Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study
Abstract
1. Introduction
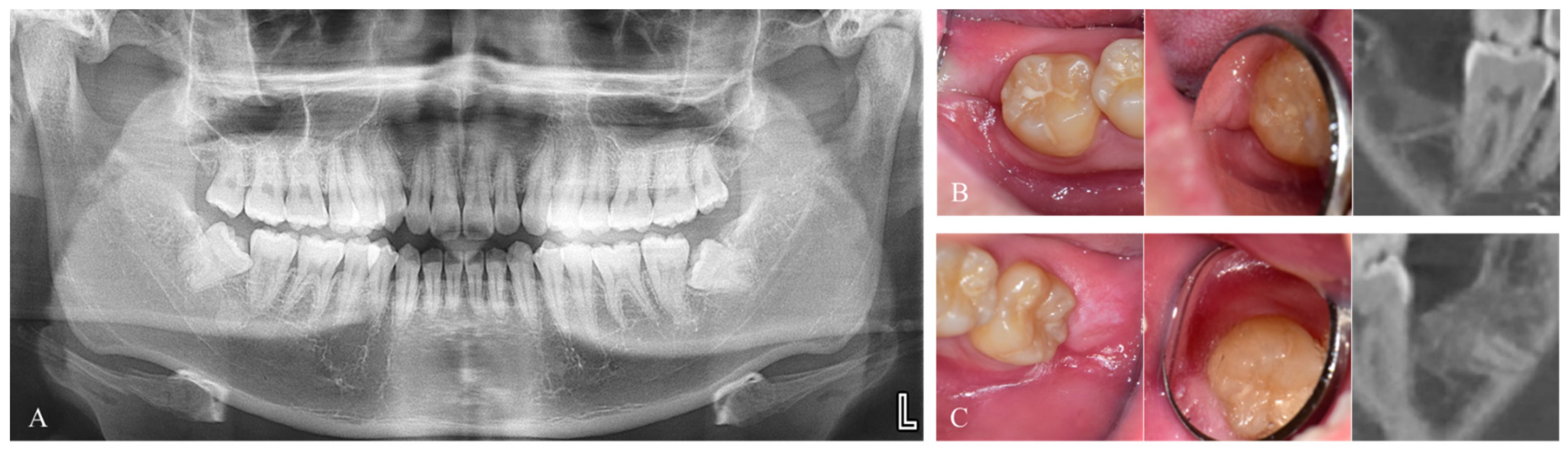
2. Materials and Methods
2.1. Study Design
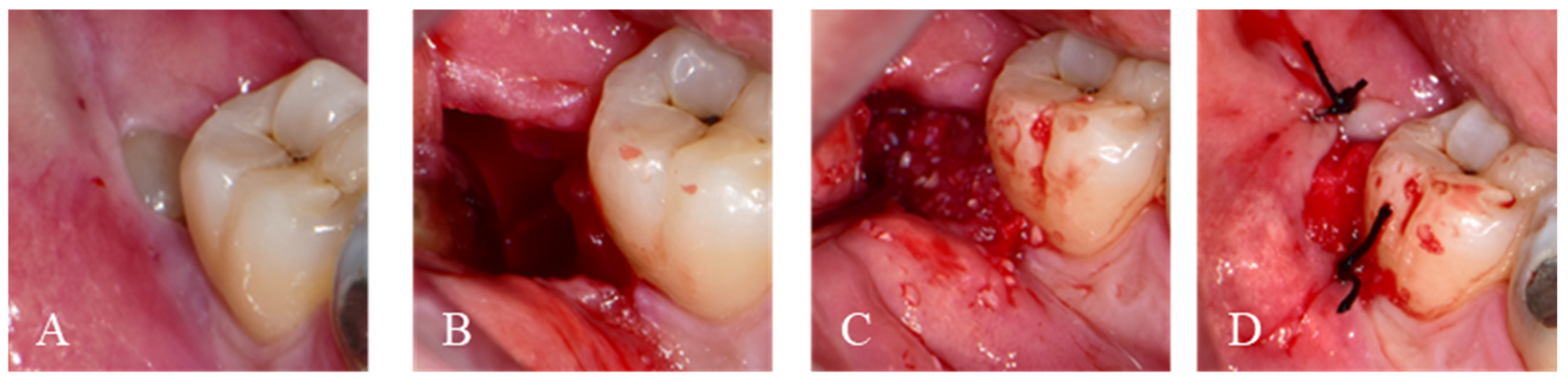
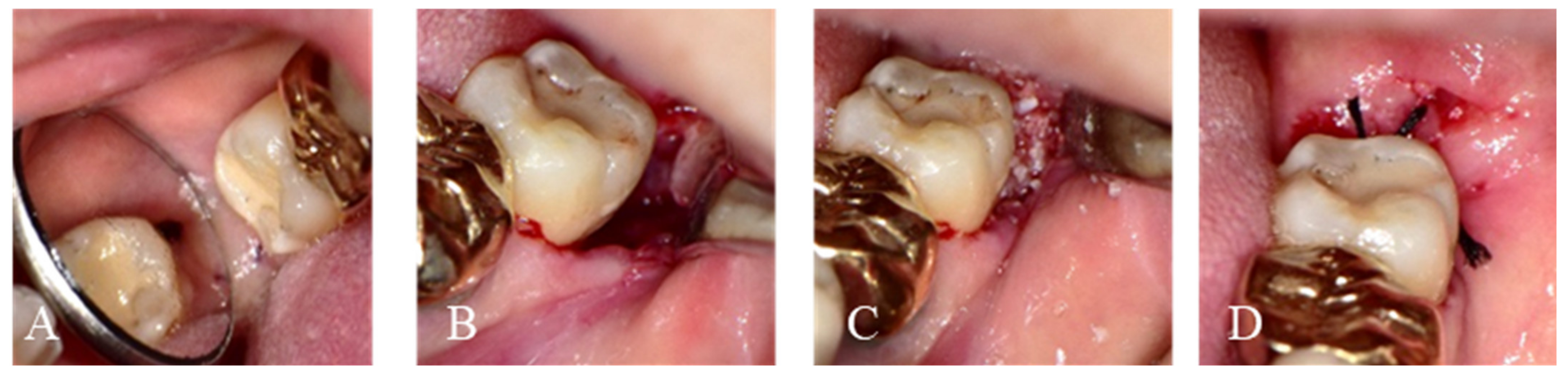
2.1.1. Immediate Graft
2.1.2. Delayed Graft
2.2. Post-Operative Surgery Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shoshani-Dror, D.; Shilo, D.; Ginini, J.G.; Emodi, O.; Rachmiel, A. Controversy regarding the need for prophylactic removal of impacted third molars: An overview. Quintessence Int. 2018, 49, 653–662. [Google Scholar] [PubMed]
- Leung, W.K.; Theilade, E.; Comfort, M.B.; Lim, P.L. Microbiology of the pericoronal pouch in mandibular third molar pericoronitis. Oral Microbiol. Immunol. 1993, 8, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, C.F.; Ahlström, U.; Ericson, S.; Hugoson, A.; Thilander, H. The influence of anatomical, pathophysiological and other factors on periodontal healing after impacted lower third molar surgery a multiple regression analysis. J. Clin. Periodontol. 1991, 18, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Low, S.H.; Lu, S.L.; Lu, H.K. Evidence-based clinical decision making for the management of patients with periodontal osseous defect after impacted third molar extraction: A systematic review and meta-analysis. J. Dent. Sci. 2021, 16, 71–84. [Google Scholar] [CrossRef]
- American Association of Oral and Maxillofacial Surgeons. Bone Grafting after Removal of Impacted Third Molars. Available online: https://www.aaoms.org/docs/practice_resources/clinical_resources/bone_grafting.pdf (accessed on 8 August 2020).
