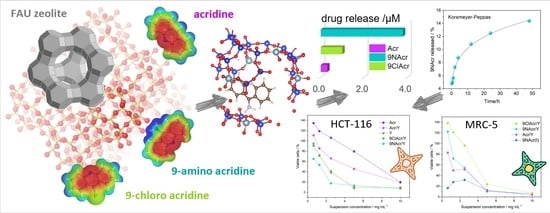
Can Zeolite-Supporting Acridines Boost Their Anticancer Performance?
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Theoretical Calculation
2.4. Cytotoxicity Study
3. Results and Discussion
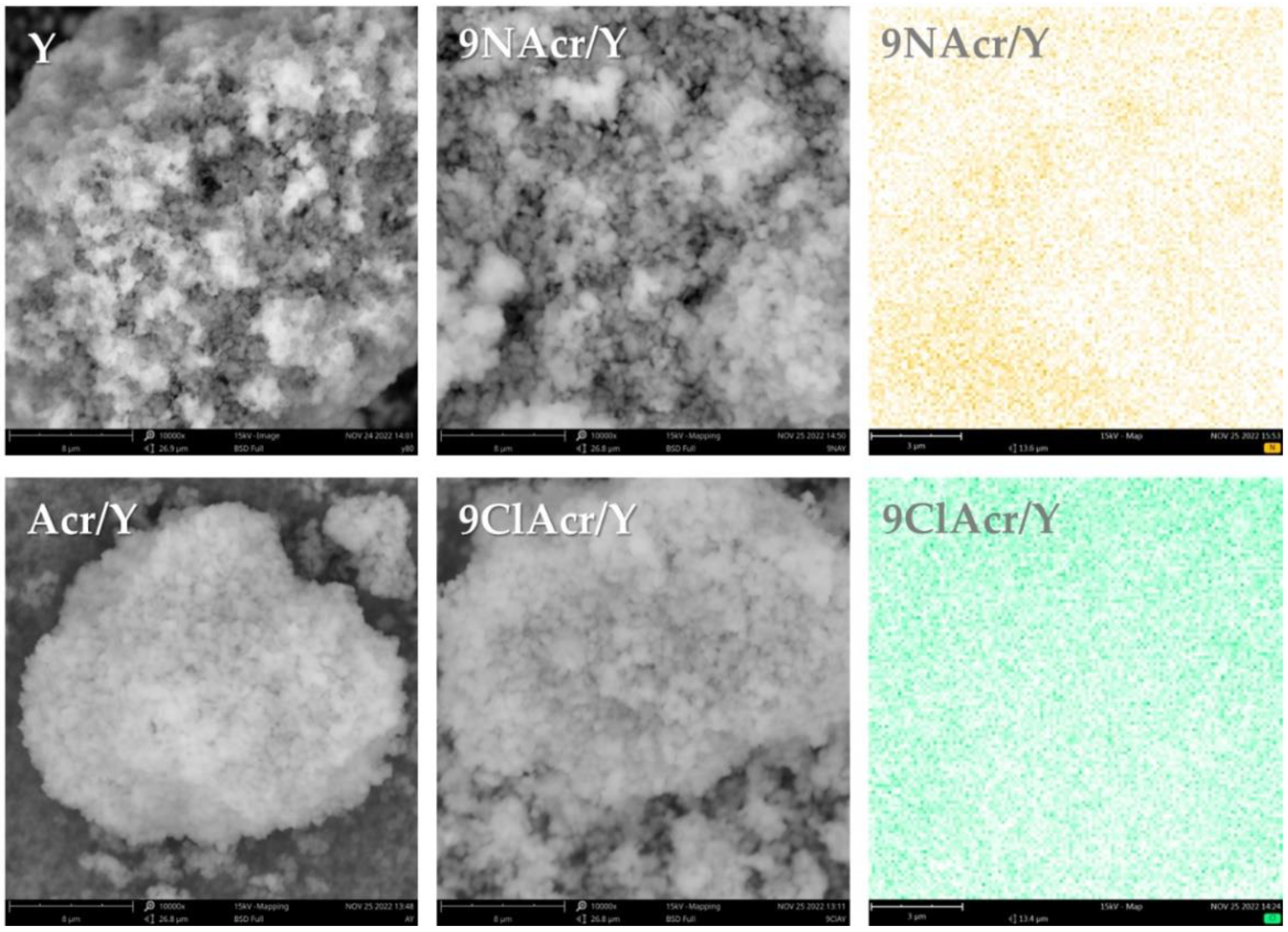
3.1. Morphology and Surface Elemental Maps
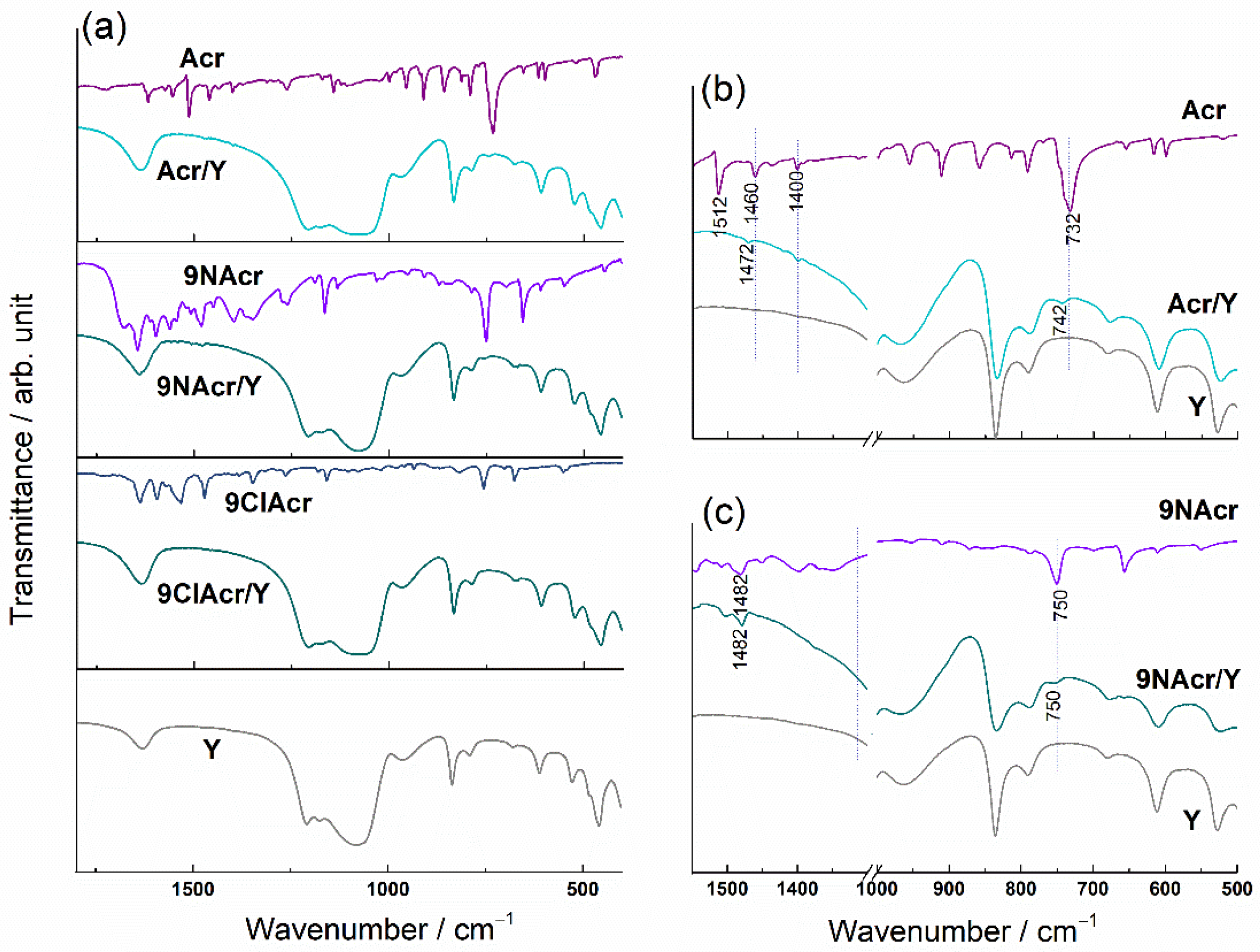
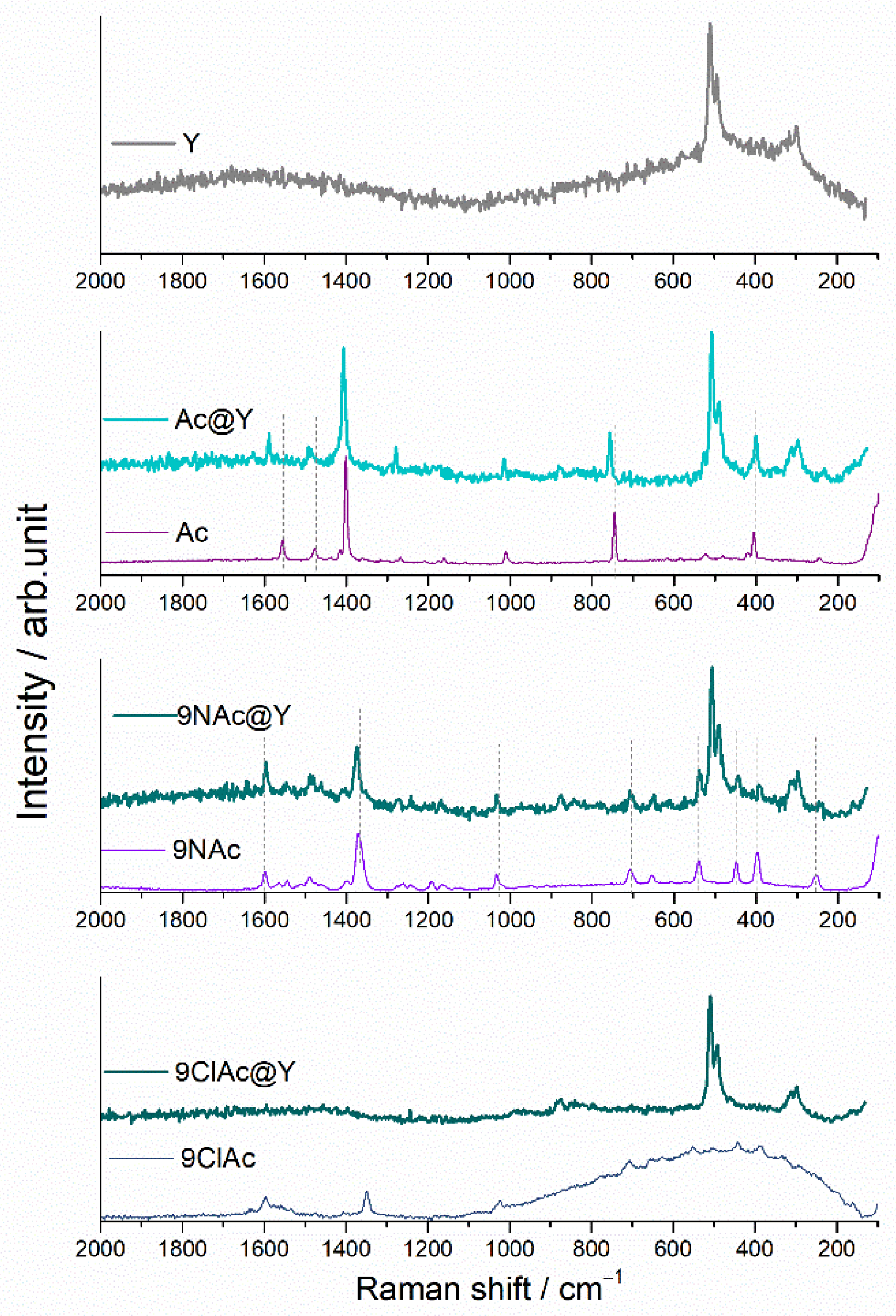
3.2. Spectral Analysis
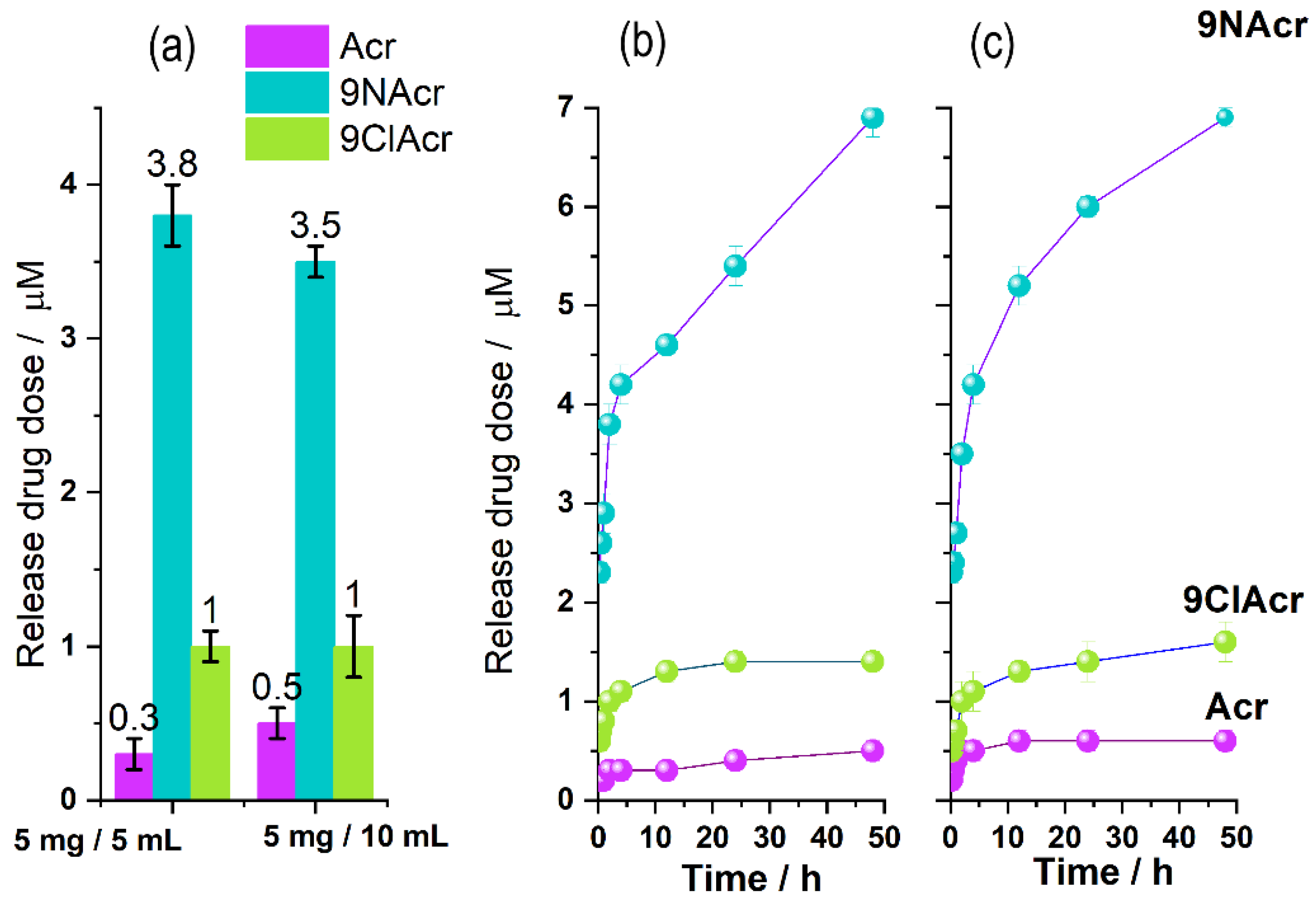
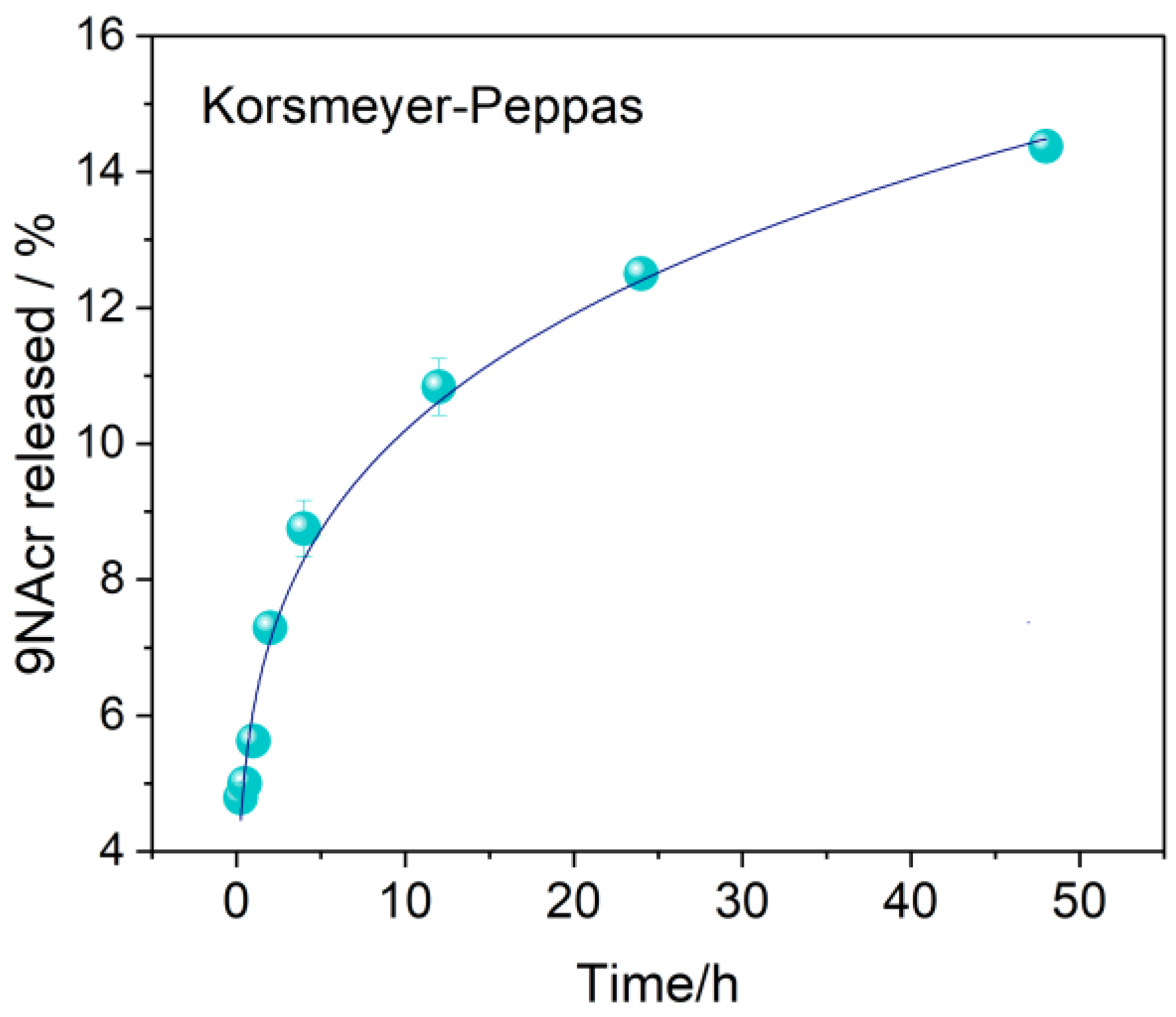
3.3. Release Study
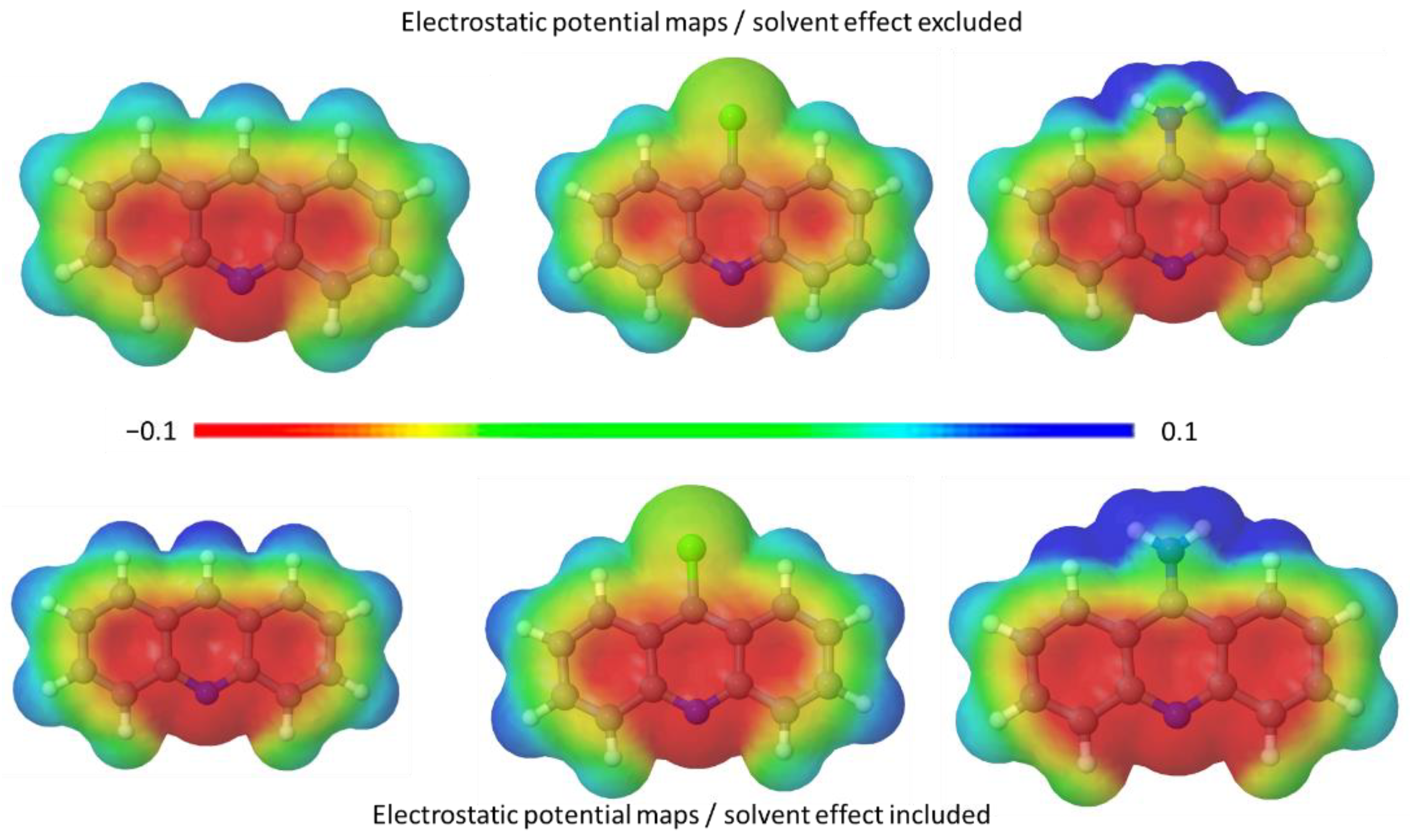
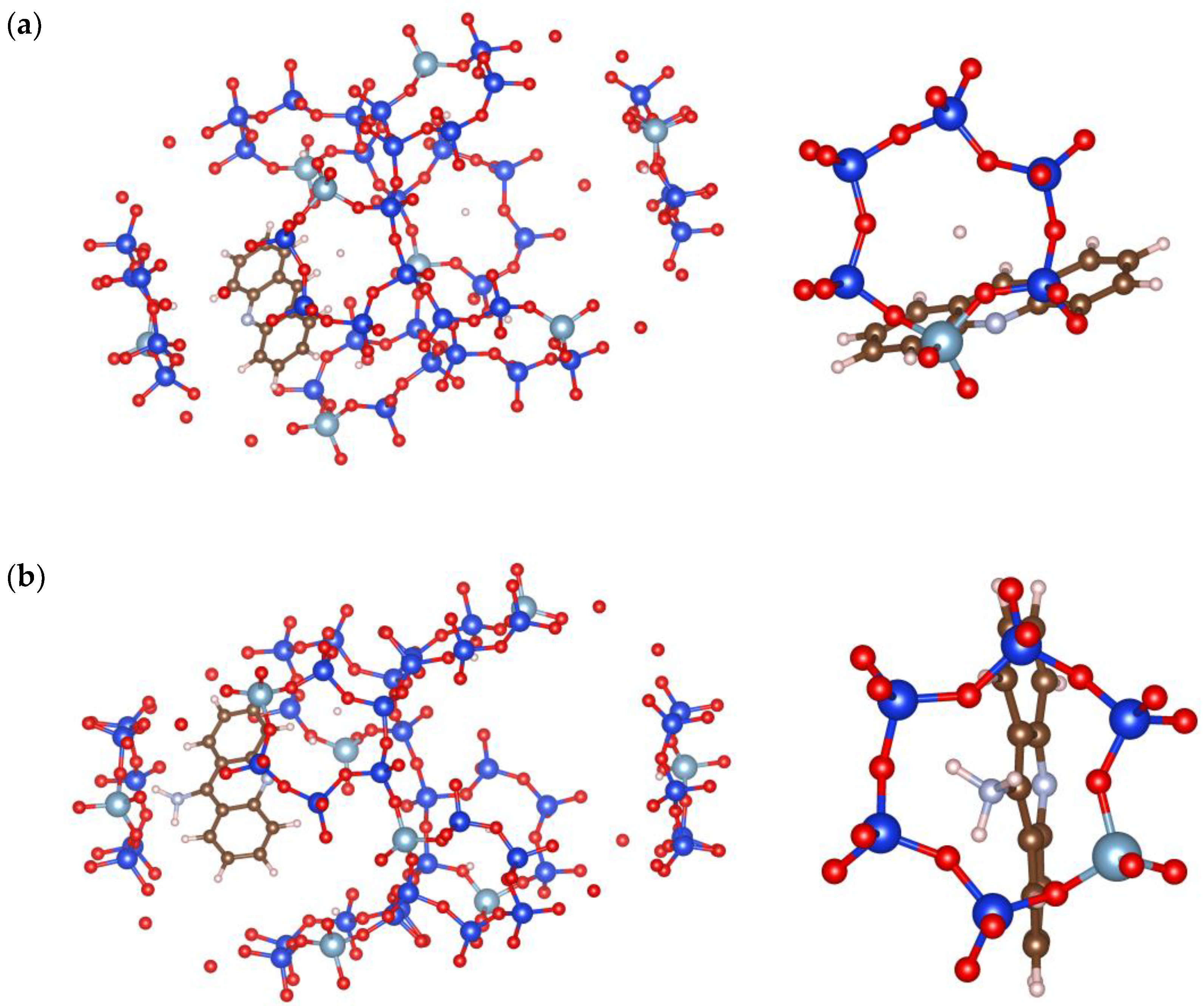
3.4. Optimization of Acridines and Zeolite Interactions
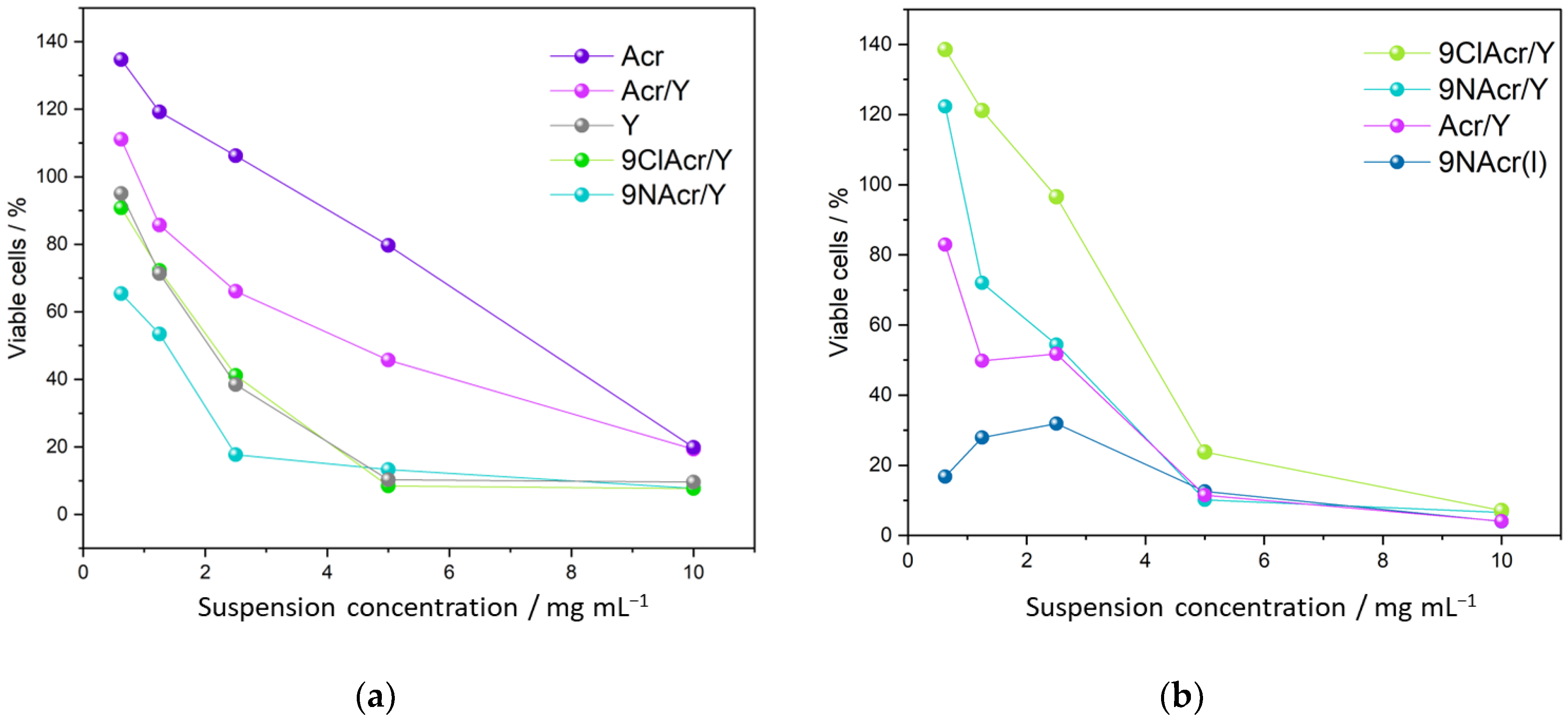
3.5. Cytotoxicity of Supported Acridines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Krajišnik, D.; Daković, A.; Milojević, M.; Malenović, A.; Kragović, M.; Bajuk-Bogdanović, D.; Dondur, V.; Milić, J. Properties of Diclofenac Sodium Sorption onto Natural Zeolite Modified with