Effect of the Abutment Rigidity on the Wear Resistance of a Lithium Disilicate Glass Ceramic: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Estimation
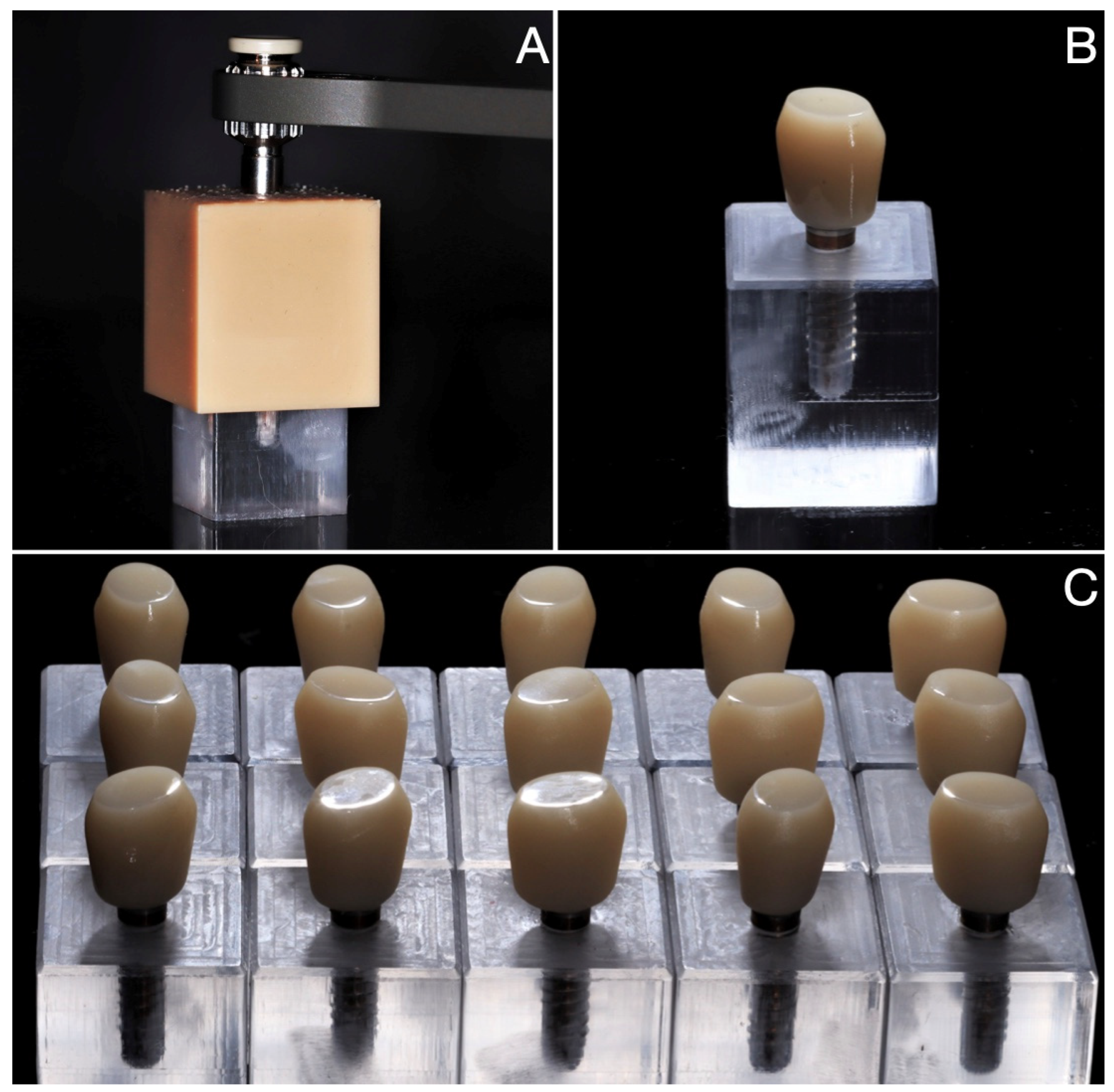
