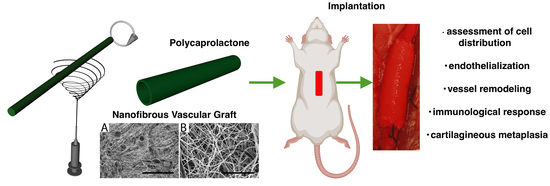
An Assessment of Blood Vessel Remodeling of Nanofibrous Poly(ε-Caprolactone) Vascular Grafts in a Rat Animal Model
Abstract
1. Introduction
| Study | Material | Assessed Timepoints | Histological Evaluation |
|---|---|---|---|
| Pektok et al. [5] | Electrospun PCL, ePTFE | 3, 6, 12, 18, 24 weeks | Cellular infiltration, endothelialization, regeneration of the vessel wall (collagen, elastin), immune response (FBGCs, macrophages), calcification |
| Nottelet et al. [15] | Electrospun PCL | 3, 6, 12 weeks | Cellular infiltration, endothelialization |
| de Valence et al. [7] | Electrospun PCL | 1.5, 3, 6, 12, 18 months | % cell invasion from the adventitia, endothelialization + vasa vasorum formation, immune response (FBGCs), regeneration of the vessel wall (smooth muscle cells, collagen, elastin), calcification |
| de Valence et al. [16] | Electrospun PCL—bi-layered grafts (no barrier, inside/outside/only barrier) | 3, 12 weeks | % cell invasion from the adventitia, endothelialization + vasa vasorum formation, intimal hyperplasia |
| de Valence et al. [17] | Electrospun PCL—plasma treated | 3 weeks | Cell penetration, cell density |
| Wang et al. [9] | Electrospun PCL—thicker fibers/thinner fibers | 7, 14, 28, 100 days | Cellular infiltration, endothelialization, regeneration of the vessel wall (smooth muscle cells, collagen, elastin), immune response (macrophages) |
| Tille et al. [18] | Electrospun PCL modified with paclitaxel/dexamethasone | 1, 3, 12 weeks | Cellular infiltration, regeneration of the vessel wall (collagen), immune response (macrophages), calcification |
| Yang et al. [19] | Poly(glycerol sebacate) core + electrospun PCL | 3, 12 months | Cellularization, endothelialization + vasa vasorum formation, regeneration of the vessel wall (smooth muscle cells, collagen, elastin), immune response (macrophages), calcification |
