Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADP-5 Synthesis
2.2. Incorporation of the ADP-5 Peptide in the GX Hydrogel
2.3. Cell Culture
2.4. Cell Metabolic Activity
2.5. Cell Morphology
2.6. Mineralization Ability of hPDL and OCM.30 Cells
2.7. Inflammatory Mediators Production
2.8. Statistical Analyses
3. Results
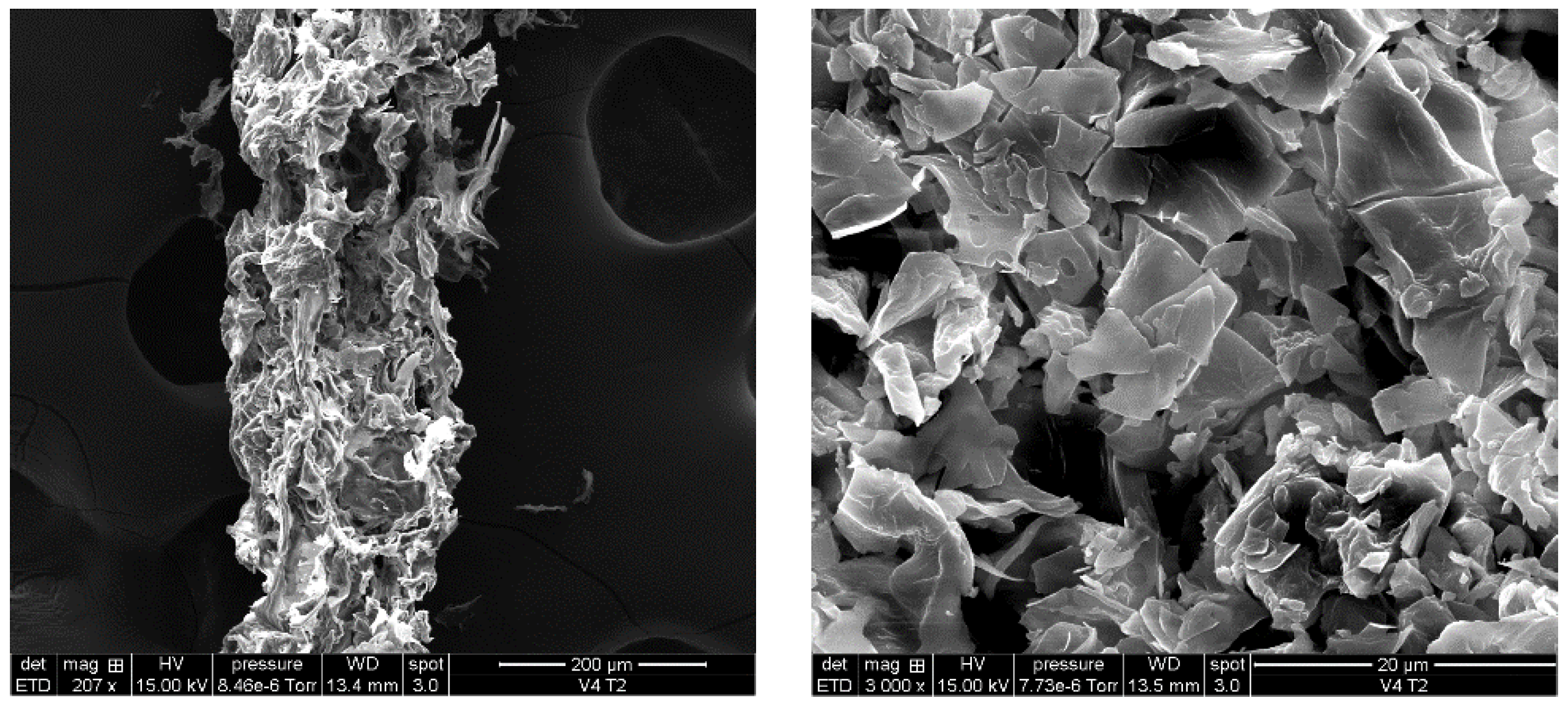
