Fluoride Retention in Root Dentin following Surface Coating Material Application
Abstract
1. Introduction
2. Materials and Methods
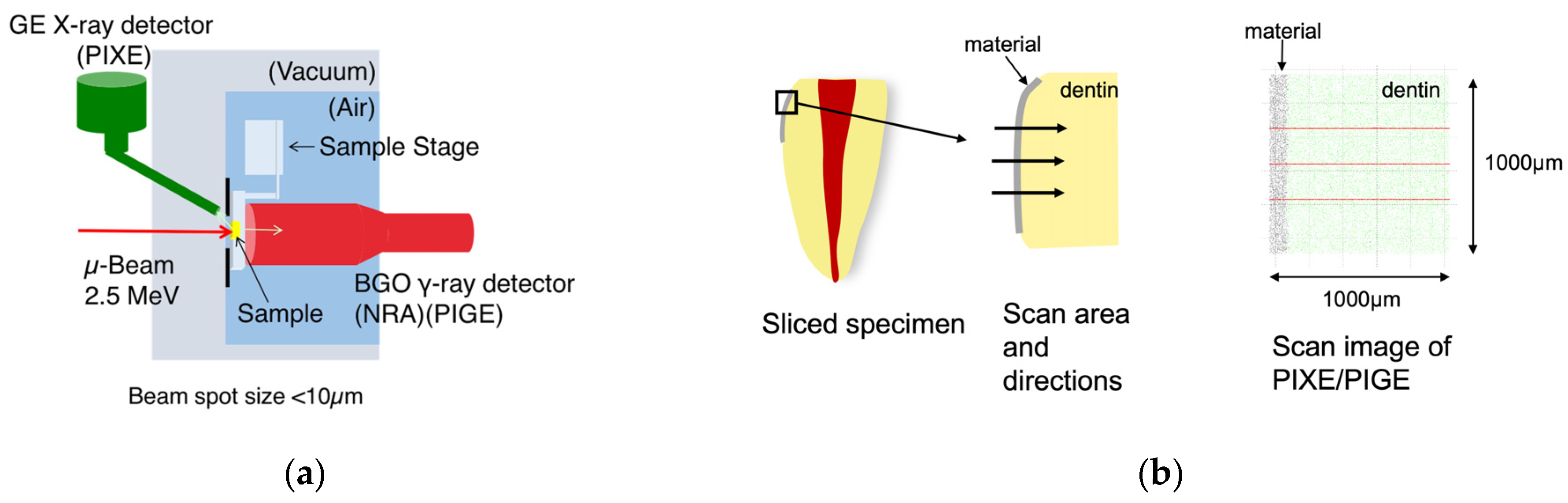
2.1. Fluoride Distribution in Root Dentin following the Application of Coating Materials
2.2. Measurement of KOH-Insoluble and Whole Fluoride in Fluoride-Treated HAP
2.3. Measurement of Fluoride Release from Materials
2.4. Data Analysis
3. Results
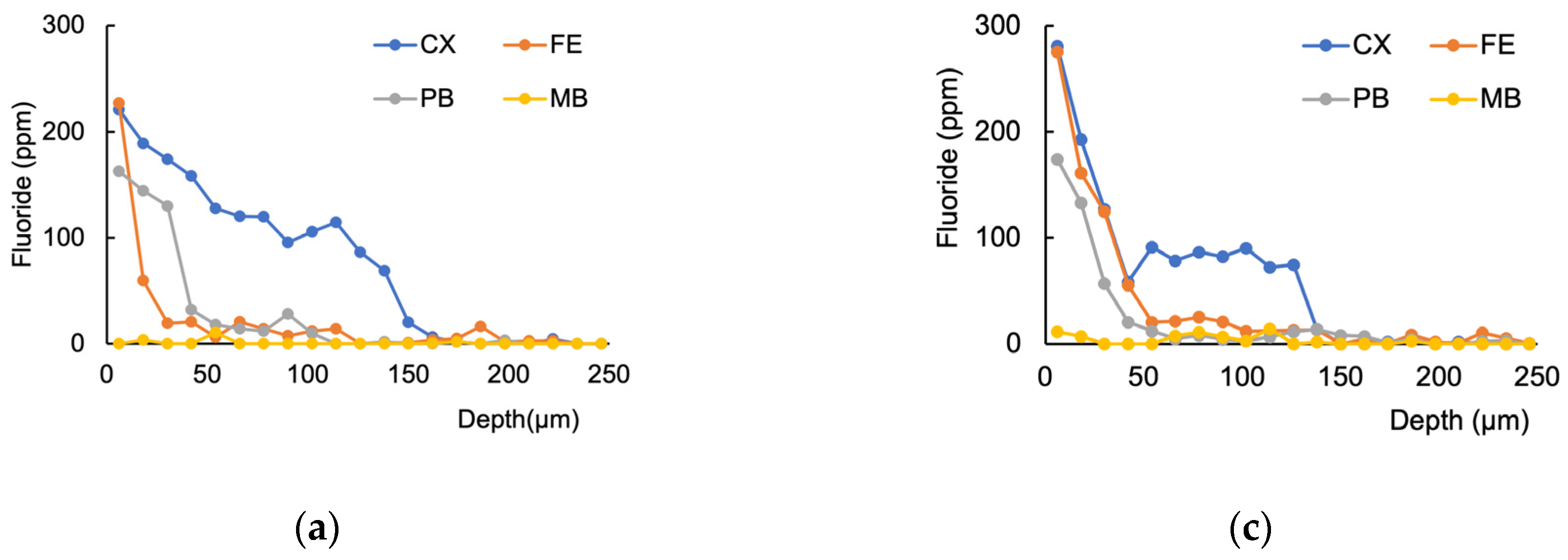
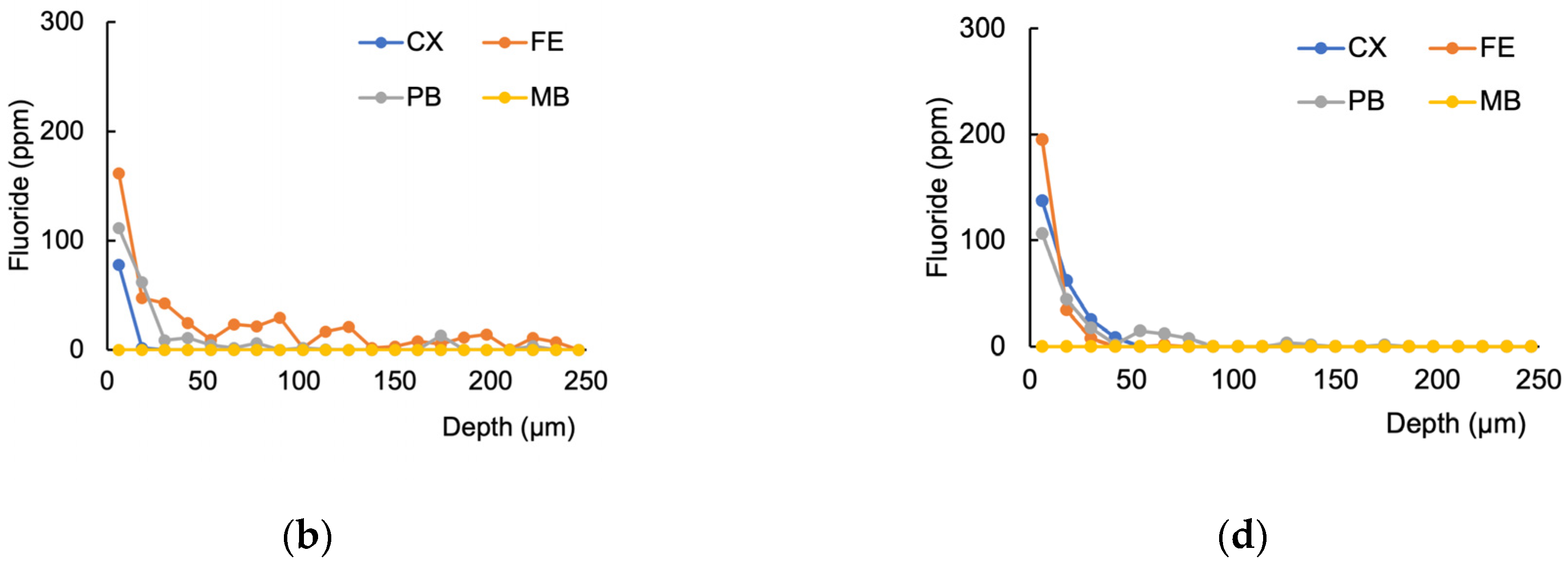
3.1. Fluoride Distribution in the Tooth Structure
3.2. W-F and T-F in Fluoride-Treated HAP
3.3. Fluoride Release from the Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Rahman, B.; El-Damanhoury, H.M.; Sheela, S.; Ngo, H.C. Effect of calcium silicate, sodium phosphate, and fluoride on dentinal tubule occlusion and permeability in comparison to desensitizing toothpaste: An in vitro study. Oper. Dent. 2021, 46, 641–649. [Google Scholar] [CrossRef]
- Oliveira Barros, A.P.; da Silva Pompeu, D.; Takeuchi, E.V.; de Melo Alencar, C.; Alves, E.B.; Silva, C.M. Effect of 1.5% potassium oxalate on sensitivity control, color change, and quality of life after at-home tooth whitening: A randomized, placebo-controlled clinical trial. PLoS ONE 2022, 17, e0277346. [Google Scholar] [CrossRef]
- Derman, S.H.M.; Lantwin, E.-M.; Barbe, A.G.; Noack, M.J. Does a pretreatment with a dentine hypersensitivity mouth-rinse compensate the pain caused by professional mechanical plaque removal? a single-blind randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 3151–3160. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, J.-C. Dentin hypersensitivity and emerging concepts for treatments. J. Oral Biosci. 2017, 59, 211–217. [Google Scholar] [CrossRef]
- Mazur, M.; Jedliński, M.; Ndokaj, A.; Ardan, R.; Janiszewska-Olszowska, J.; Nardi, G.M.; Ottolenghi, L.; Guerra, F. Long-term effectiveness of treating dentin hypersensitivity with bifluorid 10 and futurabond u: A split-mouth randomized double-blind clinical trial. J. Clin. Med. 2021, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Solati, M.; Fekrazad, R.; Vahdatinia, F.; Farmany, A.; Farhadian, M.; Hakimiha, N. Dentinal tubule blockage using nanobioglass in the presence of diode (980 Nm) and Nd:YAG lasers: An in vitro study. Clin. Oral Investig. 2022, 26, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Bönecker, M.; Altenburger, M.J.; Buzalaf, M.A.R.; Sampaio, F.C.; Lussi, A. Fluoride varnishes containing calcium glycerophosphate: Fluoride uptake and the effect on in vitro enamel erosion. Clin. Oral Investig. 