| Pan et al. [20] | Co-electrospun PCL/polydioxanone | 1, 3 months | Endothelialization, regeneration of the vessel wall (smooth muscle cells, collagen, elastin, glycosaminoglycans), calcification |
| Wang et al. [21] | Electrospun PCL modified with resveratrol | 2, 4 weeks | Cellular infiltration, endothelialization, regeneration of the vessel wall (smooth muscle cells), immune response (macrophages) |
| Wang et al. [22] | Electrospun PCL modified with proteins (VEGF, HGFI) | 1 month | Cell density, endothelialization, endothelialization + vasa vasorum formation, regeneration of the vessel wall (smooth muscle cells, collagen, elastin, glycosaminoglycans), immune response (macrophages) |
| Li et al. [23] | Electrospun PCL—bi-layered graft | 3, 18 months | Cellularization, endothelialization + vasa vasorum formation, regeneration of the vessel wall (smooth muscle cells, collagen, elastin, glycosaminoglycans), calcification |
| Wu et al. [8] | Electrospun PCL | 12 months | endothelialization, regeneration of the vessel wall (smooth muscle cells, collagen, elastin), immune response (macrophages), calcification |
| Dokuchaeva et al. [11] | Electrospun PCL | 10, 30, 60, 90 days | Cellularization, calcification |
2. Materials and Methods
2.1. Vascular Graft Preparation
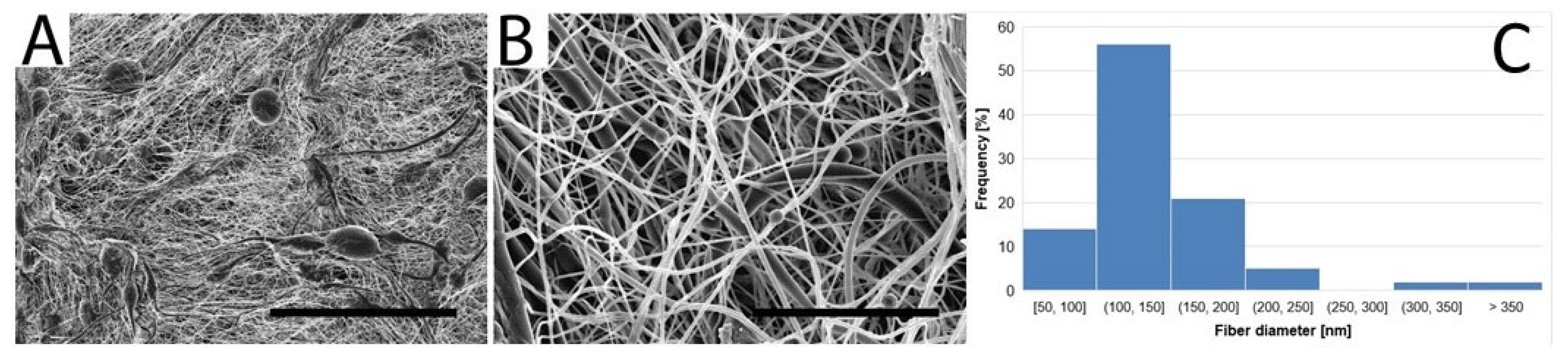
2.2. Morphological Characterization of the Vascular Graft
2.3. In Vivo Implantation
2.4. Histological Processing
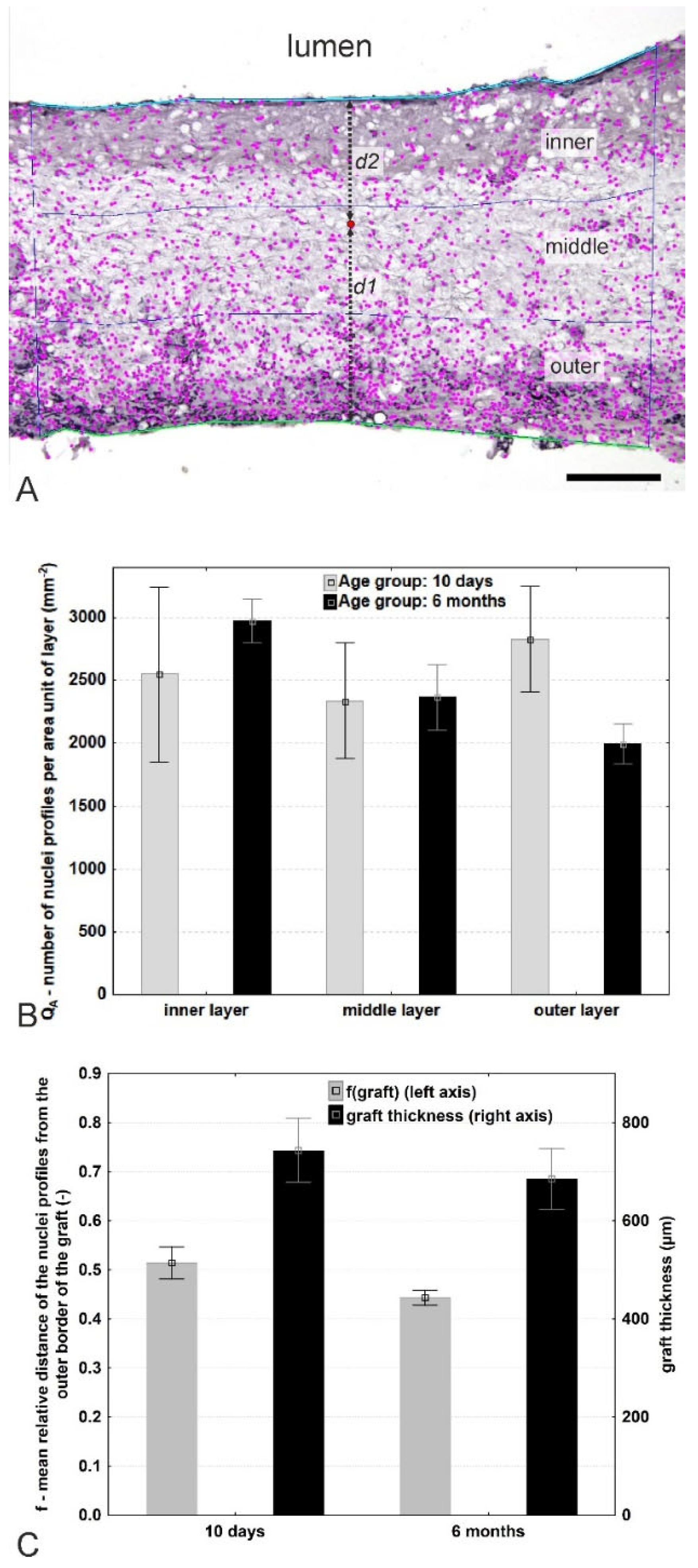
2.5. Quantification of Cellular Density within the Thickness of the Graft
3. Results
3.1. Vascular Graft Morphology
3.2. Vascular Graft Implantations and Patency
3.3. Quantification of the Cell Distribution within the Vascular Graft
3.4. Endothelialization of the Graft Lumen
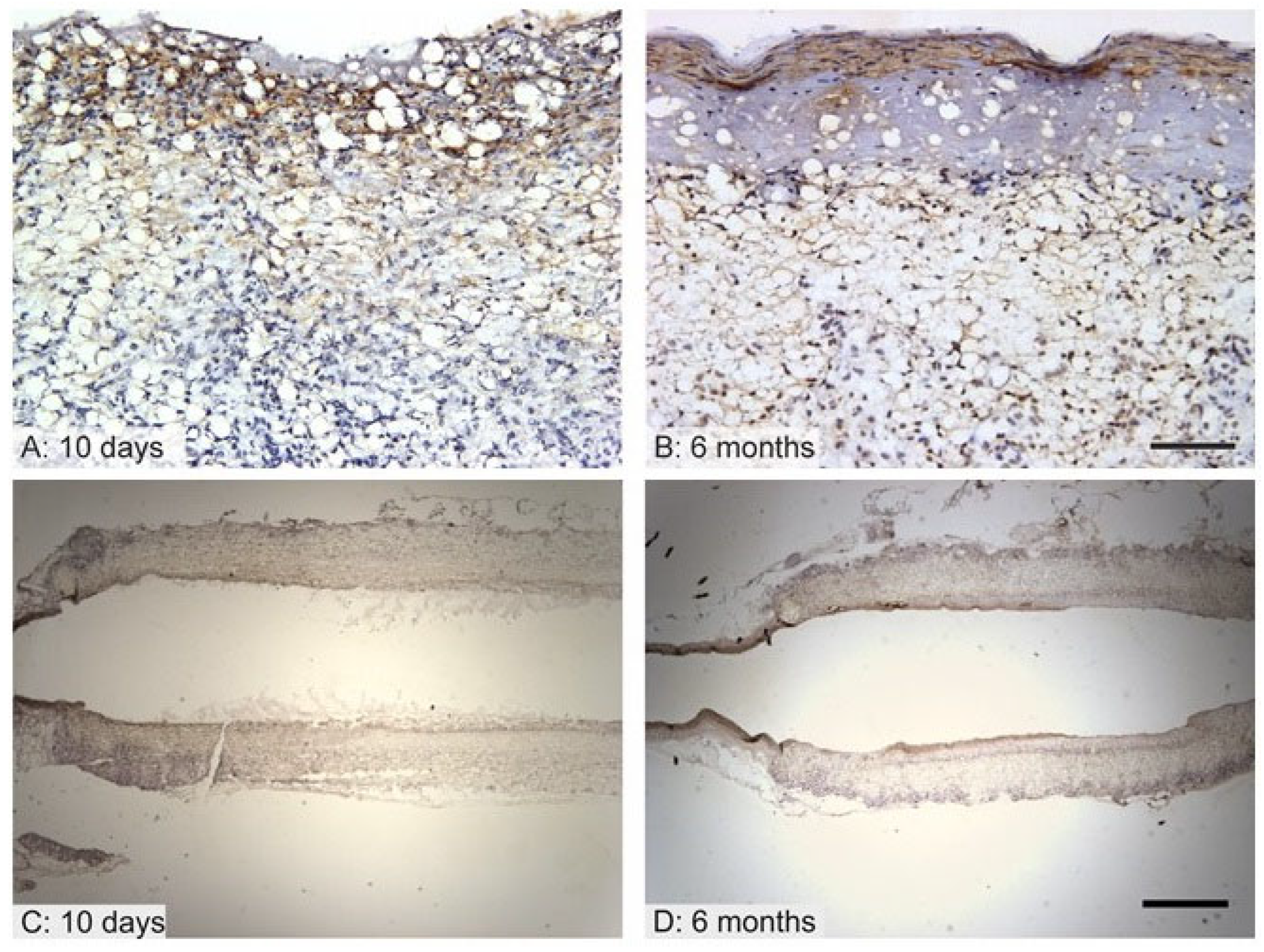
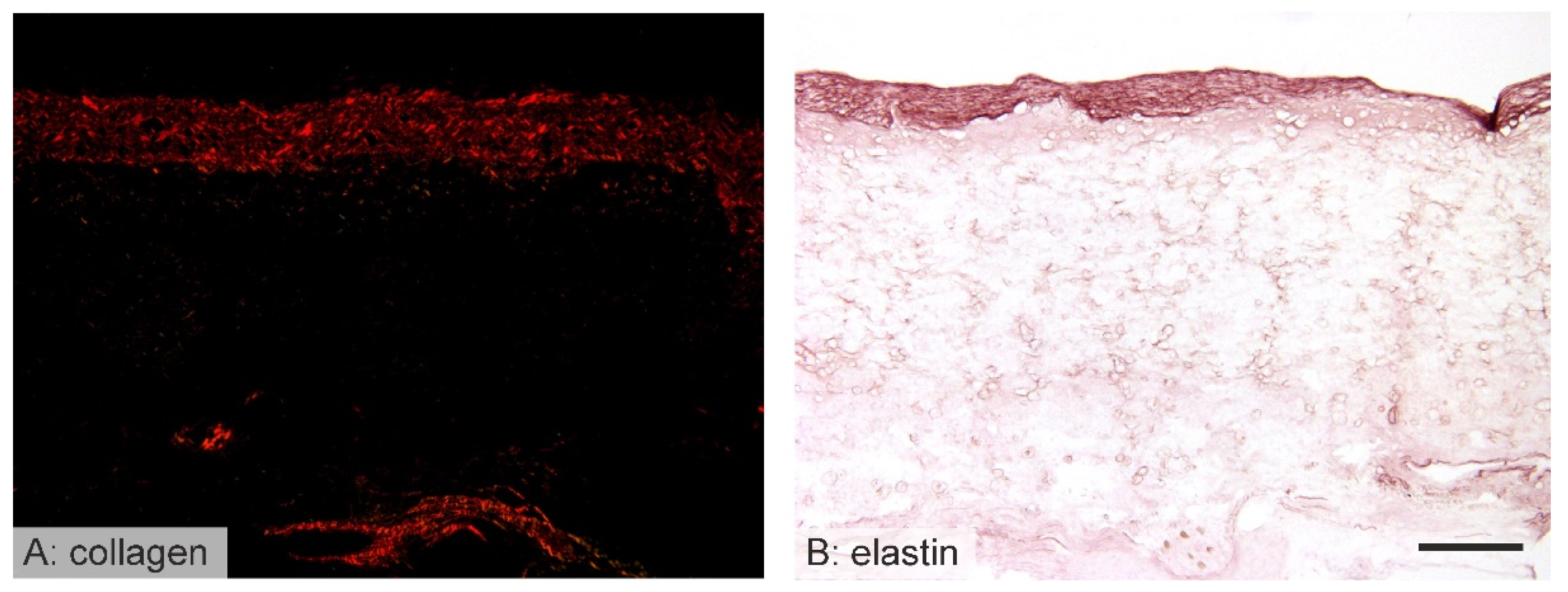
3.5. Vessel Remodeling
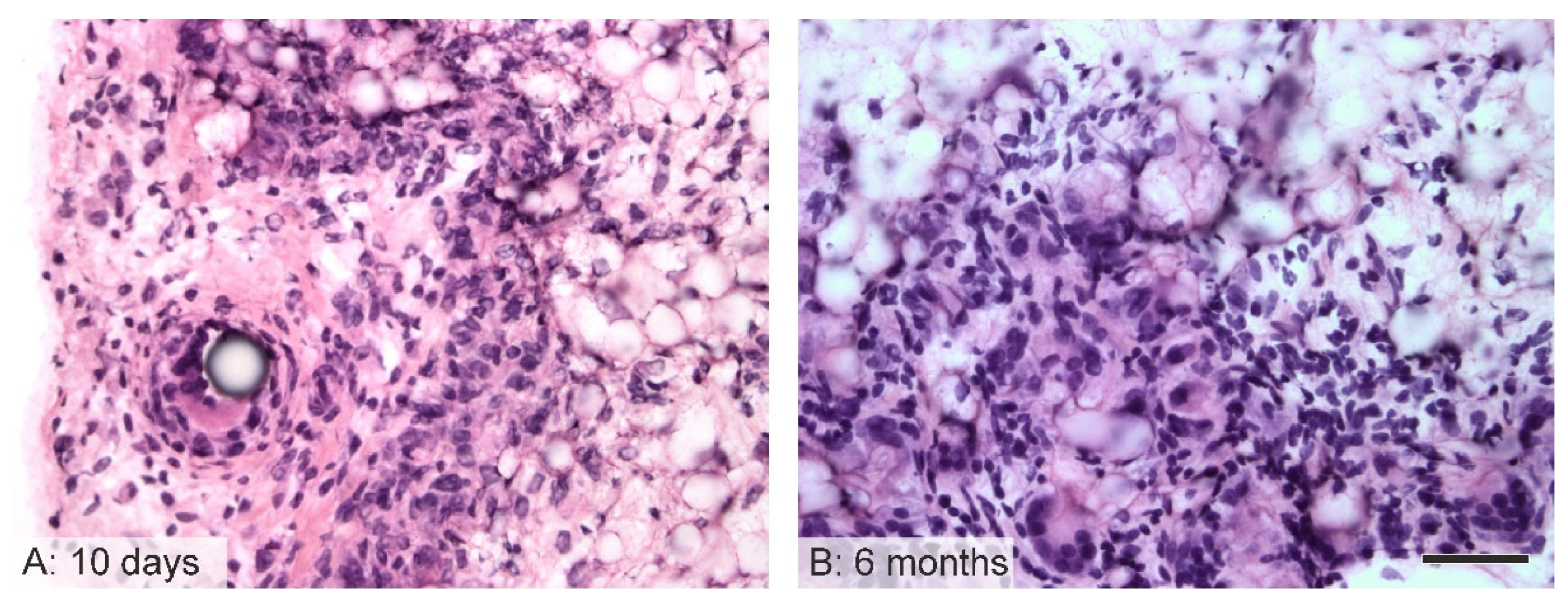
3.6. Immunological Response to the Implanted Grafts
