Abstract
Psoriasis is a typical dermal condition that has been anticipated since prehistoric times when it was mistakenly implicit in being a variant of leprosy. It is an atypical organ-specific autoimmune disorder, which is triggered by the activation of T-cells and/or B-cells. Until now, the pathophysiology of this disease is not completely explicated and still, many research investigations are ongoing. Different approaches have been investigated to treat this dreadful skin disease using various anti-psoriatic drugs of different modes of action through smart drug-delivery systems. Nevertheless, there is no ideal therapy for a complete cure of psoriasis owing to the dearth of an ideal drug-delivery system for anti-psoriatic drugs. The conventional pharmacotherapy approaches for the treatment of psoriasis demand various classes of anti-psoriatic drugs with optimum benefit/risk ratio and insignificant untoward effects. The advancement in nanoscale drug delivery had a great impact on the establishment of a nanomedicine-based therapy for better management of psoriasis in recent times. Nanodrug carriers are exploited to design and develop nanomedicine-based therapy for psoriasis. It has a promising future in the improvement of the therapeutic efficacy of conventional anti-psoriatic drugs. The present manuscript aims to discuss the pathophysiology, conventional pharmacotherapy, and contemporary research in the area of nanoscale topical drug delivery systems for better management of psoriasis including the significance of targeted pharmacotherapy in psoriasis.
1. Introduction
Psoriasis is a non-contagious autoimmune skin disorder characterized by inflammatory conditions of reverting occurrence with erythematous scaly skin lesions and hyperkeratosis plaques [1,2,3]. Psoriasis appears on the skin as patches due to the pile-up of skin cells on the surface of the skin. The International Federation of Psoriasis Association reported that psoriasis affects 2 to 3% of the world’s population [4]. The chronic effect of psoriasis can develop psoriatic arthritis in 10–30% of the cases [4]. Psoriatic arthritis is inflammatory arthritis leading to erosions of articulate cartilage and consequently sustained inflammation results in irreversible joint destruction. The American Academy of Dermatology has classified psoriasis based on the extent of an inflammatory process, patient’s severity, and rash localization as plaque psoriasis (psoriasis vulgaris), eruptive psoriasis, pustular, and erythrodermic (exfoliative psoriasis), and inverse psoriasis or intertriginous psoriasis (illustrated in Figure 1) [2,5,6]. Around 80% of the psoriatic populations are affected by the plaque psoriasis type exhibiting itchy and painful red skin lesions with silvery scales that can be noticed on all body parts including the oral cavity and genital organs [7,8,9]. Based on the degree of psoriasis coverage extent on the body, The National Psoriasis Foundation defines psoriasis as mild, moderate, and severe where psoriasis affecting less than 3% of the body, 3% to 10% and more than 10%, respectively [7]. Moreover, the influence of psoriatic lesions on a patient’s quality of life is also used as a basis to quantify the severity of psoriasis. The abstruse impact has been experienced by moderate to severe psoriasis patients on the quality of their everyday life.
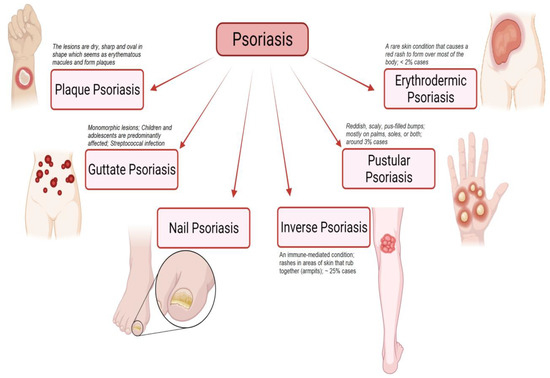
Figure 1.
Schematic illustration highlights different types of psoriasis (such as plaque psoriasis, guttate psoriasis, nail psoriasis, inverse psoriasis, pustular psoriasis, and erythrodermic psoriasis) and their characteristic features. “Image created with BioRender.com”.
The etiology of psoriasis is unclear. The under-recognized cause may be a combination of certain types of microbial infection, genetics, and or environmental factors [1,2,3,7,9]. Various non-specific triggers, like mild trauma (tattoos, piercings, and scratching), chemical irritants, and/or sunburn, may provoke psoriasis [5]. Similarly, psoriasis can be aggravated by the use of drugs such as β-blockers, lithium, antimalarial, and nonsteroidal anti-inflammatory drugs (NSAIDs) [5,10]. In the immune system, the function of T-cells is to hunt down the invading foreign organisms and provide the antibody response to keep the body healthy [1,2,3,7,9]. In psoriasis, the T-cells recognize some patches of the body’s skin as a foreign body and attack it. This attack leads to the overproduction of new T-cells, skin cells, and white blood cells [7,9]. The accumulation of dead skin cells creates hallmark scaly patches as seen in psoriatic lesions. According to the Mayo Clinic, there is a greater risk of psoriasis appearance in HIV patients, and children with frequently infected throats or other recurring infections [7]. As psoriasis often begins in the skin folds of the body, obese persons are at a higher risk. In addition, an obese person with smoking habits is also highly susceptible to psoriasis. Flare-ups may be precipitated by stress and triggered by certain drugs. These include beta-blockers, interferon, and lithium [7]. The physical and psychological stigma of psoriasis has a moderate to high impact on the life quality of psoriatic patients [11]. Approximately 60% of psoriatic patients and about 40% of psoriatic arthritis patients stated that the diseases affect their daily life psychologically and physically as well [4]. The psoriasis area and the severity index (PASI) scores are parameters for the diagnosis and can be employed to quantify the severity of the disease [5,12]. Psoriasis screening tools such as the Toronto psoriatic arthritic screening (ToPAS) questionnaire, psoriasis epidemiology screening tool (PEST), and psoriatic arthritis screening and evaluation (PASE) questionnaire can be used to aid diagnosis [5,13]. Psoriasis shows characteristic changes in skin histopathology such as epidermal acanthosis, hyperkeratosis, and parakeratosis [5]. In the dermis, the tips of dermal papillae exhibited contorted and dilated blood vessels. The most variant diagnosis of psoriasis includes tinea capitis and tinea corporis, seborrheic dermatitis, and eczema [5].
In recent years, the advancement in the nanoscale drug delivery approach had a great impact on the establishment of a nanomedicine-based therapy for better management of psoriasis. Nano drug-carrier systems are exploited to design and develop nanoscale drug delivery (nanomedicine-based therapy) to improve the therapeutic efficacy of loaded drugs in psoriasis. The present review aims to discuss the pathophysiology, current pharmacotherapy in clinical practice, and contemporary research in the area of nanoscale drug delivery systems for the better management of psoriasis including the significance of targeted pharmacotherapy in psoriasis.
2. Pathophysiology and Molecular Basis of Psoriasis
The pathophysiology of psoriasis is poorly understood [14]. Through immune system contribution, the involvement of the immune system in the eruption of psoriasis is now widely accepted [5,15,16]. The pathological advancement of psoriasis is based on a sequence of coherent events that involves the stimulation of circulating immune cells and their signaling molecules like chemokines, cytokines, and growth factors [2]. All these factors further lead to marked hyperkeratosis, congealing of the epidermis, and neovascularization. Epidermal acanthosis is the proliferation and the abnormal accelerated differentiation of keratinocytes observed in the histological examination of psoriatic skin [2,5]. In the psoriatic epidermis, keratinocytes proliferate and mature rapidly, and the terminal differentiation of squamous corneocytes is incomplete [17]. The stratum corneum (SC) of the psoriatic lesions shows hyperkeratosis and parakeratosis which are characterized by skin thickening and abnormal maturation [1,18]. Due to the abnormal maturation, SC aberrantly retains intact nuclei and releases a few extracellular lipids that typically cement the adhesion of corneocytes [17,18]. Consequently, poorly adherent SC is formed and this results in typical flakes or scales of psoriasis lesions [17,18]. Further, keratinocytes also produce vascular endothelial growth factors (VEGF), and platelet-derived growth factors (PDGF) leading to the formation and growth of blood vessels [1,19,20]. The activation of endothelial cells leads to the production of keratinocytes growth factor (KGF) which promotes keratinocytes proliferation and vasodilation of dermis capillaries, thereby granting the extravasation of circulating immune cells [21].
The interaction between keratinocytes and mononuclear leucocytes is responsible for the eruption of the psoriatic lesion [21]. Psoriasis susceptibility genes influence the gene expression program in these diverse cell types [21,22]. Keratinocytes actively participate in the recruitment and activation of leukocytes in the psoriatic lesion [21,22]. A balanced cross-talk between the activation of innate and adaptive immune cell, and different factor produced by keratinocytes directly affect the T-cells and dendritic cells (DCs) [1,2,21,22]. The relationship between the T-cells and the DCs involved in the underlying pathology of psoriasis is shown in Figure 2.
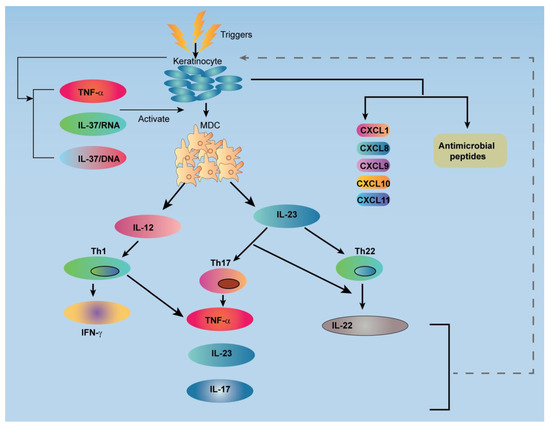
Figure 2.
Schematic illustration shows a relationship between the T-cells and the dendritic cells (DC) involved in the underlying pathology of psoriasis. TNF: Tumor Necrosis Factor; IL: Interleukin; MDC: Myeloid Dendritic Cell; Th: T-helper; IFN: Interferon; CXCL: Chemokine (C-X-C motif) Ligand.
The high level of tumor necrosis factor (TNF) and inducible nitric oxide synthase (iNOS) is expressed by CD11c+ DCs in psoriatic lesions [18,21,23,24,25]. Furthermore, CD11c+ DCs also produces cytokines IL-20 and IL-23, which in turn, potentially activate the keratinocytes and T cells, respectively [18,23,24,25]. The activation of keratinocytes by tissue necrosis factor α (TNFα) and interferon γ (INFγ) leads to the secretion of different types of cytokines (MCP-1, IL-8, CXCL1, CXCL2, CXCL3, CCL20, CXCL9, CXCL10, CXCL11), these cytokines attract many leukocytes (monocytes, neutrophils, PDCs, CCR6+, Th1, CXCR3+, Th1) from the systemic circulation to the skin area [1,17,21,22]. Further, these cytokines participate in amplifying and maintaining the inflammation response that provokes lesions. Interleukin 17 (IL-17), interleukin 22 (IL-22), and INFγ are involved in abnormal proliferation and epidermal hyperplasia [1,21,22,26]. Another important factor responsible for triggering the psoriatic lesion is the production of interferon-α (INF-α) from BDCS-2+ CD123+plasmacytoid DCs [21,27]. DC-LAMP or CD83 represent fractional maturation markers for CD11c+DCs and function as conventional DCs in terms of presenting antigens to T cells for the triggering of the acquired immune response [21,23]. Cytotoxic T cells (CD8+ T) have a great source of pro-inflammatory cytokines (INF-γ), IL-13, IL-22, and IL-17) [1,28]. IL-13 induces the expression of CCL22 in keratinocytes and matrix metalloproteinase-9 activation, leading to the migration of leukocytes into the epidermis [1,29,30].
3. Current Pharmacotherapy for the Management of Psoriasis
At present, there is no ideal pharmacotherapy for the complete cure of psoriasis. The present treatment option primarily aims to subside the symptoms and avert the disease progression and improve the patient’s quality of life. The available treatment options in the management of psoriasis include calcineurin inhibitors, vitamin D3 analogues, keratolytic agents, corticosteroids, retinoids, and biologics. The available pharmacotherapy and its applications as topical, systemic, and phototherapy in the management of psoriasis are illustrated in Figure 3.
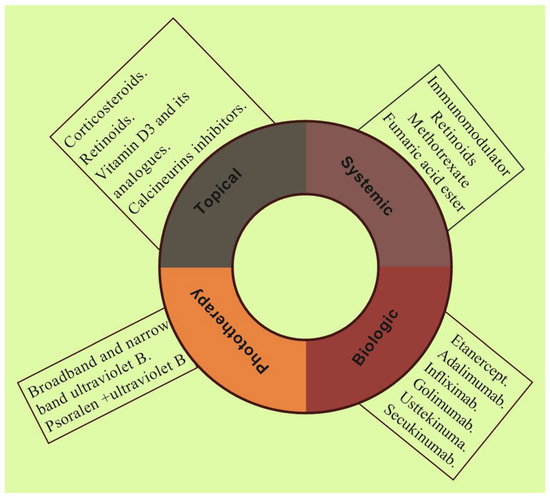
Figure 3.
Schematic illustration shows different pharmacotherapy such as topical, systemic, biologic, and phototherapy employing various therapeutics in current clinical practice for the management of psoriasis.
Further details on available drug therapy with associated characteristics and their drug action mechanism utilized in the management of psoriasis are summarized in Table 1.

Table 1.
Available pharmacotherapy for the management of psoriasis.
Combination Approach for Management of Psoriasis
Combination therapy, also called polytherapy, is the use of multiple medications or therapies in order to combat the same condition, providing low-dose, toxicity-sparing regimens of each therapeutic agent [51,52]. Different psoriasis treatments have different modes of action. Together, they can fight psoriasis on multiple fronts, and the effect is improved [7]. This approach can decrease the associated side effects and relieve the symptoms more quickly and efficiently. The combination therapies which are widely employed are methotrexate with ultraviolet B (UVB), ultraviolet A(PUVA) and UVB, acitretin with ultraviolet B (UVB) and psoralen plus ultraviolet A (PUVA), and methotrexate with cyclosporine [52]. Table 2 list the reported combination therapy for psoriasis. Another strategy for combination treatment is rotational therapy which is a practical approach to decrease the cumulative toxicity of anti-psoriasis treatments [53]. In this effort, patients are moved from one therapy to another therapy to minimize overall dosage and anticipated toxicities [54,55].

Table 2.
Available combination therapy for the management of psoriasis.
