Incorporating Moldable Demineralized Dentin Matrix into Treatment for a Jaw Cyst
Abstract
1. Introduction
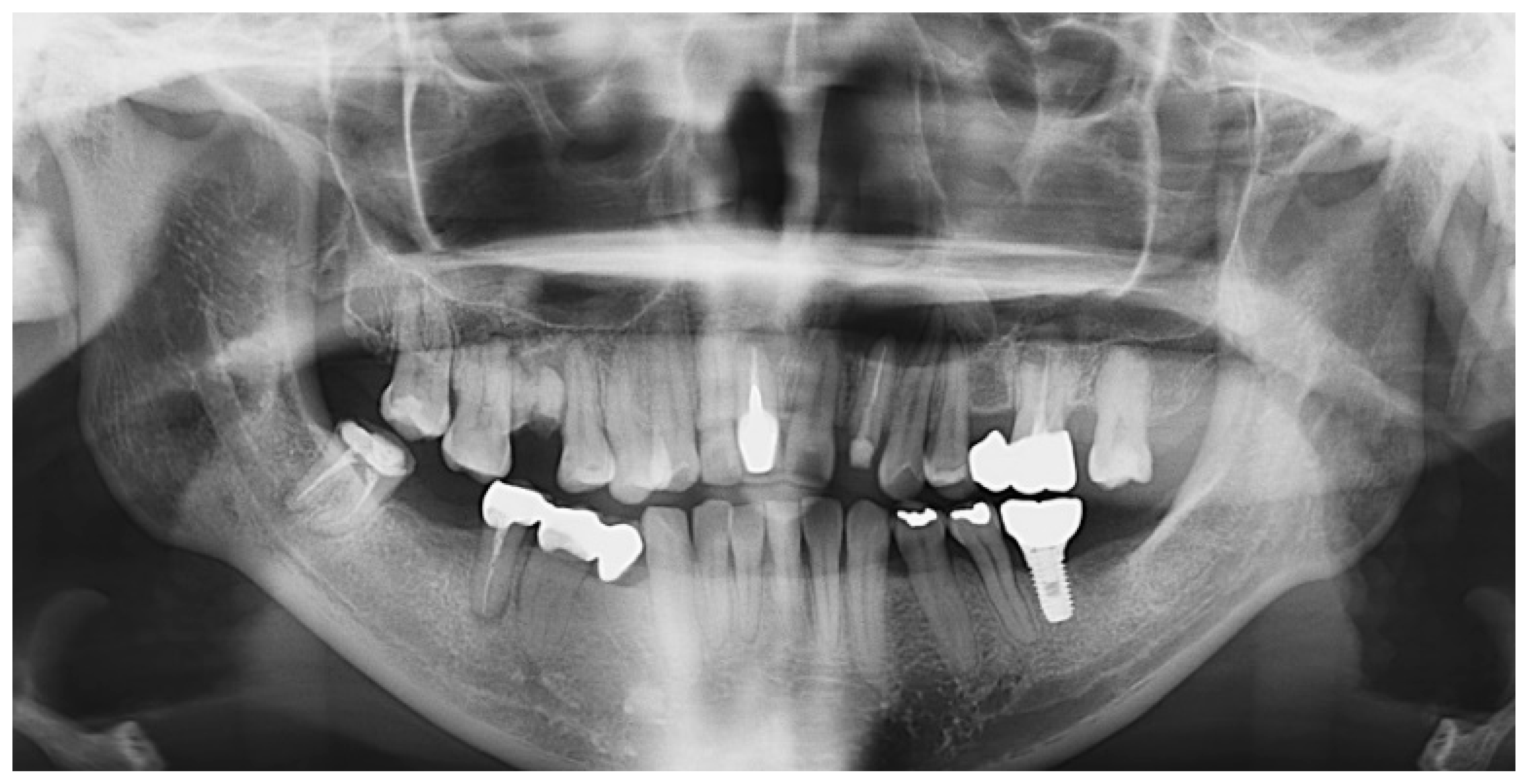
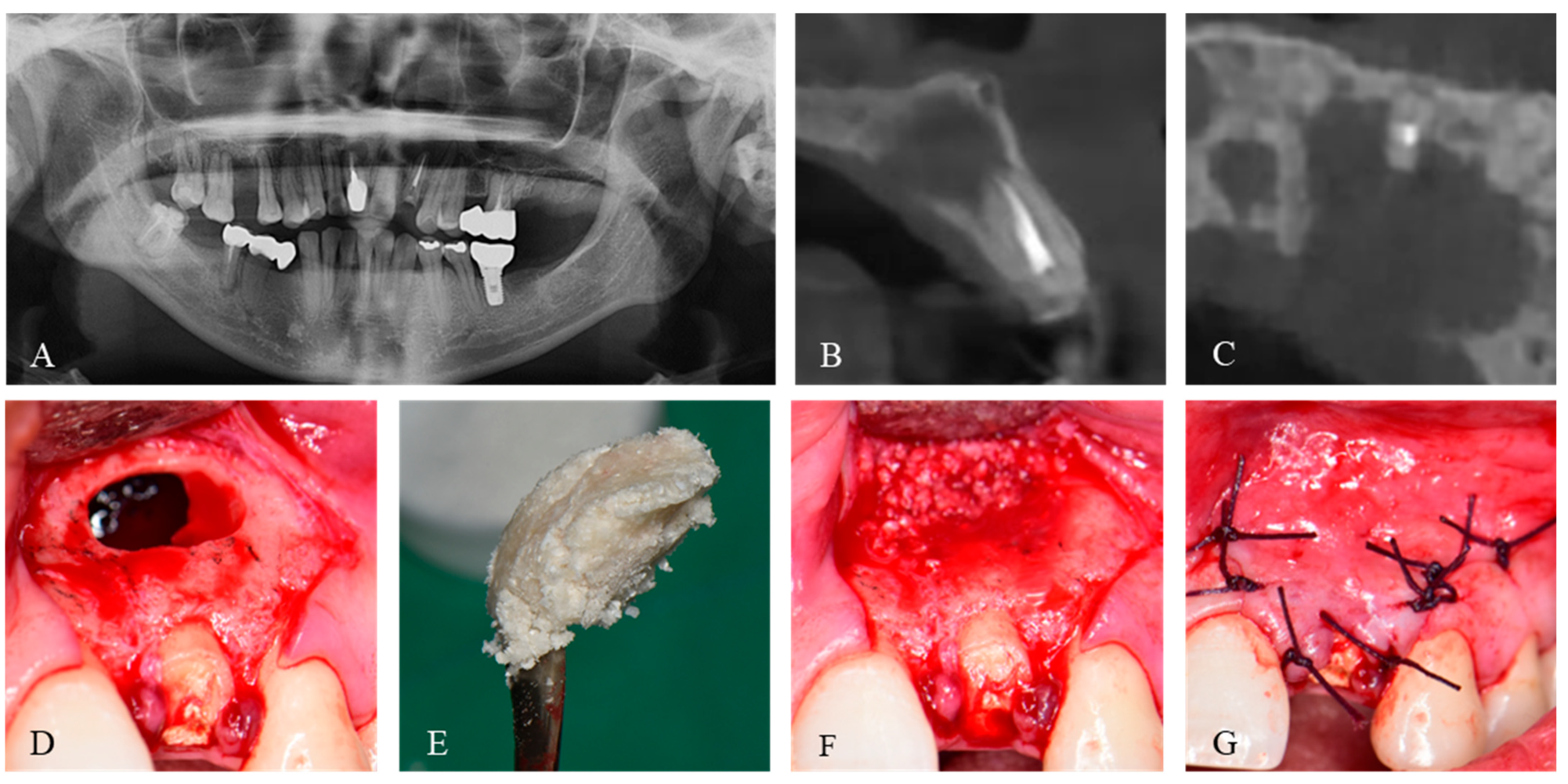
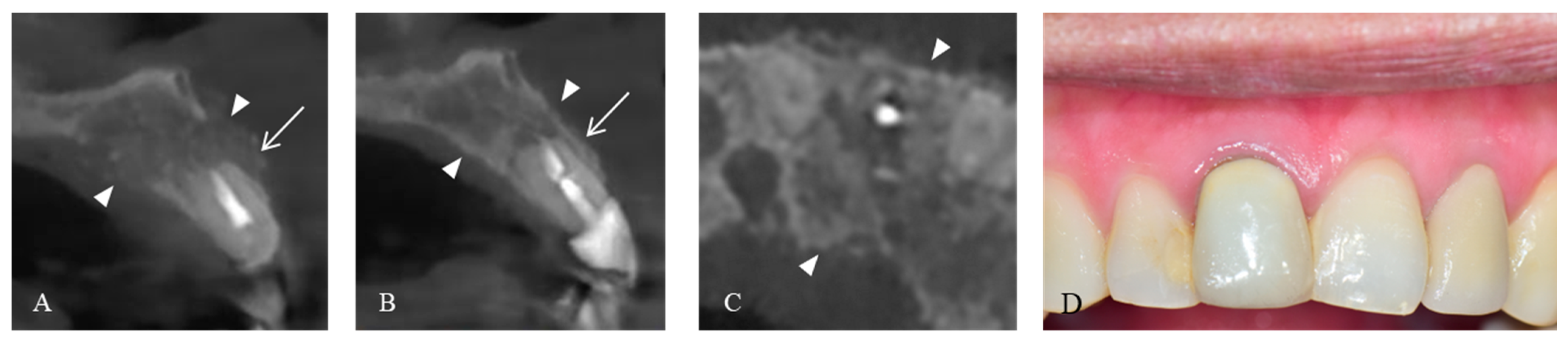
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, S.Y.; Yeo, D.S.; Lee, H.J.; Kim, H.K.; An, K.M.; Sohn, D.S. A clinicostatical study of jaw cyst between 2001~2005. J. Korean Assoc. Oral Maxillofac. Surg. 2006, 32, 588–593. [Google Scholar]
- Bertoldi, C.; Zaffe, D.; Consolo, U. Polylactide/polyglycolide copolymer in bone defect healing in humans. Biomaterials 2008, 29, 1817–1823. [Google Scholar] [CrossRef]
- Ku, J.-K.; Han, M.; Yongvikul, A.; Huh, J.-K.; Kim, J.-Y. Volumetric analysis of spontaneous bone healing after jaw cyst enucleation. Sci. Rep. 2022, 12, 14953. [Google Scholar] [CrossRef]
- Boffano, P.; Roccia, F.; Gallesio, C.; Berrone, S. Pathological mandibular fractures: A review of the literature of the last two decades. Dent. Traumatol. 2013, 29, 185–196. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Q.Y.; Zhu, H.Y. Efficacy of bone grafts in jaw cystic lesions: A systematic review. World J. Clin. Cases 2022, 10, 2801–2810. [Google Scholar] [CrossRef]
- Jang, K.; Lee, J.H.; Oh, S.H.; Ham, B.D.; Chung, S.M.; Lee, J.K.; Ku, J.K. Bone graft materials for current implant dentistry. J. Dent. Implant Res. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Ku, J.K.; Ghim, M.S.; Park, J.H.; Leem, D.H. A ramus cortical bone harvesting technique without bone marrow invasion. J. Korean Assoc. Oral Maxillofac. Surg. 2023, 49, 100. [Google Scholar] [CrossRef]
- Reininger, D.; Cobo-Vázquez, C.; Monteserín-Matesanz, M.; López-Quiles, J. Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2016, 21, e241–e249. [Google Scholar] [CrossRef]