3.7. Cartilaginous Metaplasia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaz, C.M.; Tuij, S.; Bouten, C.V.C.; Baaijens, F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, J.; Chu, C.C.; Wu, J. Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts. J. Mater. Sci. Mater. Med. 2010, 21, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Chao, W.C.; Lee, P.Y.; Huang, C.H. Construction and characterization of an electrospun tubular scaffold for small-diameter tissue-engineered vascular grafts: A scaffold membrane approach. J. Mech. Behav. Biomed. Mater. 2012, 13, 140–155. [Google Scholar] [CrossRef]
- Yalcin, I.; Horakova, J.; Mikes, P.; Gok Sadikoglu, T.; Domin, R.; Lukas, D. Design of polycaprolactone vascular grafts. J. Ind. Text. 2016, 45, 813–833. [Google Scholar] [CrossRef]
- Pektok, E.; Nottelet, B.; Tille, J.C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Vascular grafts in the rat systemic arterial circulation degradation and healing characteristics of small-diameter poly(e-caprolactone). Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- de Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Wang, Z.; Wang, J.; Zhang, C.; Li, C.; Kong, D. The regeneration og macro-porous electrospun poly(ε-caprolactone) vascular graft during long-term in situ implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1618–1627. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.L.; et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef]
- Dokuchaeva, A.A.; Mochalova, A.B.; Timchenko, T.P.; Podolskaya, K.S.; Pashkovskaya, O.A.; Karpova, E.V.; Ivanov, I.A.; Filatova, N.A.; Zhuravleva, I.Y. In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta. Polymers 2022, 14, 3313. [Google Scholar] [CrossRef] [PubMed]