2015, 19, 1429–1436. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, Y.; Feng, H. The Anti-Cariogenic Effect of fluoride iontophoresis on sound enamel under different working conditions: An in vitro study. Mater. Res. Express 2023, 10, 036301. [Google Scholar] [CrossRef]
- Zanatta, R.F.; da Silva Ávila, D.M.; Miyamoto, K.M.; Torres, C.R.G.; Borges, A.B. Influence of surfactants and fluoride against enamel erosion. Caries Res. 2019, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ogaard, B.; Rølla, G.; Ruben, J.; Arends, J. Relative cariostatic effects of KOH-soluble and KOH-insoluble fluoride in situ. J. Dent. Res. 1990, 69, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Nancollas, G.H. Physical and chemical considerations of the role of firmly and loosely bound fluoride in caries prevention. J. Dent. Res. 1990, 69, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.B.; Scaramucci, T.; Lippert, F.; Zero, D.T.; Hara, A.T. Erosion protection by calcium lactate/sodium fluoride rinses under different salivary flows in vitro. Caries Res. 2014, 48, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Nezu, T.; Tanaka, M. A warmed topical fluoride solution enhances KOH-soluble and -insoluble fluoride formation on tooth surfaces in vitro. Pediatr. Dent. J. 2014, 24, 22–26. [Google Scholar] [CrossRef]
- Wegehaupt, F.J.; Solt, B.; Sener, B.; Wiegand, A.; Schmidlin, P.R.; Attin, T. Influence of fluoride concentration and ethanol pre-treatment on the reduction of the acid susceptibility of enamel. Arch. Oral Biol. 2009, 54, 823–829. [Google Scholar] [CrossRef]
- Scaramucci, T.; Borges, A.B.; Lippert, F.; Frank, N.E.; Hara, A.T. Sodium fluoride effect on erosion–abrasion under hyposalivatory simulating conditions. Arch. Oral Biol. 2013, 58, 1457–1463. [Google Scholar] [CrossRef]
- Attin, T.; Albrecht, K.; Becker, K.; Hannig, C.; Wiegand, A. Influence of carbamide peroxide on enamel fluoride uptake. J. Dent. 2006, 34, 668–675. [Google Scholar] [CrossRef]
- Buchalla, W.; Attin, T.; Schulte-Mönting, J.; Hellwig, E. Fluoride uptake, retention, and remineralization efficacy of a highly concentrated fluoride solution on enamel lesions in situ. J. Dent. Res. 2002, 81, 329–333. [Google Scholar] [CrossRef]