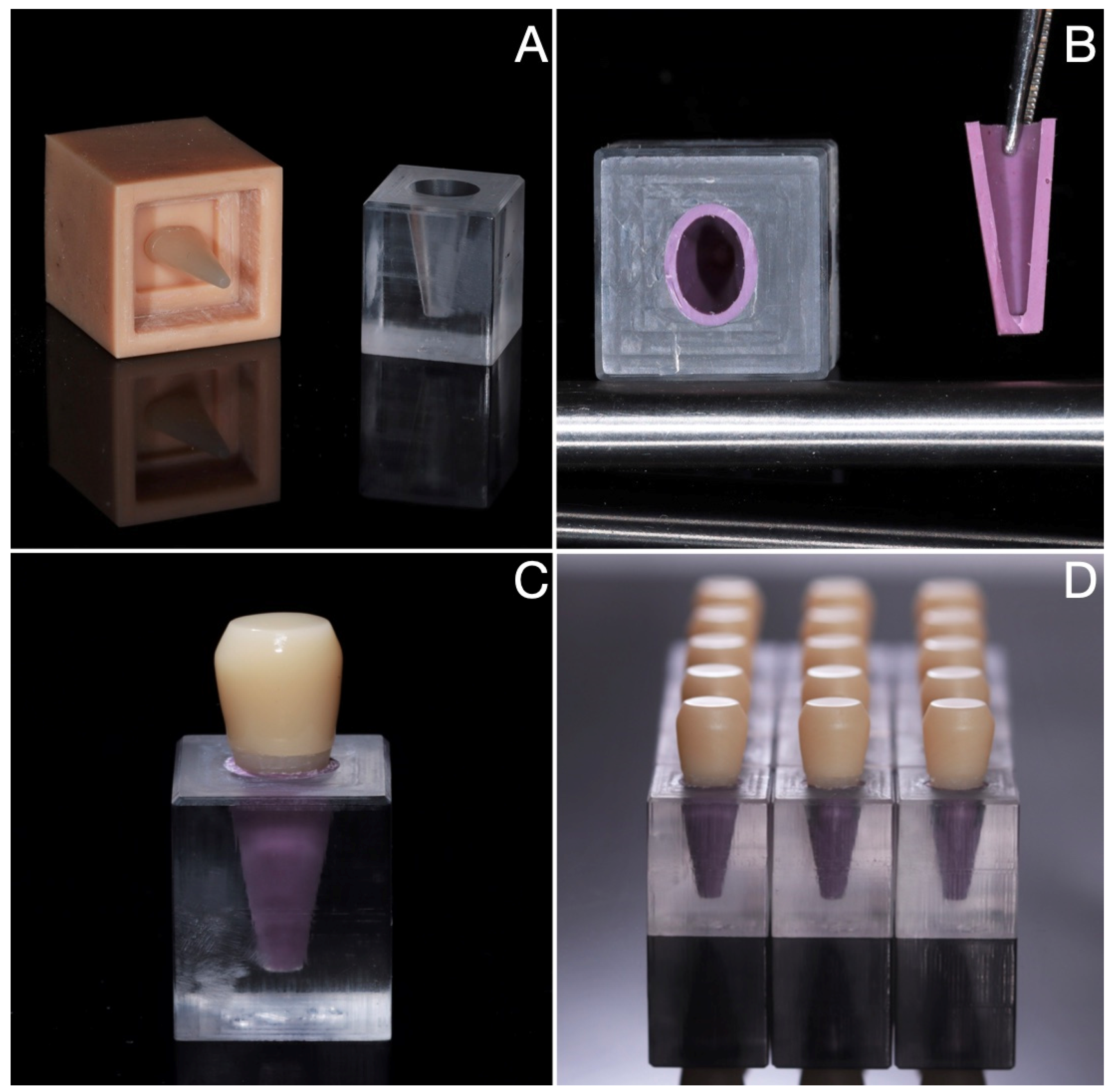
2.2. Sample Fabrication
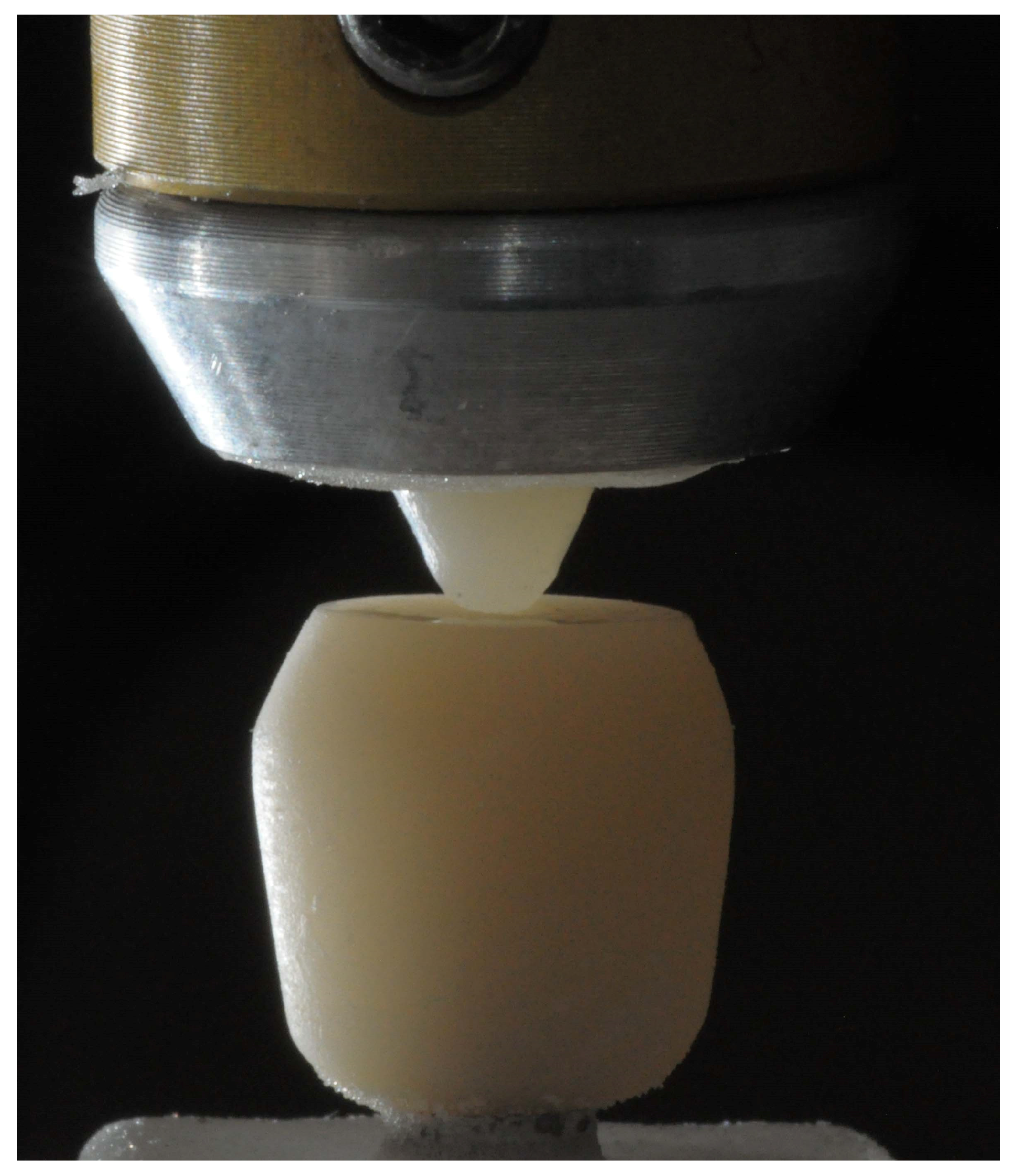
2.3. Wear Testing
2.4. Data Analysis
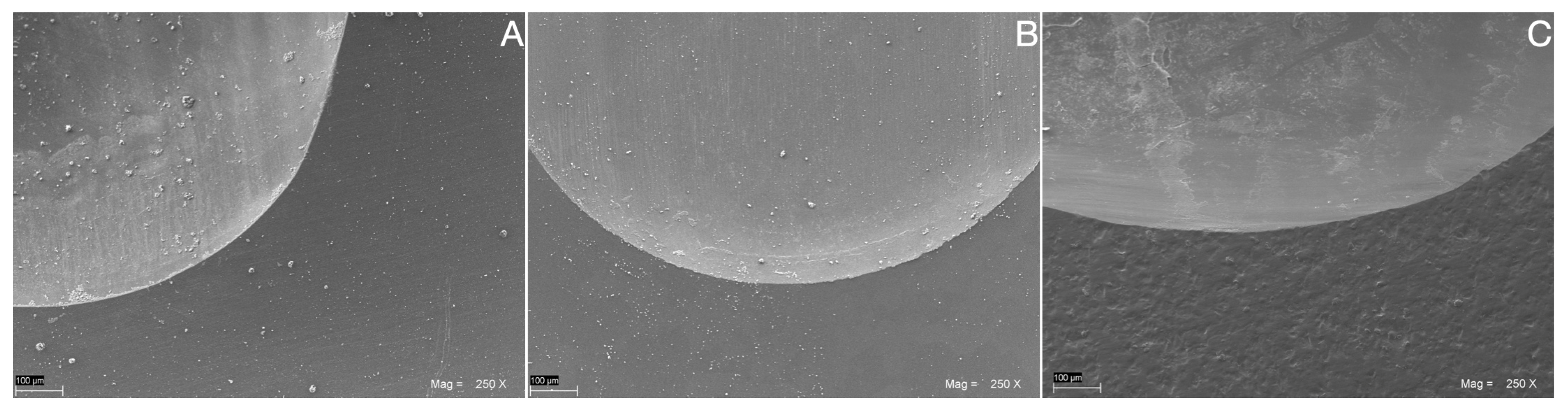
2.5. SEM Wear Facet Analysis