4. Challenges in Topical Delivery of Anti-Psoriasis Drugs
Drug delivery through the skin is one of the significant obstacles to obtaining the optimum therapeutic efficacy of the applied drugs. The stratum corneum (SC) is the outermost layer of the skin which acts as an active wall inhibiting the penetration of xenobiotics [82,83]. The most important factor associated with the percutaneous absorption of drugs is the degree of hydration of SC [82,83]. Rigidization of the normal skin, which is the absence of a normal moisturizing factor, is a common problem associated with psoriatic skin [83]. Various approaches have been tested to deliver different drugs through the skin. Nevertheless, topical administration of semisolid formulation faces considerable impediments because of low skin penetration, rapid degradation of actives or volatilization of a volatile compound, and photodegradation.
5. Nanoscale Topical Pharmacotherapy in Effective Treatment of Psoriasis
The nanotechnology-mediated pharmacotherapy provides propitious skin interactions as expected in psoriasis. The topical administration of nanoscale delivery systems combines the advantages of deeper skin penetration, due to increased surface area, as well as the local targeting that is illustrated in Figure 4. It involved the delivery of loaded therapeutics employing nanodrug carrier systems of polymeric, lipidic, and inorganic in nature [1]. The nanodrug carrier system prepared from the polymer is a nanoparticulate system of natural/synthetic origin and biodegradable/non-biodegradable in nature. In addition, nanodrug carrier systems prepared from lipids are nanoparticulate/nanovesicular systems of natural/synthetic lipids of biodegradable/non-biodegradable nature [2]. Furthermore, nanodrug carrier systems prepared from inorganic materials are nanoparticulate systems of metallic/non-metallic origin [1,2]. Nanoscale drug-delivery systems, such as vesicular systems (like liposomes, ethosomes, niosomes, and nanoemulsion), and nanoparticulate systems (like polymeric NPs, lipid-based NPs, and inorganic NPs) have been investigated to enhance the topical delivery of different therapies in various skin disorders including psoriasis [34]. The subsequent section discusses the various nanoscale drug-delivery (nanotechnology-mediated drug delivery) approaches utilized for the topical administration of anti-psoriatic drugs.
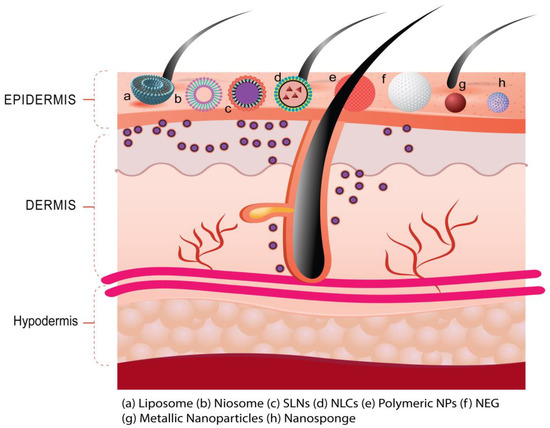
Figure 4.
Nanoscale delivery of loaded therapeutics utilizing different types of drug carrier systems (such as liposome, niosome, SLNs, NLCs, polymeric NPs, metallic NPs, etc.) for deeper skin penetration and local targeting helpful to improve the therapeutic efficacy in psoriasis.
5.1. Liposome
Out of the various available drug-delivery systems, liposomes have appeared as one of the promising drug-carrier systems for the topical administration of anti-psoriatic agents. It consists of a phospholipid bilayer surrounding an aqueous core [84]. The phospholipid bilayers of liposomes have a similar arrangement of the cellular membrane lipid composition which permits the penetration of loaded therapeutics into the epidermal skin barrier to a better extent [85].
Psoralen in combination with ultra-violate A radiation (PUVA) is an FDA-approved therapy regimen for the treatment of a severe form of recalcitrant psoriasis [86]. Doppalapudi et al. developed a psoralen-encapsulated liposomal system to improve topical efficacy and safety [86]. The designed liposomal system showed a five-fold increase in skin permeation of the psoralen liposomal system compared to the psoralen solution. The in vivo evaluation of the formulation showed a reduction in the psoriatic symptoms as well as the level of inflammatory mediators (such as TNF-α, IL-22, and IL-17) in the imiquimod-induced experimental psoriatic plaque model. In another study, Zhang et al. carried out a comparative investigation between psoralen-loaded ethosomes and liposomes for topical application in psoriasis [87]. The in vitro skin permeability profile suggested that the biopharmaceutical performance of psoralen-loaded ethosomes was better compared to the psoralen-loaded liposomes. In addition, the psoralen-loaded ethosomes exhibited better biocompatibility with human embryonic skin fibroblasts [87]. Desmet et al. investigated ‘DDC642’ as a novel liposomal carrier, capable of acting on the epidermis of impaired and intact human skin by delivering siRNA molecules, without affecting the blood capillaries or dermis. The DDC642 delivering siRNA was also tested on a psoriasis tissue model which led to the down-regulation of the human beta-defensin 2 psoriasis marker [88]. The topical antipsoriatic efficacy of mIL-4 using ultra deformable cationic liposome (UCL) applied to the K14-VEGF transgenic mouse model was reported by Li et al.; the mice treated with the above analogue displayed a mild psoriasis phenotype [89]. Furthermore, the immune histochemical analysis revealed suppression of hyperplastic and inflamed vessels [89].
Bacterial infection is one of the potential causes of the progress of chronic plaque psoriasis [84]. In this regard, Wadhwa et al. reported the formulation and evaluation of fusidic acid (FA), a steroidal antibiotic liposomal formulation. The imaging study suggested considerable cellular uptake of liposomes (loaded with fluorescent dye) [84]. Further, an in vivo study suggested better availability of the drug at a target site and improvement in the therapeutic efficacy of FA liposomal formulation in comparison to conventional formulation of FA [84]. Deformable liposomes of methotrexate entrapped in oleic acid had shown enhanced skin permeability across epidermis and dermis layers of porcine skin and exhibited a higher flux of methotrexate [90,91]. Similarly, Dubet et al. investigated the use of ethosomal nanovesicles for the transdermal delivery of methotrexate. The in vitro studies showed enhanced skin permeation and were supported by an in vivo imaging study using Rhodamine red dye [92]. The retentive nature of the formulations is also seen through some visual penetration pathways and corneocyte swelling. This highlighted the enhanced penetration profile of ethosomes through vesicle skin interaction studies [92]. Erdogan et al. investigated the delivery of liposomes-loaded tacrolimus incorporated into lotion for immune-mediated skin disorders. The radiotracer studies in a murine model showed a nine times higher concentration of tacrolimus released from the liposomal formulation at the target site compared to the systemic administration [93]. Agarwal et al. studied the liposomal/niosomal vesicular system of dithranol, an anti-psoriatic agent. The in vitro permeation studies performed on mouse abdominal skin showed a significantly improved permeation from the liposomal formulation [94,95]. Knudsen et al. investigated liposomal formulations of calcipotriol. The liposome was fabricated using lipo-polymer poly(ethylene glycol)-distearoyl phosphoethanolamine (PEG-DSPE). The study was performed to examine the protective effect of liposome stabilization on the physicochemical properties and penetration ability. The results showed a correlation between the size of the liposomes and the lipid components and the penetration ability. The smaller the unilamellar vesicles the higher the penetration rate. This suggests that the intact liposomes may penetrate through the skin with respect to the size of the vesicles. Calcipotriol showed a deeper penetration compared to the lipid components suggesting a release of a small amount of the drug from the liposomal formulation during the skin migration [96]. Jain et al. reported the topical delivery of thymoquinone through the liposphere for psoriasis treatment [97]. In vitro studies exhibited a reduction in nitric oxide level and inflammatory cytokines (TNF-α, IL-1β, IL-2, IL-6); whereas, in vivo studies supported a decreased level of TNF-α and IL-17 in psoriatic skin indicating improved efficacy in psoriasis [97]. Walunj et al. formulated cationic liposomal nanocarriers of cyclosporine (Cs) using cholesterol and 1, 2-dioleoyl-3-trimethylammonium-propane (Cs liposomal gels) for topical application and evaluated it in an imiquimod-induced experimental model of the psoriatic plaque. The reduction in psoriasis symptoms and levels of psoriatic cytokines (such as TNF-α, IL-17, and IL-22) were reduced after the topical application of the developed formulation system in the psoriatic plaque model [98]. Wang et al. successfully prepared flexible liposomes co-loaded with transretinoic acid and betamethasone and found that application in gel form can reduce the thickness of the epidermis, alleviating the symptoms of psoriasis by reducing TNF-α and IL-6 levels [99]. Saka et al. prepared a liposome gel formulation of bexarotene (retinoid X receptor agonist, and approved for anticancer activity) and the anti-psoriatic effect was evaluated in an imiquimod-induced experimental model of psoriasis in BALB/c mice. The outcome of the results suggested a reduction in scaling and inflammation including recovery from psoriasis without posing any toxic effects [100]. Further, cytokine level and histopathological analysis supported the outcomes.
Tacrolimus, an immunosuppressive agent and 10-fold more potent than cyclosporine A (CsA), has been suggested for the management of psoriasis; however, poor cutaneous bioavailability limits its use. Jindal et al. proposed the incorporation of tacrolimus in a lipid nanocarrier system, loading them in a hydrogel base for topical delivery [101]. Therefore, the proposed nanoformulation would efficiently target skin tissues and mitigate psoriatic symptoms with minimal adverse effects. Thus, such a hypothesized strategy would be considered a valued addition to the current psoriasis management strategies [101].
Clinical Investigations on Liposome-Based Pharmacotherapy
The efficacy of topical CsA in the form of lipogel was assessed in limited chronic plaque psoriasis by Kumar et al. in a single-center randomized clinical trial with 38 patients [102]. The first set consists of 24 patients treated with either cyclosporine lipogel (2.0% w/w), or placebo lipogel in a randomized way [102]. The second set comprised seven patients treated with cyclosporine lipogel (2.0% w/w), or conventional cyclosporine cream (2.0% w/w). In the third set, seven patients were randomly administered cyclosporine lipogel (2.0% w/w) or clobetasol propionate cream (0.05% w/w). Patients were assessed for dermatological sum score (DSS) twice weekly for 14 weeks, or until total clearance was observed. The dermatological sum score showed a decrease of 19% in 59% of psoriasis sites after 2 weeks of treatment with cyclosporine lipogel [102]. After eight weeks, a prominent decrease (nearly 83%) was recorded on all sites treated with cyclosporine lipogel [102]. A randomized, double-blind, study was performed by using a solvent-controlled novel liposomal formulation of dithranol in psoriasis by Saraswat et al. [103]. Around twenty patients were selected with symmetrical stable bilateral plaque psoriasis and treated with 0.5% dithranol lipogel to lesions on one side of the body [103]. On the other hand, around ten patients were treated with liposomal base randomly and further ten patients were treated with a conventional cream (containing 1.15% salicylic acid, 1.15% dithranol, and 5.3% coal tar) for 6 weeks period with a short contact regimen of 30 min [103]. Patients were assessed at baseline, 2, 4, and 6 weeks for disease severity, by observing perilesional erythema and skin staining, pruritus, and possible side effects. The clinical investigation revealed that patients treated with lipogel formulation exhibited a markedly low incidence of skin staining and perilesional erythema compared to conventional cream [103]. Agarwal et al. reported preliminary observations of a novel liposomal formulation containing dithranol in treating stable plaque psoriasis. The formulations were evaluated for their efficacy, tolerability, and cosmetic acceptability [95]. In a prospective, open-label trial, nine patients with nineteen psoriatic plaques were treated for six weeks. In this clinical study, five patients showed no lesions or perilesional irritation with complete lesion clearance, one patient exhibited more than 50% clearance and only one patient showed faint brown skin staining, which was completely reversed quickly [95]. Recently Fathalla et al. designed and developed an anthralin-loaded liposomal gel and ethosomal gel formulation to evaluate its clinical efficacy in 44-year-old male patients [104]. It was observed that both the developed formulations were helpful to minimize the psoriatic sign and symptoms after 3 weeks of treatment very effectively as shown in Figure 5.
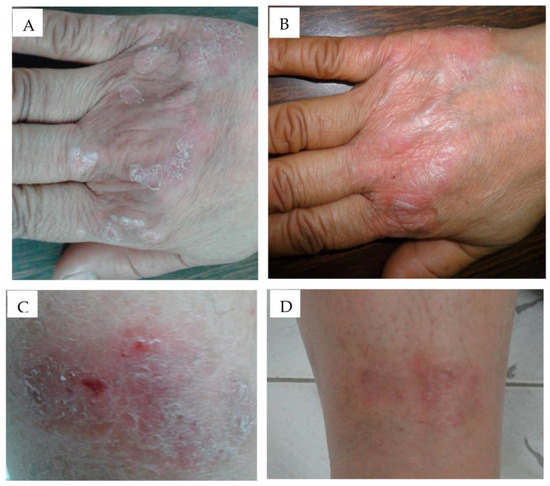
Figure 5.
Application of anthralin-loaded liposomal gel (A) before application and (B) after 3 weeks application and ethosomal gel formulation, (C) before application, (D) after 3 weeks application has shown significant improvement in psoriatic signs and symptoms in a clinical investigation. Reproduced from [104], MDPI, 2020.
5.2. Niosomes
Niosomes are non-ionic surfactant-based unilamellar or multilamellar vesicular drug-delivery systems containing a central compartment of an aqueous solution of solute surrounded by a bilayer membrane resulting from the organization of surfactant macromolecules [105]. Niosomes are considered to be a promising system for the topical delivery of therapeutics due to their biocompatibility, non-immunogenicity, and other outstanding features such as enhanced drug penetration abilities, sustained drug release, improved drug stability and capabilities to carry lipophilic as a well hydrophilic drug [106].