- Vishwakarma, A.P.; Bondarde, P.; Vishwakarma, P.; Dodamani, A.S.; Mujawar, S.; Bansal, S. Evaluation of fluoride uptake of two fluoride varnishes into and onto the enamel surface at different temperatures: An in vitro study. Int. J. Clin. Pediatr. Dent. 2022, 15, 672–679. [Google Scholar] [CrossRef]
- Soares-Yoshikawa, A.L.; Cury, J.A.; Tabchoury, C.P.M. Fluoride concentration in SDF commercial products and their bioavailability with demineralized dentine. Braz. Dent. J. 2020, 31, 257–263. [Google Scholar] [CrossRef]
- Hellwig, E. Fluoride retention in dentin after topical application of aminefluoride. J. Dent. Res. 1992, 71, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Okuyama, K.; Yamamoto, H.; Yasuda, K.; Nomachi, M.; Sugaya, Y. Tooth-bound fluoride uptake from fluoride-containing dental materials. Int. J. PIXE 2013, 23, 127–134. [Google Scholar] [CrossRef]
- Okuyama, K.; Tamaki, Y.; Matsuda, Y.; Saito, T.; Yamamoto, H.; Naito, K.; Hayashi, M.; Suzuki, K. Analysis of tooth-bound fluoride after the application of coating materials on the enamel surface with an in-air micro PIXE/PIGE. Int. J. PIXE 2019, 29, 7–16. [Google Scholar] [CrossRef]
- Scholz, K.J.; Federlin, M.; Hiller, K.-A.; Ebensberger, H.; Ferstl, G.; Buchalla, W. EDX-analysis of fluoride precipitation on human enamel. Sci. Rep. 2019, 9, 13442. [Google Scholar] [CrossRef]
- Peng, J.-Y.; Botelho, M.G.; Matinlinna, J.P.; Pan, H.-B.; Kukk, E.; Low, K.-J. Interaction of storage medium and silver diamine fluoride on demineralized dentin. J. Int. Med. Res. 2021, 49, 300060520985336. [Google Scholar] [CrossRef]
- Miyajima, H.; Ishimoto, T.; Ma, S.; Chen, J.; Nakano, T.; Imazato, S. In vitro assessment of a calcium-fluoroaluminosilicate glass-based desensitizer for the prevention of root surface demineralization. Dent. Mater. J. 2016, 35, 399–407. [Google Scholar] [CrossRef]
- Eguchi, T.; Satou, R.; Miake, Y.; Sugihara, N. Comparison of resistance of dentin to erosive acid after application of fluoride to teeth. J. Hard Tissue Biol. 2020, 29, 193–202. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Kadowaki, Y.; Matsuda, Y.; Hashimoto, N.; Oki, S.; Yamamoto, H.; Tamaki, Y.; Sano, H. Efficacy of a new filler-containing root coating material for dentin remineralization. Am. J. Dent. 2016, 29, 213–218. [Google Scholar]
- Yasuhiro, M.; Katsushi, O.; Hiroko, Y.; Hisanori, K.; Masashi, K.; Takahiro, S.; Naoki, H.; Saiko, O.; Chiharu, K.; Hidehiko, S. Fluorine uptake into the human enamel surface from fluoride-containing sealing materials during cariogenic pH cycling. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 348, 156–159. [Google Scholar] [CrossRef]