- Horakova, J.; Mikes, P.; Saman, A.; Jencova, V.; Klapstova, A.; Svarcova, T.; Ackermann, M.; Novotny, V.; Suchy, T.; Lukas, D. The effect of ethylene oxide sterilization on electrospun vascular grafts made from biodegradable polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Prosecka, E.; Rampichova, M.; Litvinec, A.; Tonar Zm Kralickova, M.; Vojtova, L.; Kochova, P.; Plencner, M.; Buzgo, M.; Mickova, A.; Jancar, J.; et al. Collagen/hydroxyapatite scaffold enriched with polycaprolactone nanofibers, thrombocyte-rich solution and mesenchymal stem cells promotes regeneration in large bone defect in vivo. J. Biomed. Mater. Res. A 2015, 103, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Rampichova, M.; Chvojka, J.; Jencova, V.; Kubikova, T.; Tonar, Z.; Erben, J.; Buzgo, M.; Dankova, J.; Litvinec, A.; Vocetkova, K.; et al. The combination of nanofibrous and microfibrous materials for enhancement of cell infiltration and in vivo bone tissue formation. Biomed. Mater. 2018, 13, 025004. [Google Scholar] [CrossRef] [PubMed]
- Nottelet, B.; Pektok, E.; Mandracchia, D.; Tille, J.C.; Walpoth, B.; Gurny, R.; Moller, M. Factorial design optimization and in vivo feasibility of poly(ε-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J. Biomed. Mater. Res. A 2009, 89, 865–875. [Google Scholar] [CrossRef]
- de Valence, S.; Tille, J.C.; Gilibert, J.P.; Mrowczynski, W.; Gurny, R.; Walpoth, B.H.; Moller, M. Advantages of bilayered vascular grafts for surgical applicability and tissue regeneration. Acta Biomater. 2012, 8, 3914–3920. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.C.; Chaabane, C.; Gurny, R.; Bochaton-Piallat, M.L.; Walpoth, B.H.; Moller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Tille, J.C.; de Valence, S.; Mandracchia, D.; Nottelet, B.; Innocente, F.; Gurny, R.; Moller, M.; Walpoth, B.H. Histological assessment of drug-eluting grafts related to implantation site. J. Dev. Biol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Yang, X.; Wei, J.; Lei, D.; Liu, Y.; Wu, W. Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation. Biomaterials 2016, 88, 34–47. