal Osteoporosis: A Literature Review. Cureus 2022, 14, e29367. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xing, X.H.; Wang, H.; Weng, X.L.; Yu, S.B.; Dong, G.Y. Dose-dependent effects of genistein on bone homeostasis in rats’ mandibular subchondral bone. Acta Pharmacol. Sin. 2012, 33, 66–74. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Assis, S.; Warri, A. Exposures to synthetic estrogens at different times during life, and their effect on breast cancer risk. J. Mammary Gland. Biol. Neoplasia 2013, 18, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 1–14. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, F.; Hu, X.; Yang, K.; Xie, D.; Yang, L.; Wu, Z.; Wei, J. Construction of a hierarchical micro & nanoporous surface for loading genistein on the composite of polyetheretherketone/tantalum pentoxide possessing antibacterial activity and accelerated osteointegration. Biomater. Sci. 2021, 9, 167–185. [Google Scholar]
- Sarasquete, C.; Úbeda-Manzanaro, M.; Ortiz-Delgado, J.B. Toxicity and non-harmful effects of the soya isoflavones, genistein and daidzein, in embryos of the zebrafish, Danio rerio. Comp. Biochem. Physiology. Toxicol. Pharmacol. CBP 2018, 211, 57–67. [Google Scholar] [CrossRef]


| Gene | Gene Reference | Forward Primer, 5′ → 3′ | Reverse Primer, 5′ → 3′ |
|---|---|---|---|
| OPG | NM_057149.2 | GCACTCCTGGTGTTCTTGGA | TTTGGTCCCAGGCAAACTGT |
| RANKL | NM_057149.1 | CGAGCGCAGATGGATCCTAA | GAGCCACGAACCTTCCATCA |
| IBSP | NM_012587.2 | GTACCGGCCACGCTACTTTC | ATCTCCAGCCTTCTTGGGTAGC |
| OCN | NM_013414.1 | CTCTGAGTCTGACAAAGCCTTCAT | GTAGCGCCGGAGTCTATTCA |
| ß-actin | NM_031144.3 | CCACCATGTACCCAGGCATT | CCTAGAAGCATTTGCGGTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, N.D.; Mulinari-Santos, G.; Batista, F.R.d.S.; Gomes, M.B.; Monteiro, N.G.; Silva, A.C.E.d.; Gruber, R.; Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Okamoto, R. Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats. J. Funct. Biomater. 2024, 15, 328. https://doi.org/10.3390/jfb15110328
Duarte ND, Mulinari-Santos G, Batista FRdS, Gomes MB, Monteiro NG, Silva ACEd, Gruber R, Lisboa-Filho PN, Gomes-Ferreira PHS, Okamoto R. Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats. Journal of Functional Biomaterials. 2024; 15(11):328. https://doi.org/10.3390/jfb15110328
Chicago/Turabian StyleDuarte, Nathália Dantas, Gabriel Mulinari-Santos, Fábio Roberto de Souza Batista, Marcelly Braga Gomes, Naara Gabriela Monteiro, Ana Cláudia Ervolino da Silva, Reinhard Gruber, Paulo Noronha Lisboa-Filho, Pedro Henrique Silva Gomes-Ferreira, and Roberta Okamoto. 2024. "Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats" Journal of Functional Biomaterials 15, no. 11: 328. https://doi.org/10.3390/jfb15110328
APA StyleDuarte, N. D., Mulinari-Santos, G., Batista, F. R. d. S., Gomes, M. B., Monteiro, N. G., Silva, A. C. E. d., Gruber, R., Lisboa-Filho, P. N., Gomes-Ferreira, P. H. S., & Okamoto, R. (2024). Sonification of Deproteinized Bovine Bone Functionalized with Genistein Enhances Bone Repair in Peri-Implant Bone Defects in Ovariectomized Rats. Journal of Functional Biomaterials, 15(11), 328. https://doi.org/10.3390/jfb15110328