Moghddam et al. developed the diacerein-entrapped niosomes formulated by the incorporation of cholesterol for the management of psoriasis [107]. The confocal laser scanning microscopic studies revealed an enhanced penetration of diacerein in the epidermal and dermal skin layers of rats [107]. This study provides a way for targeting and enhanced drug delivery through the topical application of niosomes which, in turn, reduces the adverse effects and toxicity associated with systemic administration [107]. In another study, the formulation of nanosponge and niosomes incorporated gel (NSG and NMG, respectively) containing tazarotene was demonstrated by Aggarwal et al. [108]. Their results revealed that NSG and NMG gel formulations reduce the side effects associated with conventional gel formulations and marketed formulations and improve the bioavailability of tazarotene. The prepared NSG and NMG gel formulations showed increased skin retention and local accumulation efficiency (LAE) [108]. Gupta et al. investigated the protective effect of capsaicin (CAP)-loaded niosomes, liposomes, and emulsomes for the controlled local delivery of drugs for psoriasis treatment [109]. The in vitro and in vivo studies showed prominent enhancement in skin retention of CAP from emulgel formulation [109]. Finally, their results suggested that emulgel is a plausible approach for the topical application of CAP, to achieve an effective treatment for psoriasis [109]. Parnami et al. explored the antipsoriatic activity of methotrexate and CsA in niosomal formulations for antipsoriatic activity associated with narrow and broad-band UV radiation [110]. The prepared formulations exhibited increased drug accumulation and decreased adverse effects associated with systemic delivery [110]. Bracke et al. explored the formulation of DEFB4-siRNA-containing SECosomes for topical delivery. The targeted delivery to Human β defensin-2 (hBD-2) is achieved by using a bioengineered skin-humanized mouse model for psoriasis which showed an improved psoriatic phenotype leading to normal skin structure [111]. The study revealed a filaggrin expression with the retrieval of trans-glutaminase activity and recovery of stratum corneum similar to normal human skin regeneration [111]. Bhatia et al. formulated the Tamoxifen (TAM) loaded flexible membrane vesicles (FMVs) and pluronic lecithinized organogels (PLOs) [112]. The antipsoriatic studies performed on mice tail models showed significantly higher efficacy of TAM-FMV gel and TAM-PLO compare to the conventional TAM-hydrogel [112]. Ammonium glycyrrhizinate niosomes as a potential nano-vesicular system for psoriasis treatment employing murine models was proposed by Marianecci et al. [113]. Their results suggested that drug-loaded niosomes shows reduced edema and nociception in comparison to placebo niosomes and drug alone [113]. Meng et al. developed a novel topical niosomes loaded with celastrol and, through an in vivo study, suggested its efficacy in alleviating psoriasis symptoms (erythema and scaling) in a mouse model of psoriasis [114]. Further, spleen size and the cytokines levels (IL-22, IL-23, and IL-17) were also decreased after noisome treatment, supporting their promising therapeutic prospects in psoriasis [114]. Al-Mahallawi et al. incorporate methotrexate (MTX) into ultra-permeable niosomal vesicles and their optimized formula demonstrated improved drug permeation and a significant deposition of MTX in rat dorsal skin, while histopathological evaluation confirmed the tolerability of the optimized formula in rats upon topical application [115].
Clinical Investigations on Niosomes-Based Pharmacotherapy
Lakshmi et al. conducted a double-blind placebo-controlled study and accessed the efficacy of niosomal MTX in chitosan gel on psoriasis patients [116]. Skin irritation and sensitivity are tested by the insult patch test (HRIPT) [116]. The psoriasis lesion area and severity index scoring method was used to calculate the efficacy. The niosomal methotrexate gel formulations exhibited an enhanced efficacy in comparison to placebo and marketed gel formulations [116]. Moreover, a similar approach to demonstrate the efficacy of a novel sustained release niosomal chitosan-MTX formulation in comparison to placebo gel and a plain MTX gel for different types of psoriasis treatment, especially palmoplantar psoriasis was attributed by Lakshmi et al. [117]. The treatment results showed a marked improvement in the lesions treated with niosomal chitosan-MTX formulation in comparison to plain MTX and placebo gel even though applied twice a day. The study suggests that the novel formulation did not exert any systemic toxicity and showed improved therapeutic potential without altering biochemical parameters [117]. The various preclinical/clinical investigations utilized lipid-based vesicular systems (such as liposome, ethosome, and noisome) to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in the management of psoriasis are summarized in Table 3.

Table 3.
Lipid-based vesicular system (Liposome, ethosome, and noisome) utilized to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in psoriasis.
5.3. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) have materialized as a substitute for liposomal drug-delivery systems owing to their several advantages such as enhanced physical stability, reasonably inexpensive compared to phospholipids, and simple manufacturing technique [121,122,123]. Furthermore, their potential in controlled drug delivery [121,122], topical drug delivery in epidermal targeting [124,125], and stability of active pharmaceutical ingredients have been very well established and are more or equivalent to liposomes [121]. An SLNs-loaded topical gel containing Betamethasone dipropionate and Calcipotriol delayed the abrupt growth of keratinocytes in the HaCaT cell line [126]. Furthermore, in comparison to the marketed Daivobet® ointment, the formulated SLN gel exhibited reduced epidermal depth and improved melanocyte count in an experimental mouse tail model of psoriasis [126]. Bikkad et al. reported the preparation and characterization of Halobetasol propionate (HP)-loaded SLNs gel and improved drug deposition avoiding systemic uptake and acting as a probable carrier for local delivery with reduced side effects. The ex vivo studies showed continued drug release for 12 h and the in vitro drug deposition study exhibited that HP-SLN formulations exhibited better drug accumulation [127]. Moreover, the skin irritation studies of HP loaded SLNs gel suggested better safety and tolerability as compared to commercial products [127]. Similarly, Madan et al. reported that SLNs loaded with Mometasone furoate are a potential tool for skin targeting over the conventional formulations of corticosteroid drugs [128]. Triamcinolone acetonide (TA) loaded SLNs presented extended drug release following Higuchi release kinetics. Skin distribution studies of TA-loaded SLNs demonstrated improved drug accumulation without its systemic uptake [129]. Agarwal et al. demonstrated the ability of SLN and nanostructured lipid carriers (NLCs) for skin targeting capsaicin. NLCs and SLNs proved to be potential carriers for improved local delivery exhibiting better drug accumulation in different layers of the skin [130]. SLNs loaded with dithranol were formulated by Gambhire et al. They reported that the incorporation of dithranol in SLNs reduced the rate of drug degradation [131]. Kim et al. demonstrated SLN loaded with Cyclosporin A (CsA) [132]. In vitro permeation studies of CsA-SLN showed a two-fold higher skin permeation efficiency compared to a CsA-oil mixture in viable skin [132]. Moreover, the in vivo studies in a mouse model exhibited reduced symptoms of atopic dermatitis by decreasing cytokines interleukin (IL)-4 and -5 of T helper (Th) 2 cells [132]. Shah et al. investigated the viability of tretinoin (TRE) loaded SLN gel for improved transdermal delivery of TRE [121]. TRE-loaded SLN showed significantly improved photostability [121]. Furthermore, it was also reported that SLN-based TRE gel showed decreased skin irritation compared to commercial TRE cream [121]. Similarly, Ridolfi et al. reported TRE-loaded chitosan-SLN loaded to provide a dual effect which is the anti-bacterial activity of chitosan combined with the effect of TRE for topical treatment of psoriasis [133]. The local delivery of isotretinoin from SLN (IT-SLN) was evaluated by Liu et al. [125]. The in vitro permeation studies showed no systemic uptake of isotretinoin in skins and augmented skin targeting effect by all the IT-SLN loaded formulations [125]. Trombino et al. prepared SLN, based on trehalose monooleate, loaded with cyclosporin-A for possible management of psoriasis [134]. The optimized formulation indicates the possibility of using such nanocarriers of cyclosporin-A for topical application in psoriasis, diminishing possible adverse effects due to the expected systemic translocation of cyclosporin-A and, meanwhile, augmenting its concentration at skin lesions [134].
5.4. Nanostructured Lipid Carrier
NLCs are modified unstructured-matric of SLN, i.e., consist of both solid and liquid lipid as a core matrix [135,136]. The assimilation of liquid lipids with a solid matrix of NPs facilitates the incorporation of an increased amount of drug and results in enhanced solubility, improved stability, increased permeability, and bioavailability, and most important the prolonged t1/2 and targeted drug delivery [135,136,137,138,139,140]. Furthermore, close contact of NLCs with stratum corneum and smaller particle size results in enhanced drug flux through the skin. On the other hand, the presence of solidified lipid matrix results in the controlled release of therapeutic ingredients [135,136,137,138,139,140,141]. Moreover, it has been also reported that NLCs exhibit occlusive properties and significantly increase skin hydration, and minimize transdermal water loss [141].
Agarwal et al. reported the formulation and evaluation of Acitretin-loaded NLCs. The prepared formulations exhibited higher skin deposition compared to plain acitretin gel in human cadaver skin. Moreover, the better efficacy and reduced local side effects exhibited by acitretin NLCs loaded gel in clinical studies make it a potential carrier for the topical administration of drugs in psoriasis [142]. Pinto et al. reported MTX loaded NLCs show higher skin penetration in comparison to free MTX, this signifies the role of nanocarriers in topical drug delivery [143]. Similarly, MTX-loaded NLCs (MTX-NLC) nanogel formulations showed a decreased PASI score recovering mice’s skin to normal level [144]. The combined effect of calcipotriol with MTX significantly strengthens the topical treatment of psoriasis [145]. Lin et al. proposed NLCs loaded with both lipophilic and hydrophilic drugs to demonstrate the topical delivery of Calcipotriol and MTX, respectively [145]. The skin permeation studies of NLCs loaded with dual drugs exhibited decreased skin penetration by calcipotriol in comparison to MTX [145]. The results of in vitro and in vivo studies examined by confocal laser scanning microscopy (CLSM) exhibited a good correlation [145]. Dual drug-loaded NLCs showed improved drug permeation and decreased skin irritation making it a suitable carrier system for the local administration of antipsoriatic drugs [145]. Tripathi et al. investigated and reported the influence of the carrier system on drug release and drug deposition efficiency through a carbomer gel containing MTX-loaded NLC and MTX-loaded SLN for topical administration of MTX in the management of psoriasis [146]. The skin permeation studies showed extended drug release of up to 24 h from both the carrier systems loaded with MTX through hydrogels [146]. However, among the two carrier systems, NLC hydrogels exhibited an improved skin drug deposition (28.8%) compare to SLN hydrogel (18.6%) and plain drug-loaded hydrogel (11.4%) [146]. A modified nano lipid carrier (MNLC) for topical delivery of tacrolimus was developed by Pople et al. [147]. Tacrolimus-loaded MNLC (T-MNLC) gels exhibited significantly enhanced skin penetration, and bioavailability in comparison to marketed preparation [147]. Furthermore, skin irritation studies also showed a reduced level of skin irritation from T-MNLC compared to marketed products [147]. Similarly, Thapa et al. reported the formulation and characterization of Tacrolimus-loaded liquid crystalline nanoparticles. The liquid crystalline nanoparticles resulted in extended drug release for up to two weeks and showed enhanced storage stability of Tacrolimus from nanoparticles [148]. Pradhan et al. demonstrated the Fluocinolone acetonide (FA) loaded NLCs for improvement in skin permeation efficiency of the drug in the treatment of psoriasis. In vitro skin distribution studies from NLCs and a plain suspension containing FA showed relatively better drug penetration NLCs in the skin layers [36]. The skin permeating potential of polymeric micelles loaded with TAC was demonstrated by Lapteva et al. The skin deposition studies revealed enhanced drug permeation and deposition into dermal/epidermal layers through polymeric micelles compared to conventional ointment [149]. The cutaneous distribution profile of TAC exhibited an enhanced level of TAC in the upper layer of the skin due to augmented TAC deposition in the stratum corneum, with no increased deposition in the lower deeper layers of the skin [149]. The permeation studies revealed no deeper skin deposition of the drug; thus, site targeting can be achieved with decreased adverse effects [149]. Furthermore, CLSM of the fair follicles also showed drug deposition in the follicles acting as a potential system for skin targeting with no systemic uptake and reduced side effects [149]. The first time the synergic combination was reported was by Viegas et al. by formulating a multifunctional NLC to co-administer tacrolimus and TNF-α siRNA for psoriasis treatment by targeting TNF-α [150]. The outcome of the experimental study demonstrated the efficiency of the multifunctional NLC, by reducing TNF-α expression about seven-fold and exhibiting the synergic effect of tacrolimus and TNF-α siRNA [150]. The developed system was successful in limiting the symptoms of disease in an imiquimod-induced experimental model of psoriasis. NLCs loaded with curcumin and caffeine were formulated by Iriventi et al. through a hot homogenization and ultrasonication approach and added to topical gel have provided a continued release of the drug up to 12 h [151]. An in vivo efficacy study was carried out in an imiquimod-induced mouse model of psoriasis [151] and the resulting outcome suggested improved efficacy of curcumin and caffeine in psoriasis [151]. Qadir et al. developed an NLC gel of two potential herbal agents, Smilax china and Salix alba, for safe and effective topical therapy against psoriasis [152]. During the in vivo study, they expressed a non-irritant profile of the developed system and better PASI score, and better therapeutic efficacy as compared to the marketed preparation [152]. Sharma et al. develop Squalene integrated NLC gel of tamoxifen citrate to enhance the lipid and water content of skin and to create a local depot in the skin for better efficacy in psoriasis. The outcome of the in vivo study suggested better efficacy in dampening PASI scores, pro-inflammatory cytokines level, and reinstating splenomegaly and other histological alterations to normal [153]. The clinical pertinence of this stable gel ensured its longer stay (owing to semisolid viscosity) over the skin, augmented skin hydration (due to hydrophilic gelling polymer) and lipid content (owing to the natural skin lipid composition), and better skin retention of drug and overall better efficacy in psoriasis [153]. Rapalli et al. prepared curcumin-loaded NLC to improve the penetration of curcumin (poorly soluble) onto a topical layer of skin for the management of psoriasis and Acne vulgaris [154]. An in vitro release study of Curcumin-NLC suggested an extended-release profile up to 48 h; whereas, plain curcumin released 100% drug within 4 h. Further, the ex-vivo permeation study of curcumin NLC gel exhibited more than three-fold higher drug permeation and retention in comparison to free curcumin gel [154]. The various preclinical investigations utilized lipid-based nanoparticulate systems (SLN, NLC) to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in the management of psoriasis are summarized in Table 4.

Table 4.
Lipid-based nanoparticulate system (SLN, NLC) utilized to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in psoriasis.
5.5. Polymeric Nanoparticles
In the last few decades, polymeric nanoparticles (PNPs) have attracted the great attention of formulation scientists because of their unique properties and performance due to their small size [156,157,158]. PNPs with readily tunable physicochemical properties can effectively stabilize unstable drugs, minimize their adverse effect, control the release of drugs, and potentiate the penetration of drugs across the skin barrier [159].