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J.; et al. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep. 2017, 7, 3615. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of Resveratrol on Modulation of Endothelial Cells and Macrophages for Rapid Vascular Regeneration from Electrospun Poly(ε-caprolactone) Scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Q.; Zhao, L.; Pan, Y.; Wang, T.; Zhi, D.; Ma, S.; Zhang, P.; Zhao, T.; Zhang, S.; et al. Functional Modification of Electrospun Poly(ε-caprolactone) Vascular Grafts with the Fusion Protein VEGF-HGFI Enhanced Vascular Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 11415–11427. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Xu, P.; Zhu, M.; Wu, Y.; Wang, Z.; Zhao, T.; Cheng, Q.; Wang, K.; Fan, G.; et al. Long-term evaluation of vascular grafts with circumferentially aligned microfibers in a rat abdominal aorta replacement model. J. Appl. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2596–2604. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Kocova, J. Overall staining of connective tissue and the muscular layer of vessels. Folia Morphol. 1970, 18, 293–295. [Google Scholar]
- Conklin, J.L. Staining properties of hyaline cartilage. Am. J. Anat. 1963, 112, 259–267. [Google Scholar] [CrossRef]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 4th ed.; Scion Publishing: Banbury, UK, 2008. [Google Scholar]
- Mouton, P.R. Principles and Practices of Unbiased Stereology. An Introduction for Bioscientists; The Johns Hopkins University Press: Baltimore, MD, USA, 2002. [Google Scholar]
- Tonar, Z.; Tomasek, P.; Loskot, P.; Janacek, J.; Kralickova, M.; Witter, K. Vasa vasorum in the tunica media and tunica adventitia of the porcine aorta. Ann. Anat. 2016, 205, 22–36. [Google Scholar] [CrossRef]
- Philimonenko, A.A.; Janacek, J.; Hozak, P. Statistical evaluation of colocalization patterns in immunogold labeling experiments. J. Struct. Biol. 2000, 132, 201–210. [Google Scholar] [CrossRef]