- Hassan, K.S.; Marei, H.F.; Alagl, A.S. Does grafting of third molar extraction sockets enhance periodontal measures in 30- to 35-year-old patients? J. Oral Maxillofac. Surg. 2012, 70, 757–764. [Google Scholar] [CrossRef]
- Ranganathan, M.; Balaji, M.; Krishnaraj, R.; Narayanan, V.; Thangavelu, A. Assessment of regeneration of bone in the extracted third molar sockets augmented using xenograft (collaplug(tn) zimmer) in comparison with the normal healing on the contralateral side. J. Pharm. Bioallied Sci. 2017, 9, S180–S186. [Google Scholar] [CrossRef]
- Kim, J.-W.; Seong, T.-W.; Cho, S.; Kim, S.-J. Randomized controlled trial on the effectiveness of absorbable collagen sponge after extraction of impacted mandibular third molar: Split-mouth design. BMC Oral Health 2020, 20, 77. [Google Scholar] [CrossRef]
- Ku, J.-K.; Han, M.; Yongvikul, A.; Huh, J.-K.; Kim, J.-Y. Volumetric analysis of spontaneous bone healing after jaw cyst enucleation. Sci. Rep. 2022, 12, 14953. [Google Scholar] [CrossRef]
- Ku, J.K.; Jeong, Y.K. Effectiveness of bone graft for an alveolar defect on adjacent second molar after impacted mandibular third molar extraction. J. Oral Maxillofac. Surg. 2021, 79, 756–762. [Google Scholar] [CrossRef]
- Jang, K.; Lee, J.-H.; Oh, S.-H.; Ham, B.-D.; Chung, S.-M.; Lee, J.K.; Ku, J.-K. Bone graft materials for current implant dentistry. J. Dent. Implant Res. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-K.; Murata, M.; Um, I.-W. Demineralized dentin matrix (ddm) scaffolds for alveolar bone engineering. In Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine, 2nd ed.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2017; p. 34. [Google Scholar]
- Moraes, G.F.; Caetano, R.O.; Prochnow, F.H.O.; Pupo, Y.M.; Schussel, J.L.; Schwartz-Filho, H.O. Demineralized human dentin matrix for alveolar ridge preservation using a volumetric and histologic analyses in rats. Braz. Dent. J. 2022, 33, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-U.; Jeon, T.-H.; Kang, M.-H.; Um, I.-W.; Song, I.S.; Ryu, J.