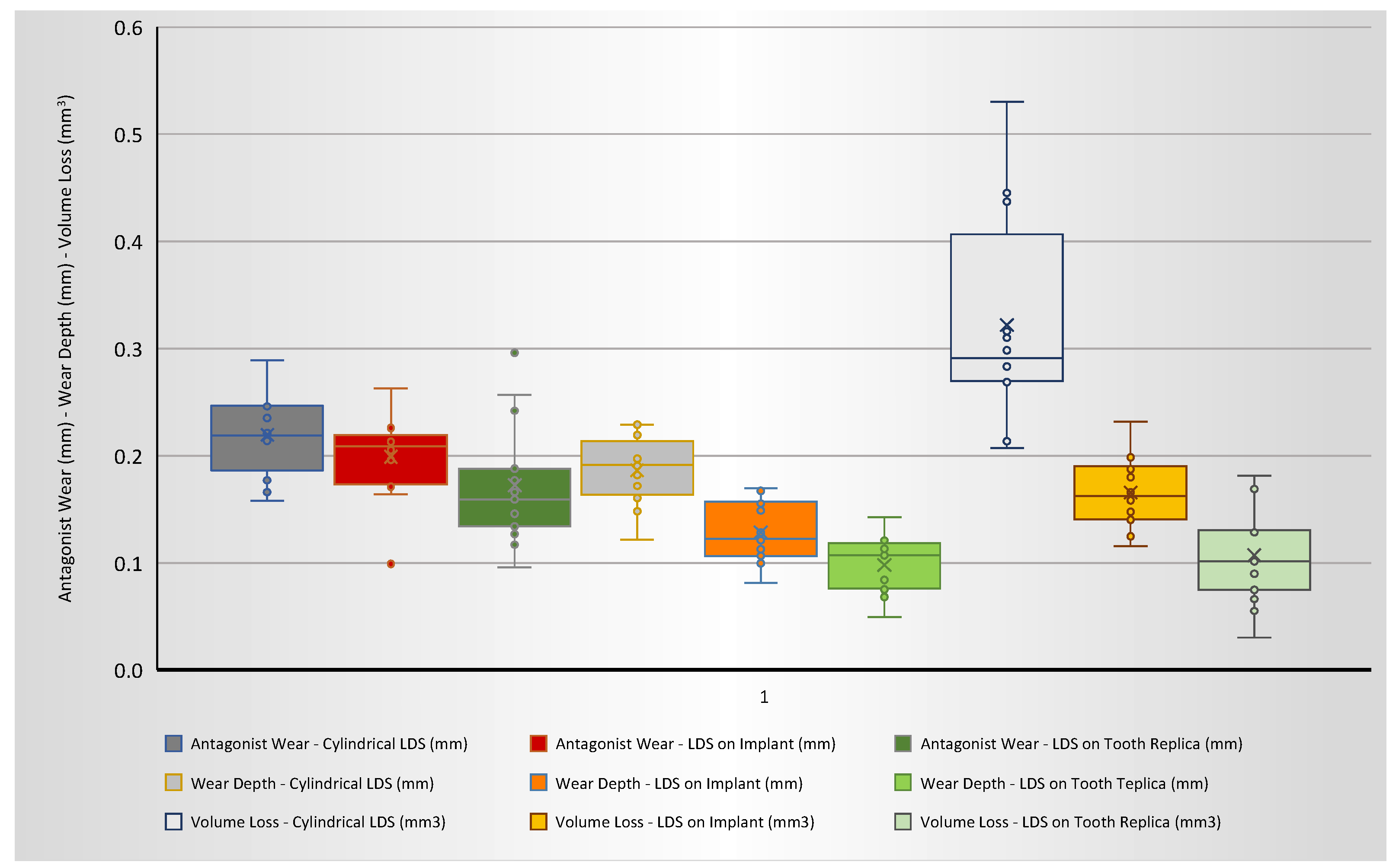
3. Results
4. Discussion
5. Conclusions
- The rigidity of the abutment is able to significantly affect the wear pattern of lithium disilicate glass ceramics, which seems to be more intense in the presence of a rigid implant-abutment support than on restorations supported by more flexible teeth replicas and in the presence of periodontal ligament.