- Gundersen, H.J. Notes on the estimation of the numerical density of arbitrary profiles: The edge effect. J. Microsc. 1977, 111, 219–223. [Google Scholar] [CrossRef]
- Sterio, D.C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984, 134, 127–136. [Google Scholar] [CrossRef]
- Gundersen, H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef]
- Foldager, C.B.; Nyengaard, J.R.; Lind, M.; Spector, M. A Stereological Method for the Quantitative Evaluation of Cartilage Repair Tissue. Cartilage 2014, 6, 123–132. [Google Scholar] [CrossRef]
- Brown, D.L.; Staup, M.; Swanson, C. Stereology of the Peripheral Nervous System. Toxicol. Pathol. 2020, 48, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Van Vré, E.A.; van Beusekom, H.M.; Vrints, C.h.J.; Bosmans, J.M.; Bult, H.; Van der Giessem, W.J. Stereology: A simplified and more time-efficient method than planimetry for the quantitative analysis of vascular structures in different models of intimal thickening. Cardiovasc. Pathol. 2007, 16, 43–50. [Google Scholar] [CrossRef]
- Ingram, D.A.; Caplice, N.M.; Yoder, M.C. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 2005, 106, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Engler, A.J.; Raredon, M.S.; Le, A.; Baevova, P.; Yoder, M.C.; Niklason, L.E. Epac agonist improves barrier function in iPSC-derived endothelial colony forming cells for whole organ tissue engineering. Biomaterials 2019, 200, 25–34. [Google Scholar] [CrossRef] [PubMed]


| Staining | Purpose |
|---|---|
| hematoxylin–eosin [24] | overall morphology of the graft, foreign-body giant cells |
| hematoxylin only | nuclei of the cells infiltrating the graft |
| Verhoeff’s hematoxylin and green trichrome [25] | overall morphology, differentiation of the connective tissue, elastin and vascular smooth muscle |
| orcein (Tanzer’s orcein, Bowley Biochemical Inc., Danvers, MA, USA) | elastin fibers |