-J.; Jun, S.H. Volumetric, radiographic, and histologic analyses of demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 for ridge preservation: A prospective randomized controlled trial in comparison with xenograft. Appl. Sci. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Sánchez-Labrador, L.; Martín-Ares, M.; Ortega-Aranegui, R.; López-Quiles, J.; Martínez-González, J.M. Autogenous dentin graft in bone defects after lower third molar extraction: A split-mouth clinical trial. Materials 2020, 13, 3090. [Google Scholar] [CrossRef]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.-i.; I, T.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef]
- Ku, J.K.; Chang, N.H.; Jeong, Y.K.; Baik, S.H.; Choi, S.K. Development and validation of a difficulty index for mandibular third molars with extraction time. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 328–334. [Google Scholar] [CrossRef]
- Ku, J.K.; Kim, I.H.; Um, I.W.; Kim, B.H.; Yun, P.Y. Effect of gamma irradiation on the osteoinductivity of demineralized dentin matrix for allografts: A preliminary study. J. Funct. Biomater. 2022, 13, 14. [Google Scholar] [CrossRef]
- Ge, J.; Yang, C.; Zheng, J.; Hu, Y. Autogenous bone grafting for treatment of osseous defect after impacted mandibular third molar extraction: A randomized controlled trial. Clin. Implant Dent. Relat. Res. 2017, 19, 572–580. [Google Scholar] [CrossRef]
- Murata, M. Collagen biology for bone regenerative surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 321–325. [Google Scholar] [CrossRef]
- Ku, J.-K.; Jeong, Y.K.; Um, I.-W. Review of allogeneic dentin graft for maxillofacial bone defects. Tissue Eng. Part C Methods 2021, 27, 472–480. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- De Biase, A.; Mazzucchi, G.; Di Nardo, D.; Lollobrigida, M.; Serafini, G.; Testarelli, L. Prevention of periodontal pocket formation after mandibular third molar extraction using dentin autologous graft: A split mouth case report. Case Rep. Dent. 2020, 2020, 1762862. [Google Scholar] [CrossRef] [PubMed]