Cetylpyridinium Chloride. Colloids Surf. B Biointerfaces 2011, 83, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Krajišnik, D.; Milojević, M.; Malenović, A.A.; Daković, A.; Ibrić, S.; Savić, S.S.; Dondur, V.; Matijašević, S.; Radulović, A.; Daniels, R.; et al. Cationic Surfactants-Modified Natural Zeolites: Improvement of the Excipients Functionality. Drug Dev. Ind. Pharm. 2010, 36, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Krajišnik, D.; Daković, A.; Malenović, A.; Milojević-Rakić, M.; Dondur, V.; Radulović, Ž.; Milić, J.; Radulović, Z.; Milić, J. Investigation of Adsorption and Release of Diclofenac Sodium by Modified Zeolites Composites. Appl. Clay Sci. 2013, 83–84, 322–326. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Mijailović, N.R.; Nedić Vasiljević, B.; Ranković, M.; Milanović, V.; Uskoković-Marković, S. Environmental and Pharmacokinetic Aspects of Zeolite/Pharmaceuticals Systems—Two Facets of Adsorption Ability. Catalysts 2022, 12, 837. [Google Scholar] [CrossRef]
- Borovcanin, M.M.; Vesić, K.; Arsenijević, D.; Milojević-Rakić, M.; Mijailović, N.R.; Jovanovic, I.P. Targeting Underlying Inflammation in Carcinoma Is Essential for the Resolution of Depressiveness. Cells 2023, 12, 710. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Zarrintaj, P.; Hosseiniamoli, H.; Mashhadzadeh, A.H.; Saeb, M.R.; Ramsey, J.D.; Ganjali, M.R.; Mozafari, M. Zeolites for Theranostic Applications. J. Mater. Chem. B 2020, 8, 5992–6012. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in Drug Delivery: Progress, Challenges and Opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Purnomo; Setyarini, P.H.; Sulistyaningsih, D. Zeolite-Based Biomaterials for Biomedical Application: A Review. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 030013. [Google Scholar]
- Nezamzadeh-Ejhieh, A.; Tavakoli-Ghinani, S. Effect of a Nano-Sized Natural Clinoptilolite Modified by the Hexadecyltrimethyl Ammonium Surfactant on Cephalexin Drug Delivery. Comptes Rendus Chim. 2014, 17, 49–61. [Google Scholar] [CrossRef]