- In vitro chewing simulation models that include a wider number of intra-oral parameters (alveolar bone, periodontal ligament, abutment rigidity/elasticity, etc.) might be closer to the actual clinical situation and seem to produce significantly different results from extremely simplified in vitro models.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Branco, A.C.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. A State-of-the-Art Review on the Wear of the Occlusal Surfaces of Natural Teeth and Prosthetic Crowns. Materials 2020, 13, 3525. [Google Scholar] [CrossRef]
- Stück, A.V.; Raith, S.; Reich, S. Twenty-four months in vivo wear of enamel antagonists to lithium disilicate implant crowns—A pilot study. J. Dent. 2022, 124, 104215. [Google Scholar] [CrossRef]
- Lambrechts, P.; Braem, M.; Vuylsteke-Wauters, M.; Vanherle, G. Quantitative in vivo wear of human enamel. J. Dent. Res. 1989, 68, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Saads Carvalho, T.; Lussi, A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Monogr. Oral. Sci. 2020, 28, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Nota, A.; Pittari, L.; Paggi, M.; Abati, S.; Tecco, S. Correlation between Bruxism and Gastroesophageal Reflux Disorder and Their Effects on Tooth Wear. A Systematic Review. J. Clin. Med. 2022, 11, 1107. [Google Scholar] [CrossRef]
- Kanzow, P.; Wegehaupt, F.J.; Attin, T.; Wiegand, A. Etiology and pathogenesis of dental erosion. Quintessence Int. 2016, 47, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Reichl, F.-X.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods —A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef]
- Chatzidimitriou, K.; Papaioannou, W.; Seremidi, K.; Bougioukas, K.; Haidich, A.B. Prevalence and association of gastroesophageal reflux disease and dental erosion: An overview of reviews. J. Dent. 2023, 133, 104520. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Mancini, A.; Inchingolo, F.; Inchingolo, A.D.; Di Venere, D.; Dipalma, G.; et al. Damage from Carbonated Soft Drinks on Enamel: A Systematic Review. Nutrients 2023, 15, 1785. [Google Scholar] [CrossRef]
- Oudkerk, J.; Grenade, C.; Davarpanah, A.; Vanheusden, A.; Vandenput, S.; Mainjot, A.K. Risk Factors of Tooth Wear in Permanent Dentition: A Scoping Review. J. Oral. Rehabil. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Gimenez-Gonzalez, B.; Setyo, C.; Picaza, M.G.; Tribst, J.P.M. Effect of defect size and tooth anatomy in the measurements of a 3D patient monitoring tool. Heliyon 2022, 8, e12103. [Google Scholar] [CrossRef]
- DeLong, R. Intra-oral restorative materials wear: Rethinking the current approaches: How to measure wear. Dent. Mater. 2006, 22, 702–711. [Google Scholar] [CrossRef]
- Luciano, M.; Francesca, Z.; Michela, S.; Tommaso, M.; Massimo, A. Lithium disilicate posterior overlays: Clinical and biomechanical features. Clin. Oral. Investig. 2020, 24, 841–848. [Google Scholar] [CrossRef]
- Loomans, B.; Opdam, N. A guide to managing tooth wear: The Radboud philosophy. Br. Dent. J. 2018, 224, 348–356. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, M.; De Angelis, F.; Mancino, M.; Frascaria, M.; Capogreco, M.; D’Arcangelo, C. Canal shaping of different single-file systems in curved root canals. J. Dent. Sci. 2017, 12, 328–332. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Minnoni, A.; Vitalone, L.M.; Carluccio, F.; Vadini, M.; Paolantonio, M.; D’Arcangelo, C. Bond strength evaluation of three self-adhesive luting systems used for cementing composite and porcelain. Oper. Dent. 2011, 36, 626–634. [Google Scholar] [CrossRef]
- De Angelis, F.; Sarteur, N.; Buonvivere, M.; Vadini, M.; Šteffl, M.; D’Arcangelo, C. Meta-analytical analysis on components released from resin-based dental materials. Clin. Oral. Investig. 2022, 26, 6015–6041. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; D’Arcangelo, C.; Buonvivere, M.; Rondoni, G.D.; Vadini, M. Shear bond strength of glass ionomer and resin-based cements to different types of zirconia. J. Esthet. Restor. Dent. 2020, 32, 806–814. [Google Scholar] [CrossRef]
- De Angelis, F.; D’Arcangelo, C.; Malíšková, N.; Vanini, L.; Vadini, M. Wear Properties of Different Additive Restorative Materials Used for Onlay/Overlay Posterior Restorations. Oper. Dent. 2020, 45, E156–E166. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; Vadini, M.; Buonvivere, M.; De Angelis, F. Safe clinical technique for increasing the occlusal vertical dimension in case of erosive wear and missing teeth. Clin. Case Rep. 2021, 9, e04747. [Google Scholar] [CrossRef]