- Wushou, A.; Zheng, Y.; Han, Y.; Yang, Z.-c.; Han, F.-k. The use of autogenous tooth bone graft powder in the treatment of osseous defects after impacted mandibular third molar extraction: A prospective split-mouth clinical pilot study. BMC Oral Health 2022, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Um, I.-W.; Ku, J.-K.; Kim, Y.-M.; Yun, P.-Y.; Chang, N.-H.; Kim, Y.-K.; Choi, Y. Allogeneic demineralized dentin matrix graft for guided bone regeneration in dental implants. Appl. Sci. 2020, 10, 4661. [Google Scholar] [CrossRef]
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Kim, J.-S.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and microstructural analysis of osteopromotive property of allogenic demineralized dentin matrix. Int. J. Oral Maxillofac. Implants 2013, 28, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Bang, K.-M.; Murata, M.; Mitsugi, M.; Um, I.-W. Retrospective clinical study of allogenic demineralized dentin matrix for alveolar bone repair. J. Hard Tissue Biol. 2017, 26, 95–102. [Google Scholar] [CrossRef]
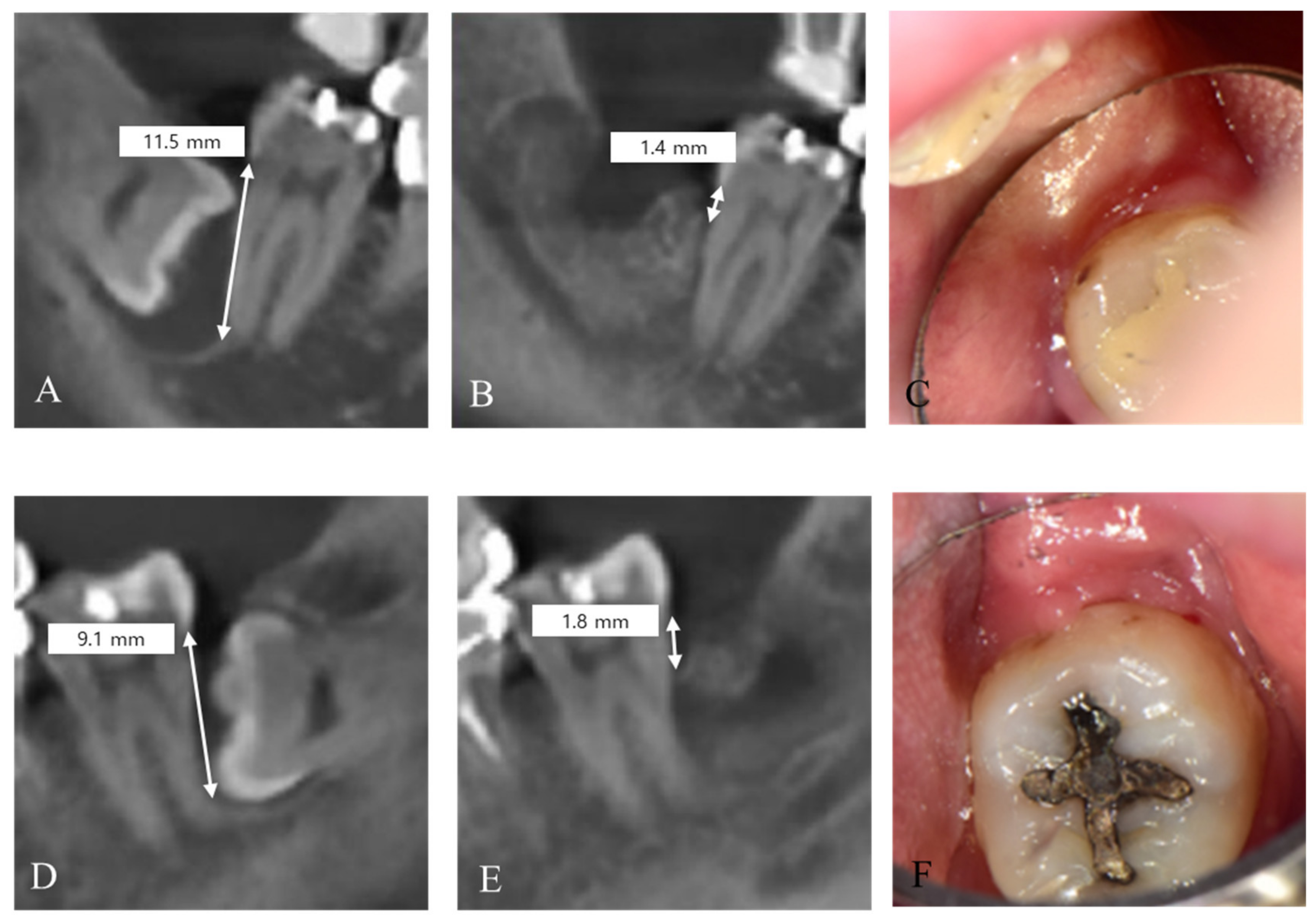
| Patients | Age | Sex | Group | Follow-Up (Months) | Pre-Operative Defect (mm) | Post-Operative Defect (mm) |
|---|---|---|---|---|---|---|
| 1 | 34 | Male | Immediate graft | 5.5 | 6.9 | 2.4 |
| 2 | 34 | Male | Immediate graft | 3.4 | 7.2 | 2.2 |
| 3 | 37 | Female | Immediate graft | 4.2 | 6.9 | 1.2 |
| 4 | 37 | Female | Control | 5.2 | 2.8 | |
| 5 | 62 | Female | Delayed graft | 8.6 | 8.2 | 2.3 |
| 6 | 47 | Male | Delayed graft | 3.5 | 15.7 | 6.0 |
| 7 | 47 | Male | Control | 1.7 | 2.3 | |
| 8 | 59 | Male | Delayed graft | 1.5 | 13.3 | 4.4 |
| 9 | 37 | Male | Immediate graft | 2.6 | 11.7 | 1.5 |
| 10 | 31 | Male | Immediate graft | 7.9 | 16.5 | 0.7 |
| 11 | 32 | Male | Delayed graft | 6.1 | 7.7 | 0.0 |
| 12 | 21 | Male | Immediate graft | 2.6 | 5.1 | 0.6 |
| 13 | 21 | Male | Control | 3.6 | 2.0 | |
| 14 | 41 | Male | Delayed graft | 4.2 | 10.8 | 1.3 |
| 15 | 41 | Male | Control | 1.4 | 1.4 | |
| 16 | 52 | Male | Immediate graft | 5.3 | 10.8 | 3.1 |
| 17 | 42 | Female | Delayed graft | 4.2 | 9.1 | 1.8 |
| 18 | 43 | Female | Delayed graft | 4.2 | 11.5 | 1.4 |
| 19 | 72 | Male | Delayed graft | 2.1 | 8.5 | 4.6 |