- Hwang, K.G.; Shim, K.S.; Yang, S.M.; Park, C.J. Partial-thickness cortical bone graft from the mandibular ramus: A non-invasive harvesting technique. J. Periodontol. 2008, 79, 941–944. [Google Scholar] [CrossRef]
- Avery, S.J.; Sadaghiani, L.; Sloan, A.J.; Waddington, R.J. Analysing the bioactive makeup of demineralised dentine matrix on bone marrow mesenchymal stem cells for enhanced bone repair. Eur. Cell. Mater. 2017, 34, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef]
- Ettl, T.; Gosau, M.; Sader, R.; Reichert, T.E. Jaw cysts-filling or no filling after enucleation? A review. J. Cranio-Maxillo-Facial Surg. 2012, 40, 485–493. [Google Scholar] [CrossRef]
- Dahlin, C.; Gottlow, J.; Linde, A.; Nyman, S. Healing of maxillary and mandibular bone defects using a membrane technique. An experimental study in monkeys. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 13–19. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Um, I.-W.; Cho, W.-J.; Murata, M.; Jun, S.-H.; Lee, J.-K. Applications of moldable autogenous tooth bone graft (m-autobt) mixed with hydroxypropylmethyl cellulose for sinus lifting. J. Hard Tissue Biol. 2015, 24, 391–396. [Google Scholar] [CrossRef]
- Ku, J.-K.; Lee, J.-K.; Min, C.-G.; Kim, Y.-M.; Um, I.-W. Moldable autogenous tooth bone graft (m-autobt) in sinus-related defects with implant: 3 case reports of long-term follow up. J. Dent. Implant. Res. 2019, 38, 24–29. [Google Scholar]
- Ku, J.K.; Um, I.W.; Jun, M.K.; Kim, I.H. Dentin-derived-barrier membrane in guided bone regeneration: A case report. Materials 2021, 14, 2166. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jung, I.Y. Complete healing of a large cystic lesion following root canal treatment with concurrent surgical drainage: A case report with 14-year follow-up. J. Endod. 2019, 45, 343–348. [Google Scholar] [CrossRef]