- De Angelis, F.; Vadini, M.; D’Amario, M.; Buonvivere, M.; D’Arcangelo, C. Effetto della mordenzatura con acido fluoridrico su flessione e adesione del disilicato di litio. Dent. Cadmos 2019, 87, 430. [Google Scholar] [CrossRef]
- Chang, H.-H.; Yeh, C.-L.; Wang, Y.-L.; Huang, Y.-C.; Tsai, S.-J.; Li, Y.-T.; Yang, J.-H.; Lin, C.-P. Differences in the biomechanical behaviors of natural teeth and dental implants. Dent. Mater. 2021, 37, 682–689. [Google Scholar] [CrossRef]
- Schwarz, M.S. Mechanical complications of dental implants. Clin. Oral. Implant. Res. 2000, 11 (Suppl. 1), 156–158. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.J.; Lee, J.Y. Comparative study of mechanical properties of dental restorative materials and dental hard tissues in compressive loads. J. Dent. Biomech. 2014, 5, 1758736014555246. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; De Angelis, F. Wear properties of dental ceramics and porcelains compared with human enamel. J. Prosthet. Dent. 2016, 115, 350–355. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Buonvivere, M.; Sorrentino, E.; Rondoni, G.D.; D’Arcangelo, C. Wear Properties of Conventional and High-Translucent Zirconia-Based Materials. Materials 2022, 15, 7324. [Google Scholar] [CrossRef]
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral. Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral. Implant. Res. 2018, 29, 351–358. [Google Scholar] [CrossRef]
- Verma, A.; Singh, S.V.; Arya, D.; Shivakumar, S.; Chand, P. Mechanical failures of dental implants and supported prostheses: A systematic review. J. Oral. Biol. Craniofacial Res. 2023, 13, 306–314. [Google Scholar] [CrossRef]
- Lee, S.J.; Alamri, O.; Cao, H.; Wang, Y.; Gallucci, G.O.; Lee, J.D. Occlusion as a Predisposing Factor for Peri-Implant Disease: A Review Article. Clin. Implant. Dent. Relat. Res. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Di Fiore, A.; Montagner, M.; Sivolella, S.; Stellini, E.; Yilmaz, B.; Brunello, G. Peri-Implant Bone Loss and Overload: A Systematic Review Focusing on Occlusal Analysis through Digital and Analogic Methods. J. Clin. Med. 2022, 11, 4812. [Google Scholar] [CrossRef]
- Preis, V.; Hahnel, S.; Behr, M.; Rosentritt, M. In vitro performance and fracture resistance of novel CAD/CAM ceramic molar crowns loaded on implants and human teeth. J. Adv. Prosthodont. 2018, 10, 300–307. [Google Scholar] [CrossRef]
- Higaki, N.; Goto, T.; Ishida, Y.; Watanabe, M.; Tomotake, Y.; Ichikawa, T. Do sensation differences exist between dental implants and natural teeth?: A meta-analysis. Clin. Oral. Implant. Res. 2014, 25, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral. Implant. Res. 2007, 18 (Suppl. 3), 97–113. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Conserva, E.; Tealdo, T.; Bevilacqua, M.; Pera, F.; Signori, A.; Pera, P. Shock absorption capacity of restorative materials for dental implant prostheses: An in vitro study. Int. J. Prosthodont. 2013, 26, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strasding, M.; Hicklin, S.P.; Todorovic, A.; Fehmer, V.; Mojon, P.; Sailer, I. A multicenter randomized controlled clinical pilot study of buccally micro-veneered lithium-disilicate and zirconia crowns supported by titanium base abutments: 1-year outcomes. Clin. Oral. Implant. Res. 2023, 34, 56–65. [Google Scholar] [CrossRef]
- Wolfart, S.; Rittich, A.; Groß, K.; Hartkamp, O.; von der Stück, A.; Raith, S.; Reich, S. Cemented versus screw-retained posterior implant-supported single crowns: A 24-month randomized controlled clinical trial. Clin. Oral. Implant. Res. 2021, 32, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Schumann, F.; Krifka, S.; Preis, V. Influence of zirconia and lithium disilicate tooth- or implant-supported crowns on wear of antagonistic and adjacent teeth. J. Adv. Prosthodont. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R. Implant-supported and tooth-supported single crowns fabricated with lithium-disilicate seem to have a high 10-year survival rate. J. Am. Dent. Assoc. 2017, 148, e48. [Google Scholar] [CrossRef]
- Teichmann, M.; Göckler, F.; Weber, V.; Yildirim, M.; Wolfart, S.; Edelhoff, D. Ten-year survival and complication rates of lithium-disilicate (Empress 2) tooth-supported crowns, implant-supported crowns, and fixed dental prostheses. J. Dent. 2017, 56, 65–77. [Google Scholar] [CrossRef]
- Spies, B.C.; Patzelt, S.B.; Vach, K.; Kohal, R.J. Monolithic lithium-disilicate single crowns supported by zirconia oral implants: Three-year results of a prospective cohort study. Clin. Oral. Implant. Res. 2016, 27, 1160–1168. [Google Scholar] [CrossRef]