| 20 | 49 | Male | Delayed graft | 3.2 | 10.6 | 4.12 |
| Control (n = 4) | Graft (n = 16) | p-Value | |
|---|---|---|---|
| Age (year) | 39.3 ± 6.3 | 42.6 ± 13.3 | 0.645 |
| Follow-up (months) | 5.5 ± 2.6 | 4.6 ± 2.0 | 0.721 |
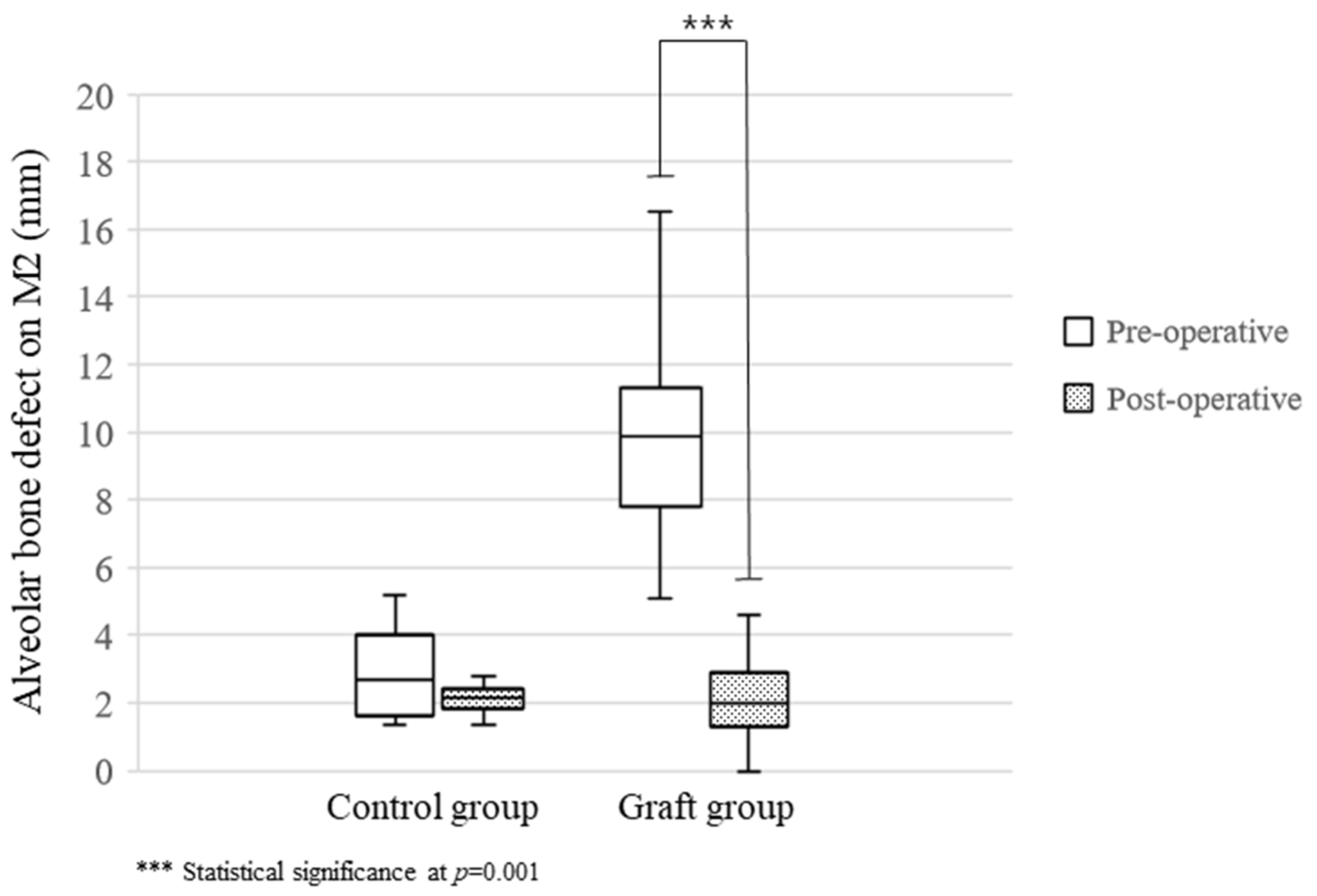
| Pre-operative | 2.98 ± 1.77 | 10.02 ± 3.22 | 0.001 * |
| Post-operative | 2.12 ± 0.59 | 2.29 ± 1.67 | 0.959 |
| Improvement (mm) | 1.00 ± 1.20 | 7.74 ± 3.19 | 0.001 * |
| Improvement ratio (%) | 22.68 ± 26.19 | 76.70 ± 15.36 | 0.001 * |
| Graft after Extraction | p-Value * | Sex | p-Value * | |||
|---|---|---|---|---|---|---|
| Immediate (n = 7) | Delayed (n = 9) | Male (n = 12) | Female (n = 4) | |||
| Age (year) | 34.8 ± 10.1 | 48.5 ± 12.8 | 0.059 | 40.9 ± 13.7 | 49.0 ± 11.3 | 0.291 |
| f/u (months) | 4.6 ± 2.1 | 4.5 ± 2.0 | 0.950 | 4.2 ± 1.8 | 5.7 ± 2.5 | 0.291 |
| Pre-operative defect (mm) | 9.70 ± 4.16 | 10.26 ± 2.59 | 0.662 | 10.14 ± 3.58 | 9.60 ± 1.71 | >0.999 |
| Post-operative defect (mm) | 1.75 ± 0.99 | 2.69 ± 2.02 | 0.573 | 2.41 ± 1.88 | 1.83 ± 0.45 | 0.885 |
| Improvement (mm) | 7.95 ± 4.46 | 7.57 ± 2.14 | 0.950 | 7.73 ± 3.50 | 7.77 ± 2.14 | 0.885 |
| Improvement ratio (%) | 79.50 ± 12.46 | 74.60 ± 17.76 | 0.662 | 75.80 ± 17.03 | 80.00 ± 7.90 | 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Ku, J.-K.; Um, I.-W.; Seok, H.; Leem, D.H. Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study. J. Funct. Biomater. 2023, 14, 4. https://doi.org/10.3390/jfb14010004
Kim Y, Ku J-K, Um I-W, Seok H, Leem DH. Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study. Journal of Functional Biomaterials. 2023; 14(1):4. https://doi.org/10.3390/jfb14010004
Chicago/Turabian StyleKim, Yesel, Jeong-Kui Ku, In-Woong Um, Hyun Seok, and Dae Ho Leem. 2023. "Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study" Journal of Functional Biomaterials 14, no. 1: 4. https://doi.org/10.3390/jfb14010004
APA StyleKim, Y., Ku, J.-K., Um, I.-W., Seok, H., & Leem, D. H. (2023). Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study. Journal of Functional Biomaterials, 14(1), 4. https://doi.org/10.3390/jfb14010004