- Komatsu, H.; Yamamoto, H.; Matsuda, Y.; Kijimura, T.; Kinugawa, M.; Okuyama, K.; Nomachi, M.; Yasuda, K.; Satoh, T.; Oikawa, S. Fluorine analysis of human enamel around fluoride-containing materials under different pH-cycling by μ-PIGE/PIXE System. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 2274–2277. [Google Scholar] [CrossRef]
- El-Safty, S.M.; El-Wakiel, N.; El-Oleimy, G.; Gaber, M.; El-Sayed, Y.S. Microhardness and fluoride release of glass ionomer cement modified with a novel Al+3 complex to enhance its antimicrobial activity. Int. J. Biomater. 2021, 2021, 1925388. [Google Scholar] [CrossRef] [PubMed]
- Morales-Valenzuela, A.A.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Garcia-Contreras, R.; Salmeron-Valdes, E.N.; Aguillón-Sol, L. Comparison of fluoride release in conventional glass-ionomer cements with a new mechanical mixing cement. Oral Health Prev. Dent. 2020, 18, 319–323. [Google Scholar] [CrossRef]
- Okuyama, K.; Murata, Y.; Pereira, P.N.R.R.; Miguez, P.A.; Komatsu, H.; Sano, H. Fluoride release and uptake by various dental materials after fluoride application. Am. J. Dent. 2006, 19, 123–127. [Google Scholar] [PubMed]
- Yasuda, K.; Nomachi, M.; Sugaya, Y.; Yamamoto, H.; Komatsu, H. Progress of in-air microbeam system at the Wakasa Wan Energy Research Center. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 2180–2183. [Google Scholar] [CrossRef]
- Chow, L.C.; Takagi, S.; Shih, S. Effect of a two-solution fluoride mouthrinse on remineralization of enamel lesions in vitro. J. Dent. Res. 1992, 71, 443–447. [Google Scholar] [CrossRef]
- Caslavska, V.; Moreno, E.C.C.; Brudevold, F. Determination of the calcium fluoride formed from in vitro exposure of human enamel to fluoride solutions. Arch. Oral Biol. 1975, 20, 333–339. [Google Scholar] [CrossRef]




| Trade Name (Company) | Composition | Code |
|---|---|---|
| PRG Barrier Coat (Shofu, Japan) | Base: S-PRG filler, polymeric monomer, water, others active: carboxylic acid monomer, phosphonic acid monomer, polymeric monomer, photo initiator, others | PB |
| Clinpro XT varnish (3M, USA) | Paste: HEMA, Bis-GMA, initiators, fluoroaluminosilicate glass, water, others Liquid: Polyacrylic acid, water, HEMA, initiators, calcium glycerophosphate, others | CX |
| Fuji IX EXTRA (GC, Japan) | Powder: Fluoroaluminosilicate glass Liquid: polyacrylic acid, polybasic carboxylic acid, water | FE |