- Amorim, R.; Vilaça, N.; Martinho, O.; Reis, R.M.; Sardo, M.; Rocha, J.; Fonseca, A.M.; Baltazar, F.; Neves, I.C. Zeolite Structures Loading with an Anticancer Compound as Drug Delivery Systems. J. Phys. Chem. C 2012, 116, 25642–25650. [Google Scholar] [CrossRef]
- Paradee, N.; Sirivat, A. Encapsulation of Folic Acid in Zeolite Y for Controlled Release via Electric Field. Mol. Pharm. 2016, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Šebestík, J.; Šafařík, M.; Stibor, I.; Hlaváček, J. Acridin-9-Yl Exchange: A Proposal for the Action of Some 9-Aminoacridine Drugs. Biopolymers 2006, 84, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Rupar, J.; Dobričić, V.; Grahovac, J.; Radulović, S.; Skok, Ž.; Ilaš, J.; Aleksić, M.; Brborić, J.; Čudina, O. Synthesis and Evaluation of Anticancer Activity of New 9-Acridinyl Amino Acid Derivatives. RSC Med. Chem. 2020, 11, 378–386. [Google Scholar] [CrossRef]
- Wang, W.; Ho, W.C.; Dicker, D.T.; MacKinnon, C.; Winkler, J.D.; Marmorstein, R.; El-Deiry, W.S. Acridine Derivatives Activate P53 and Induce Tumor Cell Death through Bax. Cancer Biol. Ther. 2005, 4, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Mangueira, V.M.; de Sousa, T.K.G.; Batista, T.M.; de Abrantes, R.A.; Moura, A.P.G.; Ferreira, R.C.; de Almeida, R.N.; Braga, R.M.; Leite, F.C.; Medeiros, K.C.d.P.; et al. A 9-Aminoacridine Derivative Induces Growth Inhibition of Ehrlich Ascites Carcinoma Cells and Antinociceptive Effect in Mice. Front. Pharmacol. 2022, 13, 963736. [Google Scholar] [CrossRef]
- Rupar, J.; Aleksić, M.M.; Dobričić, V.; Brborić, J.; Čudina, O. An Electrochemical Study of 9-Chloroacridine Redox Behavior and Its Interaction with Double-Stranded DNA. Bioelectrochemistry 2020, 135, 107579. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Prasad, D.N. Synthesis and Anticancer Study of 9-Aminoacridine Derivatives. Arab. J. Chem. 2013, 6, 79–85. [Google Scholar] [CrossRef]