- Bronkhorst, H.; Bronkhorst, E.; Kalaykova, S.; van der Meer, W.; Huysmans, M.C.; Loomans, B. Precision of In Vivo Quantitative Tooth Wear Measurement using Intra-Oral Scans. J. Vis. Exp. 2022, 185, e63680. [Google Scholar] [CrossRef]
- Charalambous, P.; O’Toole, S.; Austin, R.; Bartlett, D. The threshold of an intra oral scanner to measure lesion depth on natural unpolished teeth. Dent. Mater. 2022, 38, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Koletsi, D.; Iliadi, A.; Eliades, T.; Eliades, G. In Vitro Simulation and In Vivo Assessment of Tooth Wear: A Meta-Analysis of In Vitro and Clinical Research. Materials 2019, 12, 3575. [Google Scholar] [CrossRef] [PubMed]
- Wulfman, C.; Koenig, V.; Mainjot, A.K. Wear measurement of dental tissues and materials in clinical studies: A systematic review. Dent. Mater. 2018, 34, 825–850. [Google Scholar] [CrossRef] [PubMed]
- Elmaria, A.; Goldstein, G.; Vijayaraghavan, T.; Legeros, R.Z.; Hittelman, E.L. An evaluation of wear when enamel is opposed by various ceramic materials and gold. J. Prosthet. Dent. 2006, 96, 345–353. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; Vadini, M.; De Angelis, F. Wear Evaluation of Prosthetic Materials Opposing Themselves. Oper. Dent. 2018, 43, 38–50. [Google Scholar] [CrossRef]
- Ang, Y.; Razali, M.; Yahaya, N. Tooth Mobility Reproduction in Dental Material Research: A Scoping Review. Open Dent. J. 2020, 14, 465–473. [Google Scholar] [CrossRef]
- Re, D.; De Angelis, F.; Augusti, G.; Augusti, D.; Caputi, S.; D’Amario, M.; D’Arcangelo, C. Mechanical Properties of Elastomeric Impression Materials: An In Vitro Comparison. Int. J. Dent. 2015, 2015, 428286. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; Scharnagl, P.; Handel, G.; Kolbeck, C. Influence of Resilient Support of Abutment Teeth on Fracture Resistance of All-Ceramic Fixed Partial Dentures: An In Vitro Study. Int. J. Prosthodont. 2011, 24, 465–468. [Google Scholar]
- Al zahrani, F.; Richards, L. Micro-CT evaluation of a novel periodontal ligament simulation technique for dental experimental models. Arch. Orofac. Sci. 2018, 13, 93–103. [Google Scholar]
- Heintze, S.D.; Monreal, D.; Reinhardt, M.; Eser, A.; Peschke, A.; Reinshagen, J.; Rousson, V. Fatigue resistance of all-ceramic fixed partial dentures-Fatigue tests and finite element analysis. Dent. Mater. 2018, 34, 494–507. [Google Scholar] [CrossRef]
- Winkler, S.; Morris, H.F.; Spray, J.R. Stability of implants and natural teeth as determined by the Periotest over 60 months of function. J. Oral. Implant. 2001, 27, 198–203. [Google Scholar] [CrossRef]
- Kulkarni, V.; Duruel, O.; Ataman-Duruel, E.T.; Tözüm, M.D.; Nares, S.; Tözüm, T.F. In-depth morphological evaluation of tooth anatomic lengths with root canal configurations using cone beam computed tomography in North American population. J. Appl. Oral. Sci. 2020, 28, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef] [PubMed]
- Andrejovská, J.; Petruš, O.; Medveď, D.; Vojtko, M.; Riznič, M.; Kizek, P.; Dusza, J. Hardness and indentation modulus of human enamel and dentin. Surf. Interface Anal. 2023, 55, 270–278. [Google Scholar] [CrossRef]
- Heintze, S.D. How to qualify and validate wear simulation devices and methods. Dent. Mater. 2006, 22, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Saiki, O.; Koizumi, H.; Akazawa, N.; Kodaira, A.; Okamura, K.; Matsumura, H. Wear characteristics of polished and glazed lithium disilicate ceramics opposed to three ceramic materials. J. Oral. Sci. 2016, 58, 117–123. [Google Scholar] [CrossRef]
- Wiedenmann, F.; Böhm, D.; Eichberger, M.; Edelhoff, D.; Stawarczyk, B. Influence of different surface treatments on two-body wear and fracture load of monolithic CAD/CAM ceramics. Clin. Oral. Investig. 2020, 24, 3049–3060. [Google Scholar] [CrossRef]
- Rosentritt, M.; Schneider-Feyrer, S.; Behr, M.; Preis, V. In Vitro Shock Absorption Tests on Implant-Supported Crowns: Influence of Crown Materials and Luting Agents. Int. J. Oral. Maxillofac. Implant. 2018, 33, 116–122. [Google Scholar] [CrossRef]
- Ramos, N.d.C.; Augusto, M.G.; Alves, L.M.M.; Kleverlaan, C.J.; Piva, A.M.d.O.D. Wear of dental ceramics. Braz. Dent. Sci. 2023, 26, e3638. [Google Scholar] [CrossRef]
- Lambrechts, P.; Debels, E.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. How to simulate wear? Overview of existing methods. Dent. Mater. 2006, 22, 693–701. [Google Scholar] [CrossRef]
- Stober, T.; Lutz, T.; Gilde, H.; Rammelsberg, P. Wear of resin denture teeth by two-body contact. Dent. Mater. 2006, 22, 243–249. [Google Scholar] [CrossRef]