The therapeutic potential of Dead Sea Water (DSW) for skin diseases s have been well established [160]. DSW enriched with minerals (Ca, Mg, Na, K, Zn, and Sr) are known to utilize anti-inflammatory effects and enhance skin barrier recovery [160]. The formulation and evaluation of DSW-based PNPs were reported by Dessy et al. The resulting NPs show a time-regulated drug release and a better formulation yield [160]. Mao et al. designed the skin-permeating PNPs in a silk fibroin hydrogel-based matrix to simplify the administration of curcumin deeper in skin layers. Curcumin-NPs incorporated in silk fibroin hydrogel (CUR-NPs-gel) exhibited a steady release of curcumin as compared to CUR-gel, without affecting the skin penetration ability of CUR-NPs [161]. In in vivo studies, using an imiquimod-induced psoriatic mice model, treatment with CUR-NPs-gel exhibited a better therapeutic profile than CUR-NPs which previously demonstrated greater skin-permeation profile and efficacious in the anti-keratinization process [161]. Thus, the CUR-NPs-gel resulted in the inhibition of inflammatory cytokines expression (TNF-α, NF-κB, and IL-6) [161]. Similarly, Bilia et al. reported that CUR-PNPs potentiate the protective efficacy to act in moderate-to-severe psoriasis patients and regulate serum cholesterol levels [162]. Shah et al. developed an effective skin permeating nanogel system (SPN) for the cutaneous co-delivery of two anti-inflammatory drugs (spantide II: SP; and ketoprofen: KP) [163]. An in vitro permeation study exhibited enhanced accumulation of SP for the SP-SPN or SP+KP-SPN in the epidermis was better than SP-gel [163]. Further, the accumulation of KP for KP-SPN or SP+KP-SPN in the epidermis and dermis was better than KP-gel [163]. Simultaneously, KP permeation in KP-SPN or SP+KP-SPN was enhanced more than nine-fold in comparison to KP-gel. The thickness of the ear in the ACD model, PASI score, and IL-17 and IL-23 expression were significantly attenuated in SPN treatment in comparison to normal gel [163]. Pukale et al. reported a scalable and stable monolithic lipid–polymer hybrid nanoparticles (LPNs) of clobetasol propionate (CP) consisting of a combination of solid/liquid lipids along with amphiphilic copolymer, mPEG-PLA [164]. The prepared nanoparticles were encapsulated in the hydrophobic core of LPNs and the clobetasol-loaded LPNs (CP/LPNs) were prepared in a dermal hydrogel using carbopol 974P [164]. CP/LPNs gel exhibited a controlled in vitro drug release for 7 days with no abrupt release [164]. Further, topical administration of CP/LPNs gel on an experimental model of psoriasis-like inflammation exhibited minimal systemic penetration of clobetasol [164]. An in vivo efficacy assessment study of CP/LPNs gel resulted in prominent improvement in PASI score, and attenuation of skin damage in comparison to commercialized products (Clobetamos™) [164]. Overall, improved cellular uptake, better skin penetration with augmented dermal retention, and improved therapeutic efficacy demonstrate the promising features of these LPNs for further clinical investigations [164]. The topical delivery of dithranol through dendritic nanocarriers was reported by Agarwal et al. [165]. The polypropylene imine (PPI) dendrimers for topical administration of dithranol (DIT) were evaluated for skin deposition, encapsulation efficiency, and skin irritation [165]. The studies exhibited significantly improved permeation rate constant and decreased skin irritation with DIT-PPI compare to plain DIT [165]. The biodistribution studies of dye-loaded dendrimers revealed by confocal laser scanning microscope images exhibited enhanced drug accumulation in the epidermal and dermal layers posing it as a potential carrier system for skin targeting for psoriasis treatment [165]. The various preclinical investigations utilized polymeric nanoparticulate systems to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in the management of psoriasis are summarized in Table 5.

Table 5.
Polymeric nanoparticulate system utilized to improve the biopharmaceutical attributes/therapeutic efficacy of encapsulated drugs/actives in psoriasis.
5.6. Nanoemulsion/Microemulsion-Based System
Nanoemulgel (NEG) is an assimilated formulation of two diverse systems in which drug-loaded nanoemulsion (NE) is incorporated into a gel base [172]. NEG has been established as a promising alternative drug-delivery option with enhanced permeability and bioavailability of lipophilic drugs [173]. The amalgamation of the two different systems makes it beneficial in numerous ways, such as easy incorporation of lipophilic drugs and enhanced skin permeability of incorporated drugs [172], an enhanced retention time of drug at the targeted area, and conclusively, results in maximum therapeutic potential with less side effect [174,175].
Divya et al. reported the formulation and evaluation of topical NEG formulation of two anti-psoriatic drugs (acitretin: Act and Aloe-emodin: AE) using chitin [176]. The ex vivo study suggested enhanced dermal drug deposition [176]. The in vivo anti-psoriatic study, and skin safety study revealed the plausible therapeutic benefit of topical administration of Act and AE in psoriasis [176]. Ourique et al. reported the preparation of TRE-loaded LPN in hydrogels, which demonstrated a lower photodegradation than non-encapsulated drugs [177]. The lag time was augmented two-fold with nanoencapsulation, indicating enhanced drug retention on the skin surface [177]. The development and optimization of CP and calcipotriol (CT) NEG for the topical management of psoriasis were reported by Kaur et al. [178]. An in vitro study, using HaCaT cell lines, elicited a better uptake of the drug with enhanced penetration in stratum corneum and viable layers from NEG as compared to free drugs [178]. Imiquimod-induced experimental psoriatic BALB/c mice model suggested an improved anti-psoriatic effect of NEG in comparison to free drugs or commercialized products [178]. A comparative therapeutic efficacy study of clobetasol-loaded chitin nanogel (CLCNEG) and marketed cream were performed by Panonnummal et al. [179] and a significant anti-inflammatory effect was observed via inhibition of COX and LOX activities. Further, an in vivo anti-psoriatic study using an imiquimod-induced experimental model of psoriasis revealed the potential therapeutic benefits of NEG through topical administration of clobetasol in psoriasis [179]. Similarly, Alam et al. carried out an in vivo study of clobetasol propionate-loaded nanoemulsion through topical treatment in psoriasis and atopic dermatitis [180]. The clobetasol propionate-loaded nanoemulsion significantly elevated lymphocytic nucleoside triphosphate phosphohydrolase activity, an enzyme responsible for extracellular ATP degradation and responsible for cell differentiation, proliferation, and inflammatory cascade [180]. Baboota et al. investigated the potential of the nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for the management of psoriasis [181]. They reported enhanced drug deposition beneath the skin and improved anti-inflammatory activity against psoriasis [181]. MTX-loaded chitin nanogel (MCNEGs) in the imiquimod-induced experimental psoriatic model resulted in significant anti-psoriatic efficacy without any dermal and systemic toxicity [182]. MCNEGs have shown prominent anti-inflammatory properties via inhibition of COX-2 and LOX-5 enzyme expression in THP-1 cells [182]. Similarly improved topical delivery and anti-inflammatory activity of MTX from a nanogel were studied by Singka et al. They reported that the increased concentration gradient, high flux, and reduced prostaglandin E (2) (PGE2) synthesis is in an ex vivo study [183]. Curcumin-loaded PLGA nanoparticles (Cur-NPs) in hydrogel were prepared and delivered topically to treat imiquimod-induced psoriasis [184]. In vitro skin permeation studies revealed better drug permeation through the skin when administered as the Cur-NPs-loaded hydrogel in comparison to normal drug suspension-loaded hydrogel [184]. Similarly, Cur-NPs-loaded hydrogel showed improved anti-proliferative activity than Cur solution in the cell line study [184]. Khurana et al. prepared resveratrol-loaded polymeric micelles (PM) and converted them into gel (PMG) and in vivo efficacy was evaluated in an imiquimod-induced psoriatic-like plaque model [185]. The in vivo study represented the prominent efficacy of PMG (as apparent through reduced PASI score, cytokines levels, splenomegaly, and hyperkeratosis) in comparison to conventional gel [185]. Li et al. formulated a cyclosporine-loaded Pluronic® F127 stabilized reduced graphene oxide hydrogel to enhance permeation and retention of cyclosporine in the skin for better therapeutic efficacy in psoriasis [186]. They reported the ability of the reduced graphene oxide nanocarrier to the betterment of cyclosporine permeation, and retention in dermal layers could be used for better psoriasis management, with minimal adverse effects observed through oral/systemic routes [186]. Song et al. loaded calcipotriol in exo-polysaccharides/calcipotriol (EPS/CPT) emulsion, sunflower oil, and calcipotriol as the loaded drug, and the therapeutic effect was evaluated [187]. The in vitro and in vivo studies expressed better efficacy for the management of psoriasis vulgaris by increasing the aggregation of CPT in psoriatic lesions and attenuating pro-inflammatory cytokine levels [187].
Shinde et al. aimed to develop the mometasone furoate vesicle containing aspasomal gel for the management of psoriasis [188]. The prepared gel exhibited a longer duration of drug release and thus required a lower dosing frequency. Therefore, such approaches are capable of providing a rational future approach for the management of psoriasis [188]. Dadwal et al. prepared clobetasol propionate-loaded squarticles (nanoemulgel) for the management of psoriasis by providing better skin permeation and enhancing its therapeutic efficacy [189]. Erol et al. prepared tazarotene-loaded in situ gels using thermosensitive poloxamers via the cold method for the potential management of psoriasis [190]. The in vitro study, using LPS-stimulated RAW264.7 murine macrophage cells suggested a reduction in nitric oxide and PGE2 levels and supported its anti-inflammatory and analgesic activities. The 10% tazarotene in situ gels have shown better efficacy. Thus, it may act as a potentially safer alternative for tazarotene-based topical formulation for the management of psoriasis [190]. Recently, Guo et al. designed and developed a Salvianolic acid B-loaded microemulsion system to evaluate its preclinical efficacy in imiquimod-induced psoriasis-like dermatitis in mice models [191]. It was observed that the developed formulations were helpful to minimize the sign and symptoms of psoriasis-like dermatitis in mice after 6 days of treatment very effectively as shown in Figure 6.
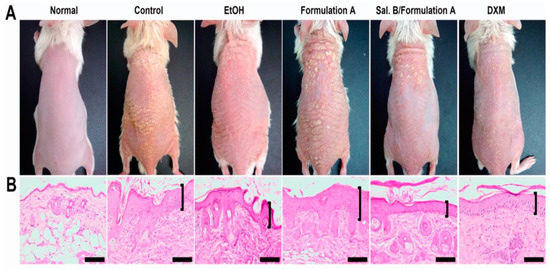
Figure 6.
(A) Salvianolic acid B loaded microemulsion system (Sal. B/formulation A) and 0.25% desoximetasone marketed ointment (DXM) improved the sign and symptoms in psoriasis-like dermatitis compared to another treatment group (control group: IMQ-induced psoriatic mice without any treatment; EtOH group: treatment with ethanol in IMQ-induced psoriatic mice; formulation A group: treatment with microemulsion base/vehicle only in IMQ-induced psoriatic mice) after 6 days of treatment. (B) The Sal. B/formulation A and DXM treatment groups exhibited fewer severe clinical and pathological features (epidermal acanthosis, parakeratosis, tortuous capillary dilatation in papillary dermis, and inflammatory cell infiltration) compared to other groups (control group; EtOH group; formulation A group) in a histopathological investigation. Scale bar = 100 μm. Reproduced from [191], MDPI, 2020.
5.7. Metallic Nanoparticles
Metallic nanoparticles (MNPs) have fascinated researchers for over a decade and are now greatly utilized for biomedical applications [192]. MNPs usually have wide utility in biomedical applications including drug-delivery vehicles in various disease conditions with good biocompatibility, affluent functionality, and controlled release of therapeutic agents [192,193]. In recent years, two-dimensional (2D) materials of metallic origin like nanosheets are also employed as a smart drug-delivery system in different disease conditions [194,195]. It has been reported that MNPs show great advantages not only as anti-aging and anti-acne treatments, and skincare products but also for the management of various skin disorders including psoriasis [196].
Gold nanoparticles (GNPs) appeared as a preferred choice for the topical application system of different drugs. Taking into the above consideration Bessar et al. reported the development and evaluation of MTX-loaded water-soluble GNP activated with sodium 3-mercapto-1-propane sulfonate (Au-3MPS) [197]. The results of in vitro studies suggested that MTX conjugated with Au-3MPS is more efficacious than MTX alone. Cerra et al. highlighted the interaction between functionalized GNP and MTX at a molecular level [198]. Monodisperse GNP was prepared by a chemical method using hydrophilic a functionalizing agent (thiol 3-mercapto-1-propane sulfonate) [198]. They suggested such a delivery system would potentiate drug efficacy and help in deciphering pharmaco-dynamic/kinetic properties for the development of effective drug-delivery systems for psoriasis [198]. Recently, Han et al. designed and developed alkyl-terminated GNP as a self-therapeutic system to treat psoriasis [199]. It was observed that the developed system is helpful to minimize the sign and symptoms of imiquimod-induced psoriasis in mice by down-regulating the interleukin-17 signaling pathway. It effectively crosses the stratum corneum and enters into keratinocytes easily due to the small dimension (<15 nm) of the developed system. In another investigation, Fereig et al. designed and developed a self-assembled hybrid system of GNP to treat psoriasis [200]. The developed formulation system was prepared by hybridization of GNP with lecithin-chitosan nanoparticles. It was observed that the developed tacrolimus-loaded system is helpful to minimize the inflammatory markers in the imiquimod-induced mouse model with a significant anti-psoriatic effect.
In another investigation, silver nanoparticles (AgNPs) using European black elderberry (Sambucus nigra, Adoxaceae family) fruit extracts were prepared and assessed for anti-psoriatic activity on HaCaT cells exposed to UVB radiation, on an acute inflammation model [201]. Their results exhibited anti-inflammatory properties by reducing inflammatory cytokines generation through UVB irradiation [201]. Further, Lai et al., in an investigation, explored the risk of applying zinc oxide nanoparticles (ZnONPs) to treat psoriasis [202]. It was observed that ZnONPs delay the recovery of psoriasis-like skin lesions by promoting inflammation and keratinocyte apoptosis via the nuclear translocation of phosphorylated NF-κB p65 and cysteine deficiency. The findings of the current investigation reveal that the application of ZnONPs as a topical drug-delivery vehicle is not desirable for pathological skin, although it has been utilized after percutaneous administration in other disease conditions.