- Sedláček, O.; Hrubý, M.; Studenovský, M.; Větvička, D.; Svoboda, J.; Kaňková, D.; Kovář, J.; Ulbrich, K. Polymer Conjugates of Acridine-Type Anticancer Drugs with PH-Controlled Activation. Bioorg. Med. Chem. 2012, 20, 4056–4063. [Google Scholar] [CrossRef]
- Zhang, H.; Gerson, T.; Varney, M.L.; Singh, R.K.; Vinogradov, S.V. Multifunctional Peptide-PEG Intercalating Conjugates: Programmatic of Gene Delivery to the Blood-Brain Barrier. Pharm. Res. 2010, 27, 2528–2543. [Google Scholar] [CrossRef]
- Ježek, J.; Hlaváček, J.; Šebestík, J. Biomedical Applications of Acridines; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2017; Volume 72, ISBN 978-3-319-63952-9. [Google Scholar]
- Denny, W. Chemotherapeutic Effects of Acridine Derivatives. Med. Chem. Rev. Online 2004, 1, 257–266. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Trendafilova, I.; Trif, L.; Mihály, J.; Makk, J.; Mavrodinova, V. Bicomponent Drug Formulation for Simultaneous Release of Ag and Sulfadiazine Supported on Nanosized Zeolite Beta. Nano-Struct. Nano-Objects 2020, 24, 100562. [Google Scholar] [CrossRef]
- Jevremović, A.; Stanojković, A.; Arsenijević, D.; Arsenijević, A.; Arzumanyan, G.; Mamatkulov, K.; Petrović, J.; Nedić Vasiljević, B.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Mitigating Toxicity of Acetamiprid Removal Techniques—Fe Modified Zeolites in Focus. J. Hazard. Mater. 2022, 436, 129226. [Google Scholar] [CrossRef] [PubMed]
- Jevremović, A.; Božinović, N.; Arsenijević, D.; Marmakov, S.; Nedić Vasiljević, B.; Uskoković-Marković, S.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Modulation of Cytotoxicity by Consecutive Adsorption of Tannic Acid and Pesticides on Surfactant Functionalized Zeolites. Environ. Sci. Process. Impacts 2020, 22, 2199–2211. [Google Scholar] [CrossRef]
- MOPAC2016, J.J.P. Stewart, Stewart Computational Chemistry: Colorado Springs, CO, USA. Available online: http://openmopac.net/ (accessed on 15 December 2022).