3.1. ADP-5 Structure by SEM
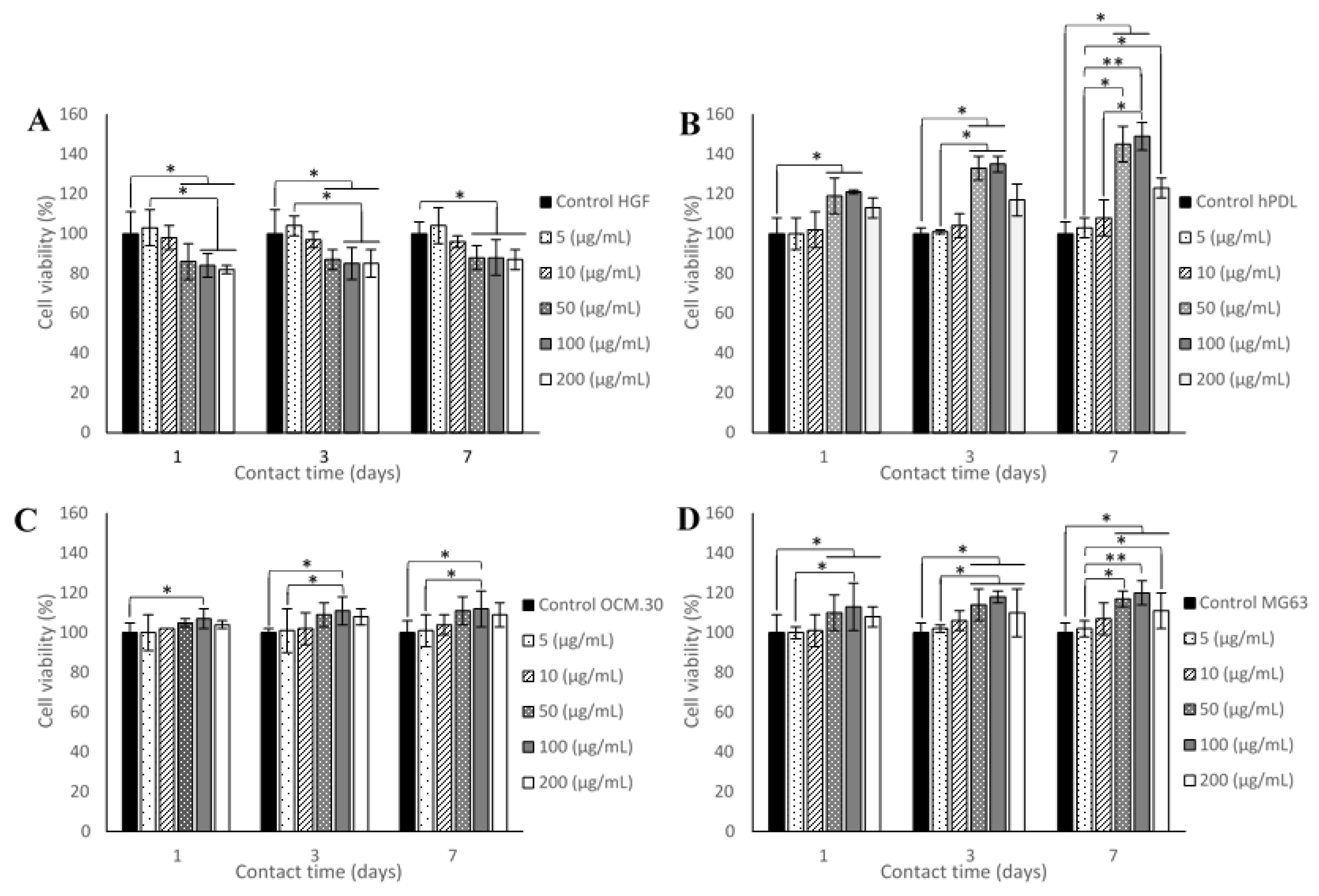
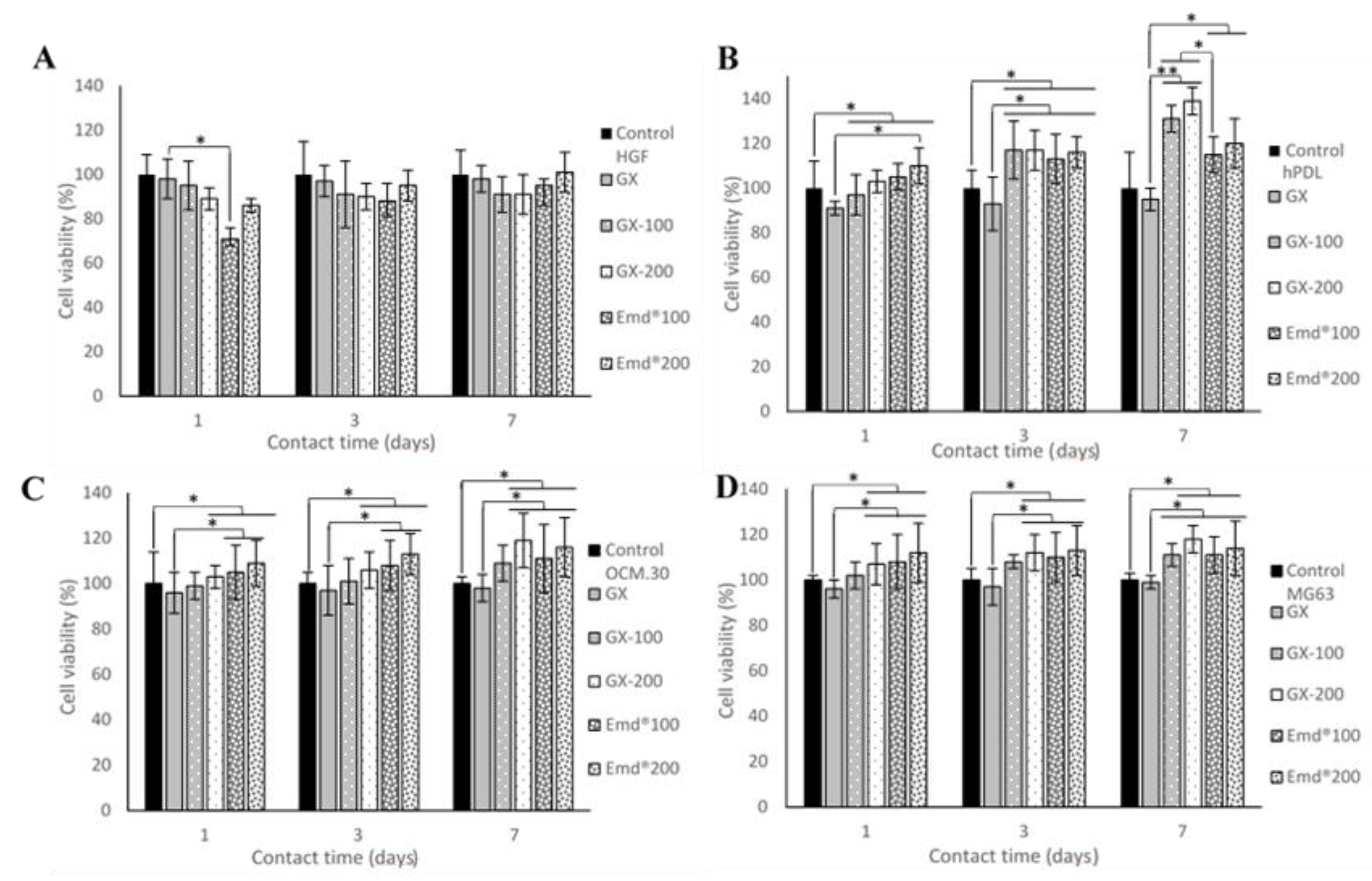
3.2. Cell Metabolic Activity