6. Targeted Pharmacotherapy in the Management of Psoriasis
Available pharmacotherapy in the management of psoriasis may be categorized into three classes dermal therapy, phototherapy, and systemic therapy. Phototherapy is normally advised in cases when the topical application is not effective. After phototherapy, systemic therapy is prescribed for better results. The limitations of phototherapy and systemic therapy are associated with various prominent adverse effects, including hepatic/nephrotoxicity, hypertension, hyperlipidemia, and skin cancer. The adverse events associated with other than dermal therapy, made topical pharmacotherapy still the preferred therapy in the management of psoriasis. We know that the currently available pharmacotherapy based on conventional dosage forms is associated with frequent dosing, chances of side effects, and the possibility of toxic events due to continuous therapy for a longer duration in the management of psoriasis [1,2].
The recent approach of targeted pharmacotherapy therapy in the management of psoriasis has advantages over conventional therapy [203,204]. It appears safe and effective in the delivery of anti-psoriatic drugs and maintains the concentration of the drug locally to better treat psoriasis. If the drug is targeted, the dose requirement is less, which eventually reduces the possibility of systemic adverse effects even though the therapy is topical. In addition, topical applications of drugs have limited release, degradation of drug, and poor efficacy due to the barrier of the stratum corneum layer. Further, the targeted formulation provides a prolonged duration of action, reduction in dosing frequency, and overall improves patient compliance. The targeting of receptors of psoriasis may also be helpful in phototherapy and systemic therapy by molecular recognition, facilitating drug formulation to specifically interact with pathogenic molecular targets [205]. Two approaches (passive and active) are commonly exploited in targeted pharmacotherapy. If the drug is accumulated in a specific site of a targeted area due to its biophysical properties, this type of targeting is called passive targeting. An example of passive targeting is the site-specific delivery of anti-psoriatic therapeutics at nanoscale dimension and their differential distribution in skin region is based on the size of the therapeutics-loaded particles [104]. That is why in this targeting approach, the delivery of the therapeutic system is manipulated at nanoscale dimension employing nanodrug carrier systems (Illustrated in Figure 4 and Figure 5). Active targeting, which is also considered molecular or biological targeting, may utilize physical forces such as electroporation, sonoporation, and magnetism including the conjugation of targeting ligands to target cells to produce therapeutic effects at the disease site. The ligand-mediated targeting can be achieved by fixing the target-specific peptides or target-specific antibodies attached to the drug-delivery system [206,207]. Targeted pharmacotherapy in the management of psoriasis is a promising strategy to deliver the loaded therapeutics to specific cells in the skin, which emerges from a combination of inflammatory, and immune responses in psoriatic skin. The recent investigation highlights the significance of targeted drug delivery of methotrexate utilizing hyaluronic acid-based albumin nanoparticles to deliver the loaded therapeutics in lymph nodes to suppress the immune cells of the acquired immune system in psoriasis through microneedle patch delivery (illustrated in Figure 7) [171].
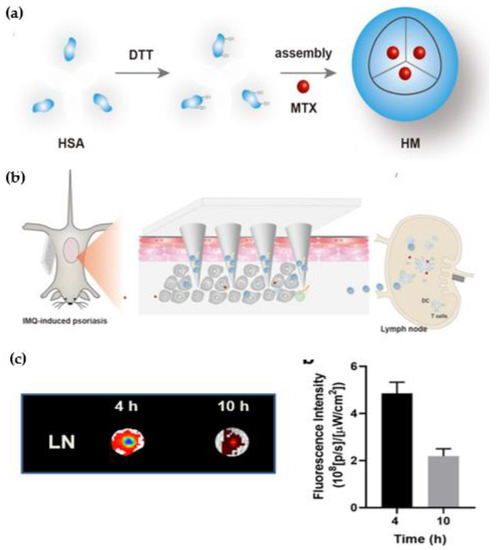
Figure 7.
Schematic illustration shows concept of targeted drug delivery in psoriasis. (a) Synthesis of hyaluronic acid-based albumin nanoparticles by hydrophobic interactions between human serum albumin and methotrexate therapeutic. (b) Delivery of methotrexate utilizing hyaluronic acid-based albumin nanoparticles for effective inhibition of dermatitis. Active targeting of developed formulation system helpful to deliver therapeutics in subcutaneous tissue and further accumulated in lymph nodes suppress the immune cells and prevent the trigger of pro-inflammatory cytokines secretion to improve the progression of psoriasis utilizing microneedle patch delivery system. (c) Fluorescence images of lymph nodes shows fluorescent intensities from the lymph nodes after administration of developed targeted formulation system at 4h and 10h. HAS: Human Serum Albumin; MTX: Methotrexate; HM: Hyaluronic-acid based microneedle patch; DC: Dendritic Cells; LN: Lymph nodes. Reproduced from Wang H et al. [171], Dove Medical Press, 2022.
6.1. Targeting through Serotonergic Receptors
Various researchers have reported the involvement of various serotonergic receptors in psoriasis [208,209,210,211]. Laberge et al. reported that 5-HT1AR is involved in the inhibition of inflammation [208] while Morita et al. reported the involvement of 5-HT7R in itching [209]. Weisshaar et al. reported the presence of 5-HT3R while Nordlind et al. suggested the presence of 5-HT1AR and 5-HT2AR expression in psoriatic skin [210,211]. The differential expression of 5-HT1A and more 5-HT2A receptors were reported [212] in the structures of psoriatic skin in an immune-histochemical investigation and suggested the possibility of targeting through serotonergic receptors could be a promising approach in the management of psoriasis.
6.2. Targeting through Dopamine Receptors
Dopamine receptor D1 (DRD1) has been reported for its anti-proliferative potential by binding to DRD1 on activated T cells and killing by causing the minimal death of resting normal human T-cells [213,214]. Fenoldopam mesylate, 6-chloro-2, 3, 4, 5-tetrahydro-l- (4- hydroxyphenyl)–1H-3-benzazepine-7, 8-diol, methane sulfonate (FD, a highly selective DRD1 agonist) therapeutic efficacy in psoriasis has been suggested by several researchers [86,119,215]. Doppalapudi et al. formulated the water-washable ointment and glycerin-based carbopol anhydrous gel of FD [216]. The developed formulation system was evaluated in the imiquimod-induced psoriasis model and suggested its utility as targeted pharmacotherapy to suppress the inflammatory condition in psoriasis [216].
6.3. Targeting through microRNAs (miRNA-Based Targeting)
It has been reported potential role of microRNA (miRNA) in the management of psoriasis opened the way for miRNA-based targeted pharmacotherapy in psoriasis. Pradyuth et al. suggested that the miRNA expression could be minimized as a potential biomarker to suppress the hyper-proliferation of keratinocytes and the production of inflammatory cytokine in the management of psoriasis [217]. The schematic illustration in Figure 8 highlights the delivery of miRNA through micelles/liposomes carrier system following the paracellular/transcellular route of absorption in skin and down-regulated epidermal homeostasis to achieve the goal of targeted pharmacotherapy through the inhibition of keratinocyte proliferation and inhibition of pro-inflammatory cytokine secretion.
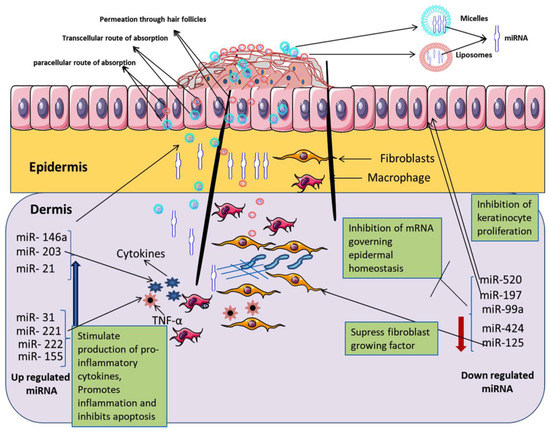
Figure 8.
Schematic illustration shows liposomes and micelles mediated targeted topical pharmacotherapy employing miRNA to decrease the pathological conditions over the skin in psoriasis. Reproduced from Pradyuth Sai et al. [217], John Wiley and Sons, 2020.
6.4. Targeting through CD44 Receptors
Lindqvist et al., in their investigation, described that the epidermis in psoriatic skin is overexpressed of CD44 receptor proteins and observed a significant reduction in hyaluronic acid distribution [218]. This receptor protein can be specifically targeted to enhance the efficacy of pharmacotherapy in psoriasis. Hyaluronic acid is a natural ligand for CD44 receptor proteins and Lv et al. reported the utilization of hyaluronic acid in targeted pharmacotherapy to improve the targeting of CD44 receptor proteins ultimately leading the drug deposition in disease sites and helping to minimize the untoward effects [219]. In another investigation, Yongtai Zhang et al. formulated curcumin-loaded hyaluronic acid-modified ethosomes to deliver the curcumin to the targeted site in psoriasis through CD44 receptor-mediated drug delivery [220]. The developed formulation system (HA-ES) was evaluated to observe the anti-psoriatic efficacy in an imiquimod-induced mouse model and compared the therapeutic efficacy of the non-targeted ethosomes system [220]. They reported that the HA-ES system effectively binds with CD44 receptor protein (abundant in inflamed psoriatic skin) for targeted delivery, thereby enhancing the accumulation of curcumin in inflamed psoriatic skin for better therapeutic efficacy compared to non-targeted ethosomes system (illustrated in Figure 9) [220].
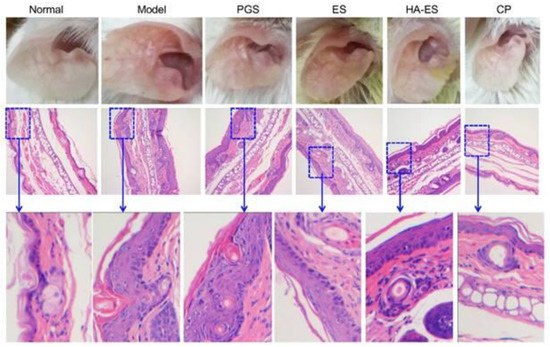
Figure 9.
Illustration shows utilization of hyaluronic acid-modified ethosomes (HA-ES) for the delivery of curcumin by active targeting approach to the targeted site in psoriasis through CD44 receptor-mediated drug-delivery approach ultimately helpful to improve the therapeutic efficacy of curcumin compared to non-targeted ethosomes system (ES). Normal: No treatment; Model: Treated with imiquimod only to induce psoriasis; PGS: Treated with curcumin in propylene glycol solution; ES: Treated with curcumin-loaded ethosomes; HA-ES: Treated with curcumin-loaded hyaluronic-modified ethosomes; CP: Treated with clobetasol propionate marketed cream. Reproduced from Zhang Y et al. [220], Ivyspring International Publisher, 2019.
7. Conclusive Remarks and Future Directions
The choice of therapy for the better management of psoriasis depends on several factors, such as disease severity, its consequence on a patient’s life, and the patient’s acuity for their disease. In terms of severity, there are three commonly employed therapeutic approaches in clinical practice, i.e., local therapy, phototherapy, and systemic therapy for the treatment of psoriasis. However, none of the currently employed therapeutic strategies are found to be potentially effective, safe, and able to completely cure psoriasis. Nowadays, antipsoriatic medicine prescribed in clinical practice is effective only to prevent the progression of the disease. The dearth of potentially effective and safer therapy for psoriasis has urged the need to design and develop nanomedicine-based therapy to make the treatment more promising and acceptable at a clinical level.
Nanotechnology-mediated drug delivery system (NDDS) plays a crucial role in the precise delivery of a variety of therapeutics including anti-psoriatic drugs. Nanomedicine is a relatively new era of nanobiotechnology; irrespective of that, the possibilities of this NDDS for pharmacotherapy seem endless. It has improved the therapeutic potential of drugs by reducing drug toxicity and/or enhancing the benefit/risk ratio by employing drug carriers-mediated delivery of loaded therapeutics including anti-psoriatic drugs. These novel intelligent systems can exploit the potency of therapeutics for psoriasis in great ways since they have the potential to promptly detect and respond to disease directly, without any impairment to a healthy cell or tissue and thereby improving the quality of a patient’s life. This novel approach is proficient in assuring the site-specific delivery of therapeutics across the skin to cure psoriatic lesions. Improved skin deposition with a targeting efficiency particularly in the epidermal/dermal layer is helpful in the success of nanomedicine. These smart delivery systems allow the administration of low doses and less dosing frequency for the same therapeutics compared to conventional drug delivery systems. In addition, the utilization of nanoscale topical pharmacotherapy for the delivery of biologics (illustrated in Figure 10) in combination with other small molecule therapeutics for targeting biomolecules to suppress the inflammatory mediators and activated immune response for better management of psoriasis is another promising strategy to achieve the aim for delivery of anti-psoriatic therapeutics for targeted pharmacotherapy.
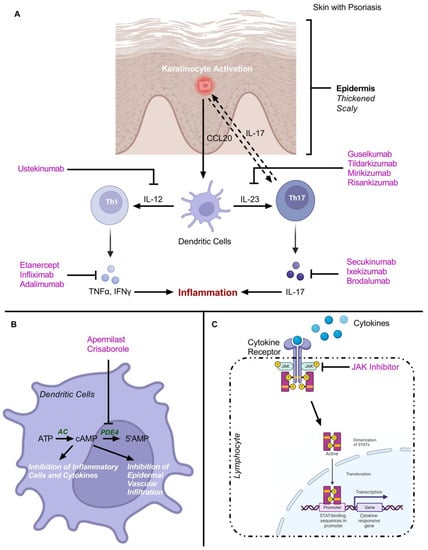
Figure 10.
Schematic illustration highlights utilization of different biologics and small molecule therapeutics for targeting biomolecules to suppress inflammatory mediators and activated immune response for better management of psoriasis. (A) Different Biologics for cytokines inhibition. (B) Small molecule therapeutics as phosphodiesterase-4 (PDE-4) inhibitor. (C) Small molecule therapeutics as Janus kinases (JAK) inhibitors. Modified from Rapalli VK et al. [205], Elsevier, 2018. “Image created with BioRender.com”.