| picrosirius red (Direct Red 80, Sigma Aldrich, Munich, Germany) | type I and type III collagen when observed under circularly polarized light |
| PAS and alcian blue [26,27] | additional staining of the samples with cartilaginous metaplasia: PAS demonstrated neutral hexoses or sialic acid, alcian blue binds to acidic glycosaminoglycans at a pH of 2.5 |
| Antibody (and Staining Purpose) | Manufacturer | Dilution | Pretreatment |
|---|---|---|---|
| Monoclonal Mouse Anti-Human Smooth Muscle Actin, Clone 1A4 (marker of smooth muscle and myofibroblasts) | DakoCytomation (Glostrup, Denmark) | 1:100 | 20 min 96 °C Dako Target Retrieval Solution, pH 6 |
| Monoclonal Anti-CD34 antibody (endothelial marker) | Abcam (Cambridge, MA, USA) | 1:250 | 20 min 96 °C Dako Target Retrieval Solution, pH 6 |
| Monoclonal Anti-CD31 antibody Clone J70A (endothelial marker) | DakoCytomation) | 1:40 | 20 min 96 °C Dako Target Retrieval Solution, pH 9 |
| Polyclonal Rabbit, Anti-Human, von Willebrand factor, Code A0082 (endothelial marker) | DakoCytomation | 1:1000 | 10 min in chilled acetone, Proteinase K |
| Quantitative Parameter Abbreviation | Definition, Reference Space, Interpretation, and Units |
|---|---|
| QA-inner layer | Number (or two-dimensional density) of nuclei profiles found within the innermost (adluminal) third of the graft thickness in a transverse section (mm−2). |
| QA-middle layer | Number (or two-dimensional density) of nuclei profiles found within the middle third of the graft thickness in a transverse section (mm−2). |
| QA-outer layer | Number (or two-dimensional density) of nuclei profiles found within the outer (abluminal) third of the graft thickness in a transverse section (mm−2). |
| QA-mean per graft | The mean number (or two-dimensional density) of nuclei profiles found within the whole graft in a transverse section (mm−2). |