| Clearfil Mega Bond (Kuraray Noritake Dental, Japan) | Primer: MDP, HEMA, hydrophilic aliphatic dimethacrylate, dl-camphorquinone, water Bond: MDP, Bis-GMA, HEMA, hydrophobic aliphatic dimethacrylate, dl-camphorquinone, initiators, accelerators; silanated colloidal silica | MB |
| Material | 7 Days Interface | 7 Days Cumulative | 28 Days Interface | 28 Days Cumulative |
|---|---|---|---|---|
| CX | 0.40 (0.01–0.97) a | 0.07 (0.00–0.21) | 0.32 (0.00–0.88) a | 0.24 (0.02–0.51) a |
| FE | 0.58 (0.09–0.87) a | 0.61 (0.07–0.86) a | 0.65 (0.06–0.93) a | 0.58 (0.06–0.80) a |
| PB | 0.43 (0.00–0.93) a | 0.49 (0.20–0.89) a | 0.65 (0.09–0.86) a | 0.43 (0.13–0.60) a |
| Conc. | Period | W-F | T-F | Ratio (T-F/W-F) | |||
|---|---|---|---|---|---|---|---|
| 1 ppmF | 7 days | 2.65 (0.04) |  | 2.55 (0.07) | 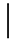 | 0.66 (0.03) a | |
| 28 days | 2.41 (0.23) * | 2.26 (0.59) * | 0.87 (0.08) b | ||||
| 10 ppmF | 7 days | 16.9 (1.07) | 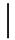 | 14.9 (0.91) |  | 0.88 (0.06) a | 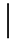 |
| 28 days | 19.1 (1.49) | 17.7 (1.35) | 0.92 (0.08) b | ||||
| 100 ppmF | 7 days | 157 (8.59) | 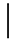 | 104 (4.51) | 0.96 (0.02) | ||
| 28 days | 152 (12.5) | 132 (10.1) | 0.82 (0.13) b | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, K.; Matsuda, Y.; Yamamoto, H.; Suzuki, K.; Shintani, K.; Saito, T.; Hayashi, M.; Tamaki, Y. Fluoride Retention in Root Dentin following Surface Coating Material Application. J. Funct. Biomater. 2023, 14, 171. https://doi.org/10.3390/jfb14030171
Okuyama K, Matsuda Y, Yamamoto H, Suzuki K, Shintani K, Saito T, Hayashi M, Tamaki Y. Fluoride Retention in Root Dentin following Surface Coating Material Application. Journal of Functional Biomaterials. 2023; 14(3):171. https://doi.org/10.3390/jfb14030171
Chicago/Turabian StyleOkuyama, Katsushi, Yasuhiro Matsuda, Hiroko Yamamoto, Kohtaku Suzuki, Kohei Shintani, Takashi Saito, Mikako Hayashi, and Yukimichi Tamaki. 2023. "Fluoride Retention in Root Dentin following Surface Coating Material Application" Journal of Functional Biomaterials 14, no. 3: 171. https://doi.org/10.3390/jfb14030171
APA StyleOkuyama, K., Matsuda, Y., Yamamoto, H., Suzuki, K., Shintani, K., Saito, T., Hayashi, M., & Tamaki, Y. (2023). Fluoride Retention in Root Dentin following Surface Coating Material Application. Journal of Functional Biomaterials, 14(3), 171. https://doi.org/10.3390/jfb14030171