- Zwahlen, R.A.; Cheung, L.K.; Zheng, L.W.; Chow, R.L.; Li, T.; Schuknecht, B.; Gratz, K.W.; Weber, F.E. Comparison of two resorbable membrane systems in bone regeneration after removal of wisdom teeth: A randomized-controlled clinical pilot study. Clin. Oral Implant. Res. 2009, 20, 1084–1091. [Google Scholar] [CrossRef]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin. Oral Implant. Res. 2016, 27, e206–e214. [Google Scholar] [CrossRef]
- Hong, I.; Khalid, A.W.; Pae, H.-C.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Jung, U.-W.; Choi, S.-H. Distinctive bone regeneration of calvarial defects using biphasic calcium phosphate supplemented ultraviolet-crosslinked collagen membrane. J. Periodontal Implant Sci. 2019, 50, 14–27. [Google Scholar] [CrossRef]
- Santamaria, J.; Garcia, A.M.; de Vicente, J.C.; Landa, S.; Lopez-Arranz, J.S. Bone regeneration after radicular cyst removal with and without guided bone regeneration. Int. J. Oral Maxillofac. Surg. 1998, 27, 118–120. [Google Scholar] [CrossRef]
- Pommer, B.; Watzek, G. Gel-pressure technique for flapless transcrestal maxillary sinus floor elevation: A preliminary cadaveric study of a new surgical technique. Int. J. Oral Maxillofac. Implant. 2009, 24, 817–822. [Google Scholar]
- Kablan, F. The use of buccal fat pad free graft in closure of soft-tissue defects and dehiscence in the hard palate. Ann. Maxillofac. Surg. 2016, 6, 241–245. [Google Scholar] [CrossRef]
- Kim, Y.; Ku, J.K.; Um, I.W.; Seok, H.; Leem, D.H. Impact of autogenous demineralized dentin matrix on mandibular second molar after third molar extraction: Retrospective study. J Funct Biomater. 2022, 14, 4. [Google Scholar] [CrossRef]