- Oh, W.S.; Delong, R.; Anusavice, K.J. Factors affecting enamel and ceramic wear: A literature review. J. Prosthet. Dent. 2002, 87, 451–459. [Google Scholar] [CrossRef]
- Preis, V.; Behr, M.; Handel, G.; Schneider-Feyrer, S.; Hahnel, S.; Rosentritt, M. Wear performance of dental ceramics after grinding and polishing treatments. J. Mech. Behav. Biomed. Mater. 2012, 10, 13–22. [Google Scholar] [CrossRef]
- Luangruangrong, P.; Cook, N.B.; Sabrah, A.H.; Hara, A.T.; Bottino, M.C. Influence of full-contour zirconia surface roughness on wear of glass-ceramics. J. Prosthodont. 2014, 23, 198–205. [Google Scholar] [CrossRef]
- Wendler, M.; Kaizer, M.R.; Belli, R.; Lohbauer, U.; Zhang, Y. Sliding contact wear and subsurface damage of CAD/CAM materials against zirconia. Dent. Mater. 2020, 36, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Janyavula, S.; Syklawer, S.; McLaren, E.A.; Burgess, J.O. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J. Dent. 2014, 42, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Swain, M.; He, L.; Lyons, K. Wear behavior of human enamel against lithium disilicate glass ceramic and type III gold. J. Prosthet. Dent. 2014, 112, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Behr, M.; Kolbeck, C.; Hahnel, S.; Handel, G.; Rosentritt, M. Wear performance of substructure ceramics and veneering porcelains. Dent. Mater. 2011, 27, 796–804. [Google Scholar] [CrossRef]
- Brosh, T.; Porat, N.; Vardimon, A.D.; Pilo, R. Appropriateness of viscoelastic soft materials as in vitro simulators of the periodontal ligament. J. Oral. Rehabil. 2011, 38, 929–939. [Google Scholar] [CrossRef]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Odin, G.; Savoldelli, C.; Bouchard, P.O.; Tillier, Y. Determination of Young’s modulus of mandibular bone using inverse analysis. Med. Eng. Phys. 2010, 32, 630–637. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Vadini, M.; Buonvivere, M.; Valerio, A.; Di Cosola, M.; Piattelli, A.; Biferi, V.; D’Arcangelo, C. In Vitro Mechanical Properties of a Novel Graphene-Reinforced PMMA-Based Dental Restorative Material. Polymers 2023, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.H.; Marshall, S.J.; Marshall, G.W. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit. Rev. Oral. Biol. Med. 2003, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]


| LDS-on-Tooth-Replica | LDS-on-Implant | |||
|---|---|---|---|---|
| Direction | Mean (PTV) | SD | Mean (PTV) | SD |
| Buccal | 2.88 | 1.13 | −1.38 | 0.84 |
| Palatal | 2.91 | 1.04 | −1.71 | 0.88 |
| Mesial | 3.04 | 0.73 | −1.69 | 0.51 |
| Distal | 3.14 | 0.75 | −1.97 | 0.57 |
| Overall | 2.99 | 0.91 | −1.69 | 0.73 |
| Number of Cycles | 120,000 |
| Force | 49 N |
| Height | 3 mm |
| Lateral movement | −0.7 mm |
| Lowering speed | 60 mm/s |
| Lifting speed | 60 mm/s |
| Advanced speed | 40 mm/s |
| Return speed | 40 mm/s |
| Frequency | 1.6 Hz |
| Antagonist Wear (mm) | Wear Depth (mm) | Volume Loss (mm3) | |
|---|---|---|---|
| LDS-Cylinders | 0.220 a | 0.186 a | 0.322 a |
| (0.038) | (0.032) | (0.098) | |
| LDS-on-Implant | 0.199 a,b | 0.128 b | 0.166 b |
| (0.038) | (0.028) | (0.033) | |
| LDS-on-Tooth-Replica | 0.172 b | 0.098 c | 0.107 c |
| (0.055) | (0.026) | (0.045) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosewski, P.; De Angelis, F.; Sorrentino, E.; Mielczarek, A.; Buonvivere, M.; D’Arcangelo, C. Effect of the Abutment Rigidity on the Wear Resistance of a Lithium Disilicate Glass Ceramic: An In Vitro Study. J. Funct. Biomater. 2023, 14, 395. https://doi.org/10.3390/jfb14080395
Kosewski P, De Angelis F, Sorrentino E, Mielczarek A, Buonvivere M, D’Arcangelo C. Effect of the Abutment Rigidity on the Wear Resistance of a Lithium Disilicate Glass Ceramic: An In Vitro Study. Journal of Functional Biomaterials. 2023; 14(8):395. https://doi.org/10.3390/jfb14080395
Chicago/Turabian StyleKosewski, Przemysław, Francesco De Angelis, Edoardo Sorrentino, Agnieszka Mielczarek, Matteo Buonvivere, and Camillo D’Arcangelo. 2023. "Effect of the Abutment Rigidity on the Wear Resistance of a Lithium Disilicate Glass Ceramic: An In Vitro Study" Journal of Functional Biomaterials 14, no. 8: 395. https://doi.org/10.3390/jfb14080395
APA StyleKosewski, P., De Angelis, F., Sorrentino, E., Mielczarek, A., Buonvivere, M., & D’Arcangelo, C. (2023). Effect of the Abutment Rigidity on the Wear Resistance of a Lithium Disilicate Glass Ceramic: An In Vitro Study. Journal of Functional Biomaterials, 14(8), 395. https://doi.org/10.3390/jfb14080395