However, the strategy for its assessment and control of the interaction of nanomaterial in in vivo systems are some of the obstacles in translating these intelligent drug delivery systems to clinical practice. Targeting specific psoriatic lesions while circumventing the major organs (such as the spleen and liver) are major challenges that need to be addressed for its clinical translation. Clinical trials in this area should be bourgeoned to delineate the place of nanomedicine in the management of psoriasis. Extensive research and advancement in characterizing tools to determine the clinical safety and efficacy of nanomedicine-based therapy including nanoscale targeted pharmacotherapy for the management of psoriasis may become a reality in near future.
Author Contributions
M.Z.A.: writing—original draft preparation, investigation, formal analysis, and validation; funding acquisition, and project administration. A.A.M.: writing—original draft preparation, investigation, formal analysis, and validation; M.S.A.: writing—review and editing, investigation, formal analysis, and validation; A.M.: writing—review and editing, investigation, formal analysis, and validation; J.A.: conceptualization, writing—review and editing, supervision, funding acquisition, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Najran University, Saudi Arabia, under the National Research Priorities funding program grant code (NU/NRP/MRC/11/5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work under the National Research Priorities funding program grant code (NU/NRP/MRC/11/5).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Act | Acitrein |
| AE | Aloe-emodin |
| AgNPs | Silver nanoparticles |
| CLSM | Confocal laser scanning microscopy |
| Cs | Cyclosporine |
| CT | Calcipotriol |
| DCs | Dendritic cells |
| DDS | Drug delivery systems |
| DSW | Dead Sea Water |
| GNPs | Gold nanoparticles |
| hBD-2 | Human β defensin-2. |
| IL | Interleukin |
| IT | Isotretinoin |
| KGF | keratinocytes growth factor. |
| MNPs | Metallic nanoparticles. |
| MTX | Methotrexate. |
| NEG | Nanoemulgel |
| NLCs | Nanostructured Lipid Carriers. |
| NPs | Nanoparticles |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PASI | Psoriasis area and the severity index |
| PDGF | Platelet-derived growth factor |
| PM | Polymeric micelles |
| PNPs | Polymeric nanoparticles |
| TAM | Tamoxifen |
| Th cell | T helper cell |
| TNF | Tumor necrosis factor |
| ToPAS | Toronto psoriatic arthritic screening |
| TRE | Tretinoin |
| UCL | Ultra deformable cationic liposome |
| ZnONPs | Zinc oxide nanoparticles |
References
- Sala, M.; Elaissari, A.; Fessi, H. Advances in psoriasis physiopathology and treatments: Up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J. Control. Release 2016, 239, 182–202. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J. Control. Release 2013, 170, 380–395. [Google Scholar] [CrossRef]
- Mabuchi, T.; Chang, T.W.; Quinter, S.; Hwang, S.T. Chemokine receptors in the pathogenesis and therapy of psoriasis. J. Dermatol. Sci. 2012, 65, 4–11. [Google Scholar] [CrossRef]
- Global Report on Psoriasis. Available online: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf.psoriasis?sequence=1 (accessed on 30 October 2022).
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Aijaz, S.F.; Khan, S.J.; Azim, F.; Shakeel, C.S.; Hassan, U. Deep Learning Application for Effective Classification of Different Types of Psoriasis. J. Healthc. Eng. 2022, 2022, 7541583. [Google Scholar] [CrossRef]
- Facts and Statistics about Psoriasis. Available online: https://www.healthline.com/health/psoriasis/facts-statistics-infographic (accessed on 21 August 2022).
- American Academy of Dermatology. Psoriasis Resource Center. Available online: https://www.aad.org/public/diseases/scaly-skin/psoriasis (accessed on 1 November 2022).
- Menter, A.; Gottlieb, A.; Feldman, S.R.; Van Voorhees, A.S.; Leonardi, C.L.; Gordon, K.B.; Lebwohl, M.; Koo, J.Y.; Elmets, C.A.; Korman, N.J.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2008, 58, 826–850. [Google Scholar] [CrossRef]
- Basavaraj, K.H.; Ashok, N.M.; Rashmi, R.; Praveen, T.K. The role of drugs in the induction and/or exacerbation of psoriasis. Int. J. Dermatol. 2010, 49, 1351–1361. [Google Scholar] [CrossRef]
- Reich, K.; Kruger, K.; Mossner, R.; Augustin, M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: A prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br. J. Dermatol. 2009, 160, 1040–1047. [Google Scholar] [CrossRef]
- Schmitt, J.; Wozel, G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005, 210, 194–199. [Google Scholar] [CrossRef]
- Coates, L.C.; Aslam, T.; Al Balushi, F.; Burden, A.D.; Burden-Teh, E.; Caperon, A.R.; Cerio, R.; Chattopadhyay, C.; Chinoy, H.; Goodfield, M.J.; et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br. J. Dermatol. 2013, 168, 802–807. [Google Scholar] [CrossRef]
- Cuesta-Montero, L.; Belinchon, I. Connective tissue diseases and psoriasis. Actas Dermosifiliogr. 2011, 102, 487–497. [Google Scholar] [CrossRef]
- Kim, J.; Krueger, J.G. The immunopathogenesis of psoriasis. Dermatol. Clin. 2015, 33, 13–23. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Qin, J.Z.; Nestle, F.O. Immunopathogenesis of psoriasis. Clin. Rev. Allergy Immunol. 2007, 33, 45–56. [Google Scholar] [CrossRef]
- Krueger, J.G.; Bowcock, A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii30–ii36. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Viac, J.; Palacio, S.; Schmitt, D.; Claudy, A. Expression of vascular endothelial growth factor in normal epidermis, epithelial tumors and cultured keratinocytes. Arch. Dermatol. Res. 1997, 289, 158–163. [Google Scholar] [CrossRef]
- Ansel, J.C.; Tiesman, J.P.; Olerud, J.E.; Krueger, J.G.; Krane, J.F.; Tara, D.C.; Shipley, G.D.; Gilbertson, D.; Usui, M.L.; Hart, C.E. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J. Clin. Investig. 1993, 92, 671–678. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Krueger, J.G. Getting under the skin: The immunogenetics of psoriasis. Nat. Rev. Immunol. 2005, 5, 699–711. [Google Scholar] [CrossRef]
- Lowes, M.A.; Chamian, F.; Abello, M.V.; Fuentes-Duculan, J.; Lin, S.L.; Nussbaum, R.; Novitskaya, I.; Carbonaro, H.; Cardinale, I.; Kikuchi, T.; et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA 2005, 102, 19057–19062. [Google Scholar] [CrossRef]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lee, E.; Lowes, M.A.; Haider, A.S.; Fuentes-Duculan, J.; Abello, M.V.; Chamian, F.; Cardinale, I.; Krueger, J.G. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: Gene regulation and cellular effects. J. Investig. Dermatol. 2006, 126, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.G. The immunologic basis for the treatment of psoriasis with new biologic agents. J. Am. Acad. Dermatol. 2002, 46, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.; Clark, R.A. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef]
- Purwar, R.; Kraus, M.; Werfel, T.; Wittmann, M. Modulation of keratinocyte-derived MMP-9 by IL-13: A possible role for the pathogenesis of epidermal inflammation. J. Investig. Dermatol. 2008, 128, 59–66. [Google Scholar] [CrossRef]
- Purwar, R.; Werfel, T.; Wittmann, M. IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: Possible role in atopic dermatitis. J. Investig. Dermatol. 2006, 126, 1043–1051. [Google Scholar] [CrossRef]
- Cather, J.C.; Abramovits, W.; Menter, A. Cyclosporine and tacrolimus in dermatology. Dermatol. Clin. 2001, 19, 119–137. [Google Scholar] [CrossRef]
- Antignac, M.; Barrou, B.; Farinotti, R.; Lechat, P.; Urien, S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br. J. Clin. Pharmacol. 2007, 64, 750–757. [Google Scholar] [CrossRef]
- Stuetz, A.; Grassberger, M.; Meingassner, J.G. Pimecrolimus (Elidel, SDZ ASM 981)—Preclinical pharmacologic profile and skin selectivity. Semin. Cutan. Med. Surg. 2001, 20, 233–241. [Google Scholar] [CrossRef]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J. Am. Acad. Dermatol. 2009, 60, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, C.O.; Burden, A.D. Current concepts in psoriasis and its treatment. Pharmacol. Ther. 2003, 99, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Singh, D.; Murthy, S.N.; Singh, M.R. Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids 2015, 101, 56–63. [Google Scholar] [CrossRef]
- Kragballe, K. Treatment of psoriasis with calcipotriol and other vitamin D analogues. J. Am. Acad. Dermatol. 1992, 27, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Federman, D.G.; Froelich, C.W.; Kirsner, R.S. Topical psoriasis therapy. Am. Fam. Physician 1999, 59, 957–962. [Google Scholar]
- Huber, C.; Christophers, E. “Keratolytic” effect of salicylic acid. Arch. Dermatol. Res. 1977, 257, 293–297. [Google Scholar] [CrossRef]
- Muller, K.H.; Pflugshaupt, C. Urea in dermatology I. Hautarzt 1989, 40 (Suppl. S9), 14–15. [Google Scholar]
- Mohandas, S.; Rai, R.; Srinivas, C.R. Halobetasol versus clobetasol: A study of potency. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 186–187. [Google Scholar]
- Fenton, C.; PLoSker, G.L. Calcipotriol/betamethasone dipropionate: A review of its use in the treatment of psoriasis vulgaris. Am. J. Clin. Dermatol. 2004, 5, 463–478. [Google Scholar] [CrossRef]
- Reid, D.C.; Kimball, A.B. Clobetasol propionate foam in the treatment of psoriasis. Expert Opin. Pharmacother. 2005, 6, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Pitocco, R.; Saraceno, R.; Nistico, S.P.; Giunta, A.; Chimenti, S. New topical treatments for psoriasis. Expert Opin. Pharmacother. 2014, 15, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Laws, P.M.; Young, H.S. Topical treatment of psoriasis. Expert Opin. Pharmacother. 2010, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Crim, C.; Pierre, L.N.; Daley-Yates, P.T. A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate. Clin. Ther. 2001, 23, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Pauporte, M.; Maibach, H.; Lowe, N.; Pugliese, M.; Friedman, D.J.; Mendelsohn, H.; Cargill, I.; Ramirez, R. Fluocinolone acetonide topical oil for scalp psoriasis. J. Dermatol. Treat. 2004, 15, 360–364. [Google Scholar] [CrossRef]
- Fowler, J.; Fowler, L. Physician and Patient Assessment of Triamcinolone Acetonide Spray for Steroid-responsive Dermatoses. J. Clin. Aesthet. Dermatol. 2010, 3, 27–31. [Google Scholar]
- Naldi, L.; Yawalkar, N.; Kaszuba, A.; Ortonne, J.P.; Morelli, P.; Rovati, S.; Mautone, G. Efficacy and safety of the Betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: A randomized, parallel-group, active-controlled, phase III study. Am. J. Clin. Dermatol. 2011, 12, 191–201. [Google Scholar] [CrossRef]
- OncoSec. What Is Combination Therapy? Available online: https://oncosec.com/2013/09/what-is-combination-therapy/ (accessed on 30 October 2022).