3.2.1. Effect of ADP-5 Alone on Cell Viability
3.2.2. Effect of GX Hydrogels Containing ADP-5 Versus Emdogain® Gels on Cell Viability
3.3. Cell Morphology and Spreading
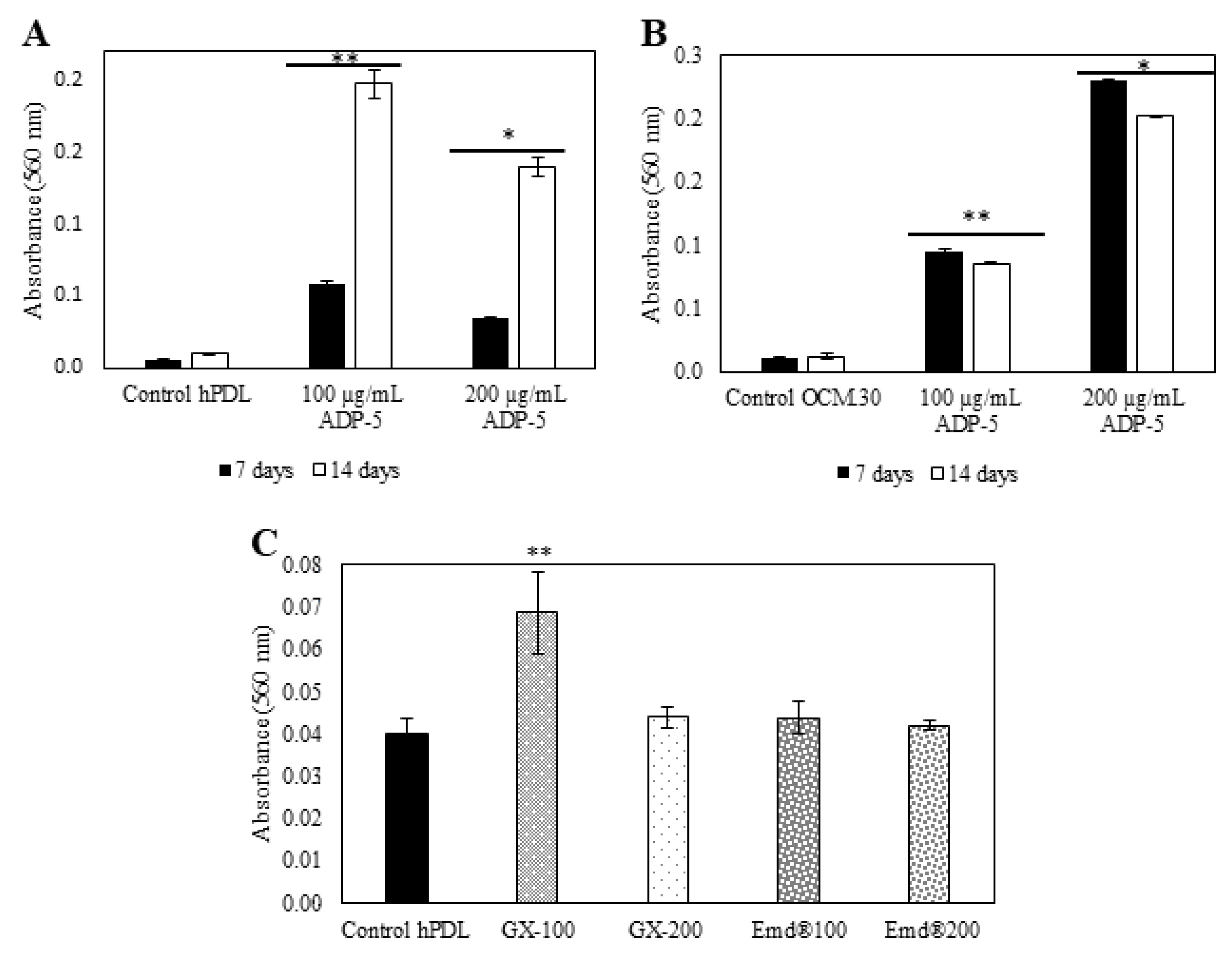
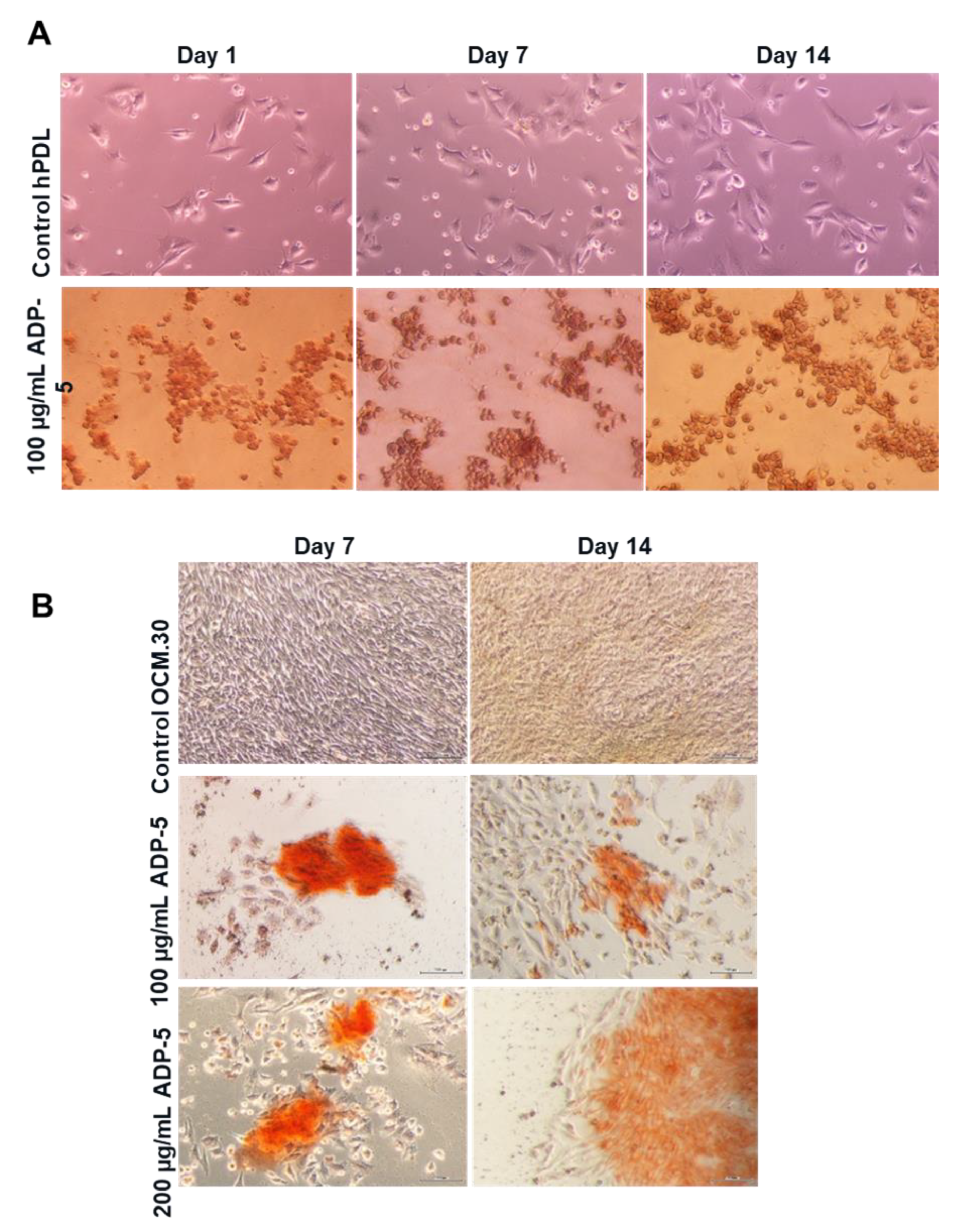
3.4. hPDL and OCM.30 Mineralization Ability by Alizarin Red Staining
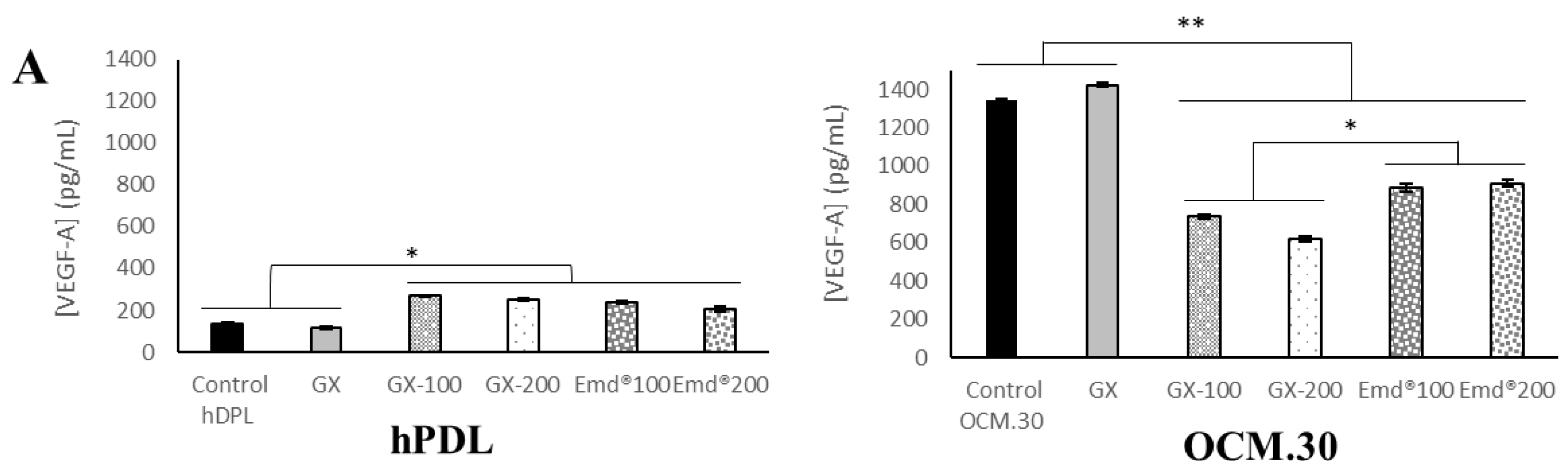