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Database of Zeolite Structures, Structure Commission of the International Zeolite Association Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases (accessed on 15 December 2022).
- Marks, D.C.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The MTT Cell Viability Assay for Cytotoxicity Testing in Multidrug-Resistant Human Leukemic Cells. Leuk. Res. 1992, 16, 1165–1173. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. Adv. Chem. 1974, 101, 201–229. [Google Scholar]
- Acheson, R.M. The Infrared Spectra of Acridines. In Chemistry of Heterocyclic Compounds: Acridines, Volume 9, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008; pp. 665–685. [Google Scholar]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.-S.S. Characterization of Aluminosilicate Zeolites by UV Raman Spectroscopy. Microporous Mesoporous Mater. 2001, 46, 23–34. [Google Scholar] [CrossRef]
- Butler, C.A.; Cooney, R.P. Normal Coordinate Analysis and MNDO Calculations: Assignment of the Vibrational Spectrum of Acridine. J. Raman Spectrosc. 1993, 24, 199–205. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary; National Center for Biotechnology Information: Bethesda, MD, USA, 2021.
- Guo, C.; Gasparian, A.V.; Zhuang, Z.; Bosykh, D.A.; Komar, A.A.; Gudkov, A.V.; Gurova, K. V 9-Aminoacridine-Based Anticancer Drugs Target the PI3K/AKT/MTOR, NF-ΚB and P53 Pathways. Oncogene 2009, 28, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zargar, P.; Ghani, E.; Mashayekhi, F.; Ramezani, A.; Eftekhar, E. Acriflavine Enhances the Antitumor Activity of the Chemotherapeutic Drug 5-fluorouracil in Colorectal Cancer Cells. Oncol. Lett. 2018, 15, 10084–10090. [Google Scholar] [CrossRef] [PubMed]
- Varakumar, P.; Rajagopal, K.; Aparna, B.; Raman, K.; Byran, G.; Gonçalves Lima, C.M.; Rashid, S.; Nafady, M.H.; Emran, T.B.; Wybraniec, S. Acridine as an Anti-Tumour Agent: A Critical Review. Molecules 2022, 28, 193. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.; Baguley, B. Dual Topoisomerase I/II Inhibitors in Cancer Therapy. Curr. Top. Med. Chem. 2003, 3, 339–353. [Google Scholar] [CrossRef]
- Yu, S.-H.; Kumar, M.; Kim, I.W.; Rimer, J.D.; Kim, T.-J. A Comparative Analysis of In Vitro Toxicity of Synthetic Zeolites on IMR-90 Human Lung Fibroblast Cells. Molecules 2021, 26, 3194. [Google Scholar] [CrossRef] [PubMed]
- Troulinaki, K.; Tavernarakis, N. Endocytosis and Intracellular Trafficking Contribute to Necrotic Neurodegeneration in C. elegans. EMBO J. 2012, 31, 654–666. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranković, M.; Jevremović, A.; Janošević Ležaić, A.; Arsenijević, A.; Rupar, J.; Dobričić, V.; Nedić Vasiljević, B.; Gavrilov, N.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Can Zeolite-Supporting Acridines Boost Their Anticancer Performance? J. Funct. Biomater. 2023, 14, 173. https://doi.org/10.3390/jfb14030173
Ranković M, Jevremović A, Janošević Ležaić A, Arsenijević A, Rupar J, Dobričić V, Nedić Vasiljević B, Gavrilov N, Bajuk-Bogdanović D, Milojević-Rakić M. Can Zeolite-Supporting Acridines Boost Their Anticancer Performance? Journal of Functional Biomaterials. 2023; 14(3):173. https://doi.org/10.3390/jfb14030173
Chicago/Turabian StyleRanković, Maja, Anka Jevremović, Aleksandra Janošević Ležaić, Aleksandar Arsenijević, Jelena Rupar, Vladimir Dobričić, Bojana Nedić Vasiljević, Nemanja Gavrilov, Danica Bajuk-Bogdanović, and Maja Milojević-Rakić. 2023. "Can Zeolite-Supporting Acridines Boost Their Anticancer Performance?" Journal of Functional Biomaterials 14, no. 3: 173. https://doi.org/10.3390/jfb14030173
APA StyleRanković, M., Jevremović, A., Janošević Ležaić, A., Arsenijević, A., Rupar, J., Dobričić, V., Nedić Vasiljević, B., Gavrilov, N., Bajuk-Bogdanović, D., & Milojević-Rakić, M. (2023). Can Zeolite-Supporting Acridines Boost Their Anticancer Performance? Journal of Functional Biomaterials, 14(3), 173. https://doi.org/10.3390/jfb14030173