| f(graft) | Mean relative distance of the nuclei profiles found within the graft from the outer border of the graft. A dimensionless parameter ranging between 0 and 1, where the value = 0 refers to the nuclei profiles directly on the outer graft border, and the value = 1 refers to the nuclei on the luminal border of the neointima (-). |
| Graft thickness | Thickness of the graft measured as the mean distance between the adluminal (neointimal) surface profile (line 1) and the outer abluminal (outer) border profile (line 2) (µm). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horakova, J.; Blassova, T.; Tonar, Z.; McCarthy, C.; Strnadova, K.; Lukas, D.; Mikes, P.; Bowen, P.; Guillory, R., II; Frost, M.; et al. An Assessment of Blood Vessel Remodeling of Nanofibrous Poly(ε-Caprolactone) Vascular Grafts in a Rat Animal Model. J. Funct. Biomater. 2023, 14, 88. https://doi.org/10.3390/jfb14020088
Horakova J, Blassova T, Tonar Z, McCarthy C, Strnadova K, Lukas D, Mikes P, Bowen P, Guillory R II, Frost M, et al. An Assessment of Blood Vessel Remodeling of Nanofibrous Poly(ε-Caprolactone) Vascular Grafts in a Rat Animal Model. Journal of Functional Biomaterials. 2023; 14(2):88. https://doi.org/10.3390/jfb14020088
Chicago/Turabian StyleHorakova, Jana, Tereza Blassova, Zbynek Tonar, Connor McCarthy, Katerina Strnadova, David Lukas, Petr Mikes, Patrick Bowen, Roger Guillory, II, Megan Frost, and et al. 2023. "An Assessment of Blood Vessel Remodeling of Nanofibrous Poly(ε-Caprolactone) Vascular Grafts in a Rat Animal Model" Journal of Functional Biomaterials 14, no. 2: 88. https://doi.org/10.3390/jfb14020088
APA StyleHorakova, J., Blassova, T., Tonar, Z., McCarthy, C., Strnadova, K., Lukas, D., Mikes, P., Bowen, P., Guillory, R., II, Frost, M., & Goldman, J. (2023). An Assessment of Blood Vessel Remodeling of Nanofibrous Poly(ε-Caprolactone) Vascular Grafts in a Rat Animal Model. Journal of Functional Biomaterials, 14(2), 88. https://doi.org/10.3390/jfb14020088