- Lebwohl, M.; Menter, A.; Koo, J.; Feldman, S.R. Combination therapy to treat moderate to severe psoriasis. J. Am. Acad. Dermatol. 2004, 50, 416–430. [Google Scholar] [CrossRef]
- van de Kerkhof, P.C. Therapeutic strategies: Rotational therapy and combinations. Clin. Exp. Dermatol. 2001, 26, 356–361. [Google Scholar] [CrossRef]
- Menter, M.A.; See, J.A.; Amend, W.J.; Ellis, C.N.; Krueger, G.G.; Lebwohl, M.; Morison, W.L.; Prystowsky, J.H.; Roenigk, H.H., Jr.; Shupack, J.L.; et al. Proceedings of the Psoriasis Combination and Rotation Therapy Conference. Deer Valley, Utah, 7–9 October 1994. J. Am. Acad. Dermatol. 1996, 34, 315–321. [Google Scholar] [CrossRef]
- Weinstein, G.D.; White, G.M. An approach to the treatment of moderate to severe psoriasis with rotational therapy. J. Am. Acad. Dermatol. 1993, 28, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P.; Kaufmann, R.; Lecha, M.; Goodfield, M. Efficacy of treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol for the treatment of psoriasis vulgaris: A randomised, double-blind trial. Dermatology 2004, 209, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Guenther, L.; Boyden, B.; Larsen, F.G.; Harvima, R.J.; Guilhou, J.J.; Kaufmann, R.; Rogers, S.; van de Kerkhof, P.C.; Hanssen, L.I.; et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J. Am. Acad. Dermatol. 2003, 48, 48–54. [Google Scholar] [CrossRef]
- Ruzicka, T.; Lorenz, B. Comparison of calcipotriol monotherapy and a combination of calcipotriol and betamethasone valerate after 2 weeks’ treatment with calcipotriol in the topical therapy of psoriasis vulgaris: A multicentre, double-blind, randomized study. Br. J. Dermatol. 1998, 138, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Austad, J.; Bjerke, J.R.; Gjertsen, B.T.; Helland, S.; Livden, J.K.; Morken, T.; Mork, N.J. Clobetasol propionate followed by calcipotriol is superior to calcipotriol alone in topical treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 19–24. [Google Scholar] [CrossRef]
- Katoh, N.; Kishimoto, S. Combination of calcipotriol and clobetasol propionate as a premixed ointment for the treatment of psoriasis. Eur. J. Dermatol. 2003, 13, 382–384. [Google Scholar]
- Salmhofer, W.; Maier, H.; Soyer, H.P.; Honigsmann, H.; Hodl, S. Double-blind, placebo-controlled, randomized, right-left study comparing calcipotriol monotherapy with a combined treatment of calcipotriol and diflucortolone valerate in chronic plaque psoriasis. Acta Derm. Venereol. Suppl. 2000, 80, 5–8. [Google Scholar] [CrossRef][Green Version]
- Rim, J.H.; Park, J.Y.; Choe, Y.B.; Youn, J.I. The efficacy of calcipotriol + acitretin combination therapy for psoriasis: Comparison with acitretin monotherapy. Am. J. Clin. Dermatol. 2003, 4, 507–510. [Google Scholar] [CrossRef]
- van de Kerkhof, P.C.; Cambazard, F.; Hutchinson, P.E.; Haneke, E.; Wong, E.; Souteyrand, P.; Damstra, R.J.; Combemale, P.; Neumann, M.H.; Chalmers, R.J.; et al. The effect of addition of calcipotriol ointment (50 micrograms/g) to acitretin therapy in psoriasis. Br. J. Dermatol. 1998, 138, 84–89. [Google Scholar] [CrossRef]
- Grossman, R.M.; Thivolet, J.; Claudy, A.; Souteyrand, P.; Guilhou, J.J.; Thomas, P.; Amblard, P.; Belaich, S.; de Belilovsky, C.; de la Brassinne, M.; et al. A novel therapeutic approach to psoriasis with combination calcipotriol ointment and very low-dose cyclosporine: Results of a multicenter placebo-controlled study. J. Am. Acad. Dermatol. 1994, 31, 68–74. [Google Scholar] [CrossRef]
- de Jong, E.M.; Mork, N.J.; Seijger, M.M.; De La Brassine, M.; Lauharanta, J.; Jansen, C.T.; Guilhou, J.J.; Guillot, B.; Ostrojic, A.; Souteyrand, P.; et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: Results of a multicentre placebo-controlled randomized trial. Br. J. Dermatol. 2003, 148, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Even-Chen, Z.; Lipets, I.; Pritulo, O.A.; Svyatenko, T.V.; Andrashko, Y.; Lebwohl, M.; Gottlieb, A. Pilot, multicenter, double-blind, randomized placebo-controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. J. Am. Acad. Dermatol. 2010, 63, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Guenther, L.C.; Poulin, Y.P.; Pariser, D.M. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clin. Ther. 2000, 22, 1225–1238. [Google Scholar] [CrossRef]
- Green, L.; Sadoff, W. A clinical evaluation of tazarotene 0.1% gel, with and without a high- or mid-high-potency corticosteroid, in patients with stable plaque psoriasis. J. Cutan. Med. Surg. 2002, 6, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tiplica, G.S.; Salavastru, C.M. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 905–912. [Google Scholar] [CrossRef] [PubMed]
- van der Rhee, H.J.; Tijssen, J.G.; Herrmann, W.A.; Waterman, A.H.; Polano, M.K. Combined treatment of psoriasis with a new aromatic retinoid (Tigason) in low dosage orally and triamcinolone acetonide cream topically: A double-blind trial. Br. J. Dermatol. 1980, 102, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Ortonne, J.P.; Chimenti, S.; Ghislain, P.D.; Arenberger, P.; Kragballe, K.; Saurat, J.H.; Khemis, A.; Sprogel, P.; Esslinger, H.U.; et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: The BELIEVE study. Br. J. Dermatol. 2010, 163, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Prystowsky, J.H.; Bourget, T.; Edelstein, J.; Nychay, S.; Armstrong, R. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J. Am. Acad. Dermatol. 1991, 24, 591–594. [Google Scholar] [CrossRef]
- Lauharanta, J.; Juvakoski, T.; Lassus, A. A clinical evaluation of the effects of an aromatic retinoid (Tigason), combination of retinoid and PUVA, and PUVA alone in severe psoriasis. Br. J. Dermatol. 1981, 104, 325–332. [Google Scholar] [CrossRef]
- Green, C.; Lakshmipathi, T.; Johnson, B.E.; Ferguson, J. A comparison of the efficacy and relapse rates of narrowband UVB (TL-01) monotherapy vs. etretinate (re-TL-01) vs. etretinate-PUVA (re-PUVA) in the treatment of psoriasis patients. Br. J. Dermatol. 1992, 127, 5–9. [Google Scholar] [CrossRef]
- Mahajan, R.; Kaur, I.; Kanwar, A. Methotrexate/narrowband UVB phototherapy combination vs. narrowband UVB phototherapy in the treatment of chronic plaque-type psoriasis—A randomized single-blinded placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Roussaki-Schulze, A.V.; Kouskoukis, C.; Klimi, E.; Zafiriou, E.; Galanos, A.; Rallis, E. Calcipotriol monotherapy versus calcipotriol plus UVA1 versus calcipotriol plus narrow-band UVB in the treatment of psoriasis. Drugs Exp. Clin. Res. 2005, 31, 169–174. [Google Scholar] [PubMed]
- Torras, H.; Aliaga, A.; Lopez-Estebaranz, J.L.; Hernandez, I.; Gardeazabal, J.; Quintanilla, E.; Mascaro, J.M. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J. Dermatol. Treat. 2004, 15, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Jacobe, H.; Winterfield, L.; Kim, F.; Huet-Adams, B.; Cayce, R. The role of narrowband UV-B plus alefacept combination therapy in the treatment of psoriasis. Arch. Dermatol. 2008, 144, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Kowalzick, L.; Christophers, E.; Schill, W.B.; Schopf, E.; Stander, M.; Wolff, H.H.; Altmeyer, P. Calcitriol 3 microg g-1 ointment in combination with ultraviolet B phototherapy for the treatment of plaque psoriasis: Results of a comparative study. Br. J. Dermatol. 2001, 144, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.K.; McKenna, K.E. Combination TL01 ultraviolet B phototherapy and topical calcipotriol for psoriasis: A prospective randomized placebo-controlled clinical trial. Br. J. Dermatol. 2003, 149, 146–150. [Google Scholar] [CrossRef]
- Ramsay, C.A.; Schwartz, B.E.; Lowson, D.; Papp, K.; Bolduc, A.; Gilbert, M. Calcipotriol cream combined with twice weekly broad-band UVB phototherapy: A safe, effective and UVB-sparing antipsoriatric combination treatment. The Canadian Calcipotriol and UVB Study Group. Dermatology 2000, 200, 17–24. [Google Scholar] [CrossRef]
- Morganti, P.; Ruocco, E.; Wolf, R.; Ruocco, V. Percutaneous absorption and delivery systems3. Clin. Dermatol. 2001, 19, 489–501. [Google Scholar] [CrossRef]
- Katare, O.P.; Raza, K.; Singh, B.; Dogra, S. Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 612–621. [Google Scholar] [CrossRef]
- Wadhwa, S.; Singh, B.; Sharma, G.; Raza, K.; Katare, O.P. Liposomal fusidic acid as a potential delivery system: A new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016, 23, 1204–1213. [Google Scholar] [CrossRef]
- Du Plessis, J.; Egbaria, K.; Weiner, N. Influence of formulation factors on the deposition of liposomal components into the different strata of the skin. Soc. Cosmet. Chem. 1992, 43, 93–100. [Google Scholar]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Desmet, E.; Bracke, S.; Forier, K.; Taevernier, L.; Stuart, M.C.; De Spiegeleer, B.; Raemdonck, K.; Van Gele, M.; Lambert, J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int. J. Pharm. 2016, 500, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhang, Y.; Zhou, X.K.; Yang, H.S.; Chen, X.C.; Wang, Y.S.; Wei, Y.Q.; Chen, L.J.; Hu, H.Z.; et al. Gene therapy for psoriasis in the K14-VEGF transgenic mouse model by topical transdermal delivery of interleukin-4 using ultradeformable cationic liposome. J. Gene Med. 2010, 12, 481–490. [Google Scholar] [CrossRef]
- Trotta, M.; Peira, E.; Carlotti, M.E.; Gallarate, M. Deformable liposomes for dermal administration of methotrexate. Int. J. Pharm. 2004, 270, 119–125. [Google Scholar] [CrossRef]
- Srisuk, P.; Thongnopnua, P.; Raktanonchai, U.; Kanokpanont, S. Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int. J. Pharm. 2012, 427, 426–434. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef]
- Erdogan, M.; Wright, J.R., Jr.; McAlister, V.C. Liposomal tacrolimus lotion as a novel topical agent for treatment of immune-mediated skin disorders: Experimental studies in a murine model. Br. J. Dermatol. 2002, 146, 964–967. [Google Scholar] [CrossRef]
- Agarwal, R.; Katare, O.P.; Vyas, S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Agarwal, R.; Saraswat, A.; Kaur, I.; Katare, O.P.; Kumar, B. A novelliposomal formulation of dithranol for psoriasis: Preliminary results. J. Dermatol. 2002, 29, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.O.; Ronholt, S.; Salte, R.D.; Jorgensen, L.; Thormann, T.; Basse, L.H.; Hansen, J.; Frokjaer, S.; Foged, C. Calcipotriol delivery into the skin with PEGylated liposomes. Eur. J. Pharm. Biopharm. 2012, 81, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017, 13, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Walunj, M.; Doppalapudi, S.; Bulbake, U.; Khan, W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J. Liposome Res. 2020, 30, 68–79. [Google Scholar] [CrossRef]
- Wang, W.; Shu, G.F.; Lu, K.J.; Xu, X.L.; Sun, M.C.; Qi, J.; Huang, Q.L.; Tan, W.Q.; Du, Y.Z. Flexible liposomal gel dual-loaded with all-trans retinoic acid and betamethasone for enhanced therapeutic efficiency of psoriasis. J. Nanobiotechnol. 2020, 18, 80. [Google Scholar] [CrossRef]
- Saka, R.; Jain, H.; Kommineni, N.; Chella, N.; Khan, W. Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. J. Drug Deliv. Sci. Technol. 2020, 58, 101691. [Google Scholar] [CrossRef]
- Jindal, S.; Awasthi, R.; Singhare, D.; Kulkarni, G.T. Topical delivery of Tacrolimus using liposome containing gel: An emerging and synergistic approach in management of psoriasis. Med. Hypotheses 2020, 142, 109838. [Google Scholar] [CrossRef]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar] [CrossRef]
- Saraswat, A.; Agarwal, R.; Katare, O.P.; Kaur, I.; Kumar, B. A randomized, double-blind, vehicle-controlled study of a novel liposomal dithranol formulation in psoriasis. J. Dermatol. Treat. 2007, 18, 40–45. [Google Scholar] [CrossRef]
- Fathalla, D.; Youssef, E.M.K.; Soliman, G.M. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A controlled and novel drug delivery system. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Rahimpour, Y.; Kouhsoltani, M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin. Drug Deliv. 2013, 10, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Moghddam, S.R.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Nagpal, M.; Kaur, G. Development and Comparison of Nanosponge and Niosome based Gel for the Topical Delivery of Tazarotene. Pharm. Nanotechnol. 2016, 4, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 825–834. [Google Scholar] [CrossRef]
- Parnami, N.; Garg, T.; Rath, G.; Goyal, A.K. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2014, 42, 406–412. [Google Scholar] [CrossRef]
- Bracke, S.; Carretero, M.; Guerrero-Aspizua, S.; Desmet, E.; Illera, N.; Navarro, M.; Lambert, J.; Del Rio, M. Targeted silencing of DEFB4 in a bioengineered skin-humanized mouse model for psoriasis: Development of siRNA SECosome-based novel therapies. Exp. Dermatol. 2014, 23, 199–201. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, B.; Wadhwa, S.; Raza, K.; Katare, O.P. Novel phospholipid-based topical formulations of tamoxifen: Evaluation for antipsoriatic activity using mouse-tail model. Pharm. Dev. Technol. 2014, 19, 160–163. [Google Scholar] [CrossRef]
- Marianecci, C.; Rinaldi, F.; Di Marzio, L.; Mastriota, M.; Pieretti, S.; Celia, C.; Paolino, D.; Iannone, M.; Fresta, M.; Carafa, M. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int. J. Nanomed. 2014, 9, 635–651. [Google Scholar] [CrossRef]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Fares, A.R.; Abd-Elsalam, W.H. Enhanced Permeation of Methotrexate via Loading into Ultra-permeable Niosomal Vesicles: Fabrication, Statistical Optimization, Ex Vivo Studies, and In Vivo Skin Deposition and Tolerability. AAPS PharmSciTech 2019, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.K.; Devi, G.S.; Bhaskaran, S.; Sacchidanand, S. Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 157–161. [Google Scholar] [CrossRef]
- Lakshmi, P.; Devi, S.; Bhaskaran, S. Clinical management of psoriasis using 0.25% niosomal methotrexate gel: A placebo controlled double blind study. Int. J. Dermatol. 2006, 3, 3. [Google Scholar]
- Ali, M.F.; Salah, M.; Rafea, M.; Saleh, N. Liposomal methotrexate hydrogel for treatment of localized psoriasis: Preparation, characterization and laser targeting. Med. Sci. Monit. 2008, 14, PI66–PI74. [Google Scholar] [PubMed]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and curcumin co-loaded liposphere gel: Synergistic combination towards management of psoriasis. J. Control. Release 2016, 243, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Shah, K.A.; Date, A.A.; Joshi, M.D.; Patravale, V.B. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wissing, S.A.; Muller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Santos Maia, C.; Mehnert, W.; Schaller, M.; Korting, H.C.; Gysler, A.; Haberland, A.; Schafer-Korting, M. Drug targeting by solid lipid nanoparticles for dermal use. J. Drug Target. 2002, 10, 489–495. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, R.; Harde, H.; Katariya, M.; Agrawal, S.; Jain, S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis. Expert Opin. Drug Deliv. 2014, 11, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Bikkad, M.L.; Nathani, A.H.; Mandlik, S.K.; Shrotriya, S.N.; Ranpise, N.S. Halobetasol propionate-loaded solid lipid nanoparticles (SLN) for skin targeting by topical delivery. J. Liposome Res. 2014, 24, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif. Cells Nanomed. Biotechnol. 2016, 44, 392–400. [Google Scholar] [CrossRef]