- Um, I.-W.; Ku, J.-K.; Lee, B.K.; Yun, P.-Y.; Lee, J.K.; Nam, J.-H. Postulated release profile of recombinant human bone morphogenetic protein-2 (rhbmp-2) from demineralized dentin matrix. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 123–128. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-K.; Kim, S.-G.; Lim, S.-C. Histomorphometric study of sinus bone graft using various graft material. J. Dent. Rehabil. Appl. Sci. 2011, 27, 141–147. [Google Scholar]
- Jeong, K.-I.; Kim, S.-G.; Kim, Y.-K.; Oh, J.-S.; Jeong, M.-A.; Park, J.-J. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant. Dent. 2011, 20, 471–475. [Google Scholar] [CrossRef]
- Jung, G.-U.; Jeon, T.-H.; Kang, M.-H.; Um, I.-W.; Song, I.-S.; Ryu, J.-J.; Jun, S.-H. Volumetric, radiographic, and histologic analyses of demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 for ridge preservation: A prospective randomized controlled trial in comparison with xenograft. Appl. Sci. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Oguić, M.; Čandrlić, M.; Tomas, M.; Vidaković, B.; Blašković, M.; Jerbić Radetić, A.T.; Zoričić Cvek, S.; Kuiš, D.; Cvijanović Peloza, O. Osteogenic potential of autologous dentin graft compared with bovine xenograft mixed with autologous bone in the esthetic zone: Radiographic, histologic and immunohistochemical evaluation. Int. J. Mol. Sci. 2023, 24, 6440. [Google Scholar] [CrossRef]
- Ma, J.L.; Pan, J.L.; Tan, B.S.; Cui, F.Z. Determination of critical size defect of minipig mandible. J. Tissue Eng. Regen. Med. 2009, 3, 615–622. [Google Scholar] [CrossRef]
- Um, I.; Jun, S.-H.; Yun, P.-Y.; Kim, Y.-K. Histological comparison of autogenous and allogenic demineralized dentin matrix loaded with recombinant human bone morphogenetic protein-2 for alveolar bone repair: A preliminary report. J. Hard Tissue Biol. 2017, 26, 417–424. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Bajantai, N.V.; Sriram, S.K.; Sriram, R.R.; Rao, V.K.; Desai, P.D. Observer strategy and radiographic classification of healing after grafting of cystic defects in maxilla: A radiological appraisal. J. Contemp. Dent. Pract. 2013, 14, 227–232. [Google Scholar]
- Vuletić, M.; Knežević, P.; Jokić, D.; Rebić, J.; Žabarović, D.; Macan, D. Alveolar bone grafting in cleft patients from bone defect to dental implants. Acta Stomatol. Croat. 2014, 48, 250–257. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.; Um, I.W.; Kim, K.W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef]
- Um, I.-W.; Kim, Y.-K.; Park, J.-C.; Lee, J.-H. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin. Implant Dent. Relat. Res. 2019, 21, 4–10. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of bmp-2 and vegf in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef]
- Um, I.W.; Ku, J.K.; Kim, Y.K.; Lee, B.K.; Leem, D.H. Histological review of demineralized dentin matrix as a carrier of rhbmp-2. Tissue Eng. Part B Rev. 2020, 26, 284–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.-K.; Kwak, H.-W.; Um, I.-W. Incorporating Moldable Demineralized Dentin Matrix into Treatment for a Jaw Cyst. J. Funct. Biomater. 2023, 14, 258. https://doi.org/10.3390/jfb14050258
Ku J-K, Kwak H-W, Um I-W. Incorporating Moldable Demineralized Dentin Matrix into Treatment for a Jaw Cyst. Journal of Functional Biomaterials. 2023; 14(5):258. https://doi.org/10.3390/jfb14050258
Chicago/Turabian StyleKu, Jeong-Kui, Han-Wool Kwak, and In-Woong Um. 2023. "Incorporating Moldable Demineralized Dentin Matrix into Treatment for a Jaw Cyst" Journal of Functional Biomaterials 14, no. 5: 258. https://doi.org/10.3390/jfb14050258
APA StyleKu, J.-K., Kwak, H.-W., & Um, I.-W. (2023). Incorporating Moldable Demineralized Dentin Matrix into Treatment for a Jaw Cyst. Journal of Functional Biomaterials, 14(5), 258. https://doi.org/10.3390/jfb14050258