- Agrawal, U.; Gupta, M.; Vyas, S.P. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef]
- Gambhire, M.; Bhalekar, M.; Shrivastava, B.J.P.C.J. Investigations in photostability of dithranol incorporated in solid lipid nanoparticles. Pharm. Chem. J. 2012, 46, 256–261. [Google Scholar] [CrossRef]
- Kim, S.T.; Jang, D.J.; Kim, J.H.; Park, J.Y.; Lim, J.S.; Lee, S.Y.; Lee, K.M.; Lim, S.J.; Kim, C.K. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation. Pharmazie 2009, 64, 510–514. [Google Scholar]
- Ridolfi, D.M.; Marcato, P.D.; Justo, G.Z.; Cordi, L.; Machado, D.; Duran, N. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloids Surf. B Biointerfaces 2012, 93, 36–40. [Google Scholar] [CrossRef]
- Trombino, S.; Russo, R.; Mellace, S.; Varano, G.P.; Laganà, A.S.; Marcucci, F.; Cassano, R. Solid lipid nanoparticles made of trehalose monooleate for cyclosporin—A topic release. J. Drug Deliv. Sci. Technol. 2019, 49, 563–569. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles and nanostructured lipid carriers. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2004; Volume 9, pp. 43–56. [Google Scholar]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Basri, M.; Ismail, R.; Lau, H.; Tejo, B.; Kanthimathi, M.; Hassan, H.; Choo, Y. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int. J. Nanomed. 2013, 8, 13–22. [Google Scholar] [CrossRef]
- Agrawal, Y.; Petkar, K.C.; Sawant, K.K. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010, 401, 93–102. [Google Scholar] [CrossRef]
- Pinto, M.F.; Moura, C.C.; Nunes, C.; Segundo, M.A.; Costa Lima, S.A.; Reis, S. A new topical formulation for psoriasis: Development of methotrexate-loaded nanostructured lipid carriers. Int. J. Pharm. 2014, 477, 519–526. [Google Scholar] [CrossRef]
- Avasatthi, V.; Pawar, H.; Dora, C.P.; Bansod, P.; Gill, M.S.; Suresh, S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 2016, 21, 554–562. [Google Scholar] [CrossRef]
- Lin, Y.K.; Huang, Z.R.; Zhuo, R.Z.; Fang, J.Y. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int. J. Nanomed. 2010, 5, 117–128. [Google Scholar]
- Tripathi, P.; Kumar, A.; Jain, P.K.; Patel, J.R. Carbomer gel bearing methotrexate loaded lipid nanocontainers shows improved topical delivery intended for effective management of psoriasis. Int. J. Biol. Macromol. 2018, 120, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Pople, P.V.; Singh, K.K. Development and evaluation of colloidal modified nanolipid carrier: Application to topical delivery of tacrolimus. Eur. J. Pharm. Biopharm. 2011, 79, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Baskaran, R.; Madheswaran, T.; Kim, J.O.; Yong, C.S.; Yoo, B.K. Technology. Preparation, characterization, and release study of tacrolimus-loaded liquid crystalline nanoparticles. J. Dispers. Sci. Technol. 2013, 34, 72–77. [Google Scholar] [CrossRef]
- Lapteva, M.; Mondon, K.; Moller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Iriventi, P.; Gupta, N.V. Topical delivery of curcumin and caffeine mixture-loaded nanostructured lipid carriers for effective treatment of psoriasis. Pharmacogn. Mag. 2020, 16, 206. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: Systematic optimization, dermatokinetic and preclinical evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Sharma, A.; Upadhyay, D.K.; Sarma, G.S.; Kaur, N.; Gupta, G.D.; Narang, R.K.; Rai, V.K. Squalene integrated NLC based gel of tamoxifen citrate for efficient treatment of psoriasis: A preclinical investigation. J. Drug Deliv. Sci. Technol. 2020, 56, 101568. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef]
- Kalariya, M.; Padhi, B.K.; Chougule, M.; Misra, A. Clobetasol propionate solid lipid nanoparticles cream for effective treatment of eczema: Formulation and clinical implications. Indian J. Exp. Biol. 2005, 43, 233–240. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsai, P.C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef]
- Dessy, A.; Kubowicz, S.; Alderighi, M.; Bartoli, C.; Piras, A.M.; Schmid, R.; Chiellini, F. Dead Sea Minerals loaded polymeric nanoparticles. Colloids Surf. B Biointerfaces 2011, 87, 236–242. [Google Scholar] [CrossRef]
- Mao, K.L.; Fan, Z.L.; Yuan, J.D.; Chen, P.P.; Yang, J.J.; Xu, J.; Zhuge, D.L.; Jin, B.H.; Zhu, Q.Y.; Shen, B.X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef]
- Shah, P.P.; Desai, P.R.; Patel, A.R.; Singh, M.S. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials 2012, 33, 1607–1617. [Google Scholar] [CrossRef]
- Pukale, S.S.; Sharma, S.; Dalela, M.; Singh, A.K.; Mohanty, S.; Mittal, A.; Chitkara, D. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020, 115, 393–409. [Google Scholar] [CrossRef]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef]
- Suzuki, I.L.; de Araujo, M.M.; Bagnato, V.S.; Bentley, M. TNFα siRNA delivery by nanoparticles and photochemical internalization for psoriasis topical therapy. J. Control. Release 2021, 338, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.I.; Khan, D.; Rehman, A.U.; Elaissari, A.; Ahmed, N. Development and In Vitro/In Vivo Evaluation of pH-Sensitive Polymeric Nanoparticles Loaded Hydrogel for the Management of Psoriasis. Nanomaterials 2021, 11, 3433. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.P.D.; Rants’o, T.A.; Mdanda, S.; Mohlomi, L.M.; Choonara, Y.E. A Poly (Caprolactone)-Cellulose Nanocomposite Hydrogel for Transdermal Delivery of Hydrocortisone in Treating Psoriasis Vulgaris. Polymers 2022, 14, 2633. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhai, Y.Y.; Sun, L.; Wang, Z.; Xia, X.; Yao, Q.; Kou, L. Alantolactone-loaded chitosan/hyaluronic acid nanoparticles suppress psoriasis by deactivating STAT3 pathway and restricting immune cell recruitment. Asian J. Pharm. Sci. 2022, 17, 268–283. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Wu, C.; Tong, X.; Shi, Y.; Chen, S. Microneedle Patch Delivery of Methotrexate-Loaded Albumin Nanoparticles to Immune Cells Achieves a Potent Antipsoriatic Effect. Int. J. Nanomed. 2022, 17, 3841–3851. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F.S.; Daneshamuz, S.; Moghimi, H. Pharmaceutical nanoemulsions and their potential topical and transdermal applications. Iran. J. Pharm. Sci. 2011, 7, 139–150. [Google Scholar]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Brownlow, B.; Nagaraj, V.J.; Nayel, A.; Joshi, M.; Elbayoumi, T. Development and In Vitro Evaluation of Vitamin E-Enriched Nanoemulsion Vehicles Loaded with Genistein for Chemoprevention Against UVB-Induced Skin Damage. J. Pharm. Sci. 2015, 104, 3510–3523. [Google Scholar] [CrossRef]
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ourique, A.F.; Melero, A.; de Bona da Silva, C.; Schaefer, U.F.; Pohlmann, A.R.; Guterres, S.S.; Lehr, C.M.; Kostka, K.H.; Beck, R.C. Improved photostability and reduced skin permeation of tretinoin: Development of a semisolid nanomedicine. Eur. J. Pharm. Biopharm. 2011, 79, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine 2017, 13, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef]
- Alam, M.S.; Ali, M.S.; Alam, N.; Siddiqui, M.R.; Shamim, M.; Safhi, M. In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent. Today 2013, 5, 8–12. [Google Scholar] [CrossRef]
- Baboota, S.; Alam, M.S.; Sharma, S.; Sahni, J.K.; Kumar, A.; Ali, J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int. J. Pharm. Investig. 2011, 1, 139–147. [Google Scholar] [CrossRef]
- Panonnummal, R.; Sabitha, M. Anti-psoriatic and toxicity evaluation of methotrexate loaded chitin nanogel in imiquimod induced mice model. Int. J. Biol. Macromol. 2018, 110, 245–258. [Google Scholar] [CrossRef]
- Singka, G.S.; Samah, N.A.; Zulfakar, M.H.; Yurdasiper, A.; Heard, C.M. Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur. J. Pharm. Biopharm. 2010, 76, 275–281. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Khurana, B.; Arora, D.; Narang, R.K. QbD based exploration of resveratrol loaded polymeric micelles based carbomer gel for topical treatment of plaque psoriasis: In vitro, ex vivo and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 59, 101901. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.; Qi, X.; Wei, F.; Chen, H.; Wang, T. Pluronic® F127 stabilized reduced graphene oxide hydrogel for the treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 195, 111246. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Song, R.; Cheng, M.; Chu, H.; Yan, F.; Wang, Y. Preparation of Calcipotriol Emulsion Using Bacterial Exopolysaccharides as Emulsifier for Percutaneous Treatment of Psoriasis Vulgaris. Int. J. Mol. Sci. 2019, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Shinde, G.; Desai, P.; Shelke, S.; Patel, R.; Bangale, G.; Kulkarni, D. Mometasone furoate-loaded aspasomal gel for topical treatment of psoriasis: Formulation, optimization, in vitro and in vivo performance. J. Dermatol. Treat. 2020, 33, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Mishra, N.; Rawal, R.K.; Narang, R.K. Development and characterisation of clobetasol propionate loaded Squarticles as a lipid nanocarrier for treatment of plaque psoriasis. J. Microencapsul. 2020, 37, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Erol, İ.; Üstündağ Okur, N.; Orak, D.; Sipahi, H.; Aydın, A.; Özer, Ö. Tazarotene-loaded in situ gels for potential management of psoriasis: Biocompatibility, anti-inflammatory and analgesic effect. Pharm. Dev. Technol. 2020, 25, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Cheng, Y.P.; Liu, C.Y.; Thong, H.Y.; Huang, C.J.; Lo, Y.; Wu, C.Y.; Jee, S.H. Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Pharmaceutics 2020, 12, 457. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Manisekaran, R.; García-Contreras, R.; Rasu Chettiar, A.D.; Serrano-Díaz, P.; Lopez-Ayuso, C.A.; Arenas-Arrocena, M.C.; Hernández-Padrón, G.; López-Marín, L.M.; Acosta-Torres, L.S. 2D Nanosheets—A New Class of Therapeutic Formulations against Cancer. Pharmaceutics 2021, 13, 1803. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, T.; Chen, W.; Li, Y.; Wang, B. Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact. Mater. 2020, 5, 1071–1086. [Google Scholar] [CrossRef]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cerra, S.; Matassa, R.; Beltrán, A.M.; Familiari, G.; Battocchio, C.; Pis, I.; Sciubba, F.; Scaramuzzo, F.A.; Del Giudice, A.; Fratoddi, I. Insights about the interaction of methotrexate loaded hydrophilic gold nanoparticles: Spectroscopic, morphological and structural characterizations. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111337. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ho, L.W.C.; Bai, Q.; Chan, C.K.W.; Lee, L.K.C.; Choi, P.C.; Choi, C.H.J. Alkyl-Terminated Gold Nanoparticles as a Self-Therapeutic Treatment for Psoriasis. Nano Lett. 2021, 21, 8723–8733. [Google Scholar] [CrossRef]
- Fereig, S.; El-Zaafarany, G.M.; Arafa, G.; Abdel-Mottaleb, M.M.A. Boosting the anti-inflammatory effect of self-assembled hybrid lecithin-chitosan nanoparticles via hybridization with gold nanoparticles for the treatment of psoriasis: Elemental mapping and in vivo modeling. Drug Deliv. 2022, 29, 1726–1742. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Lai, X.; Wang, M.; Zhu, Y.; Feng, X.; Liang, H.; Nie, L.; Li, L.; Shao, L. ZnO NPs Delay the Recovery of Psoriasis-like Skin Lesions through Promoting Inflammation and Keratinocyte Apoptosis via Nuclear Translocation of Phosphorylated NF-κB p65 and Cysteine Deficiency. J. Hazard. Mater. 2021, 410, 124566. [Google Scholar] [CrossRef]
- Maurelli, M.; Gisondi, P.; Girolomoni, G. Tailored biological treatment for patients with moderate-to-severe psoriasis. Expert Rev. Clin. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Yasmeen, N.; Sawyer, L.M.; Malottki, K.; Levin, L.; Didriksen Apol, E.; Jemec, G.B. Targeted therapies for patients with moderate-to-severe psoriasis: A systematic review and network meta-analysis of PASI response at 1 year. J. Dermatol. Treat. 2022, 33, 204–218. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Singhvi, G.; Dubey, S.K.; Gupta, G.; Chellappan, D.K.; Dua, K. Emerging landscape in psoriasis management: From topical application to targeting biomolecules. Biomed. Pharmacother. 2018, 106, 707–713. [Google Scholar] [CrossRef]
- Gungor, S.; Rezigue, M. Nanocarriers Mediated Topical Drug Delivery for Psoriasis Treatment. Curr. Drug Metab. 2017, 18, 454–468. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: An enticing approach to offset psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Laberge, S.; Cruikshank, W.W.; Beer, D.J.; Center, D.M. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J. Immunol. 1996, 156, 310–315. [Google Scholar] [PubMed]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.; Wong, J.F.; Stucky, C.L.; et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Ziethen, B.; Gollnick, H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm. Res. 1997, 46, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Nordlind, K.; Thorslund, K.; Lonne-Rahm, S.; Mohabbati, S.; Berki, T.; Morales, M.; Azmitia, E.C. Expression of serotonergic receptors in psoriatic skin. Arch. Dermatol. Res. 2006, 298, 99–106. [Google Scholar] [CrossRef]
- Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. Current and Future Therapies for Psoriasis with a Focus on Serotonergic Drugs. Mol. Neurobiol. 2020, 57, 2391–2419. [Google Scholar] [CrossRef]
- Levite, M. Killing Human Lymphoma and Leukemia Cancer Cells and Tcr-Activated Normal Human Cells by Dopamine D1r Agonists. U.S. Patent 11/997,848, 18 December 2008. [Google Scholar]
- Levite, M.; Domb, A. Fenoldopam Formulations and Pro-Drug Derivatives. U.S. Patent 8,859,001, 14 October 2014. [Google Scholar]
- Doppalapudi, S.; Jain, A.; Bulbake, U.; Khan, W.J.P.N. Nanotherapeutics: Emerging trends in management of psoriasis. Pharm. Nanotechnol. 2016, 4, 267–283. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Fenoldopam mesylate for treating psoriasis: A new indication for an old drug. Int. J. Pharm. 2020, 573, 118726. [Google Scholar] [CrossRef]
- Pradyuth, S.; Rapalli, V.K.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Singhvi, G. Insightful exploring of microRNAs in psoriasis and its targeted topical delivery. Dermatol. Ther. 2020, 33, e14221. [Google Scholar] [CrossRef]
- Lindqvist, U.; Phil-Lundin, I.; Engström-Laurent, A. Dermal distribution of hyaluronan in psoriatic arthritis; coexistence of CD44, MMP3 and MMP9. Acta Derm. Venereol. 2012, 92, 372–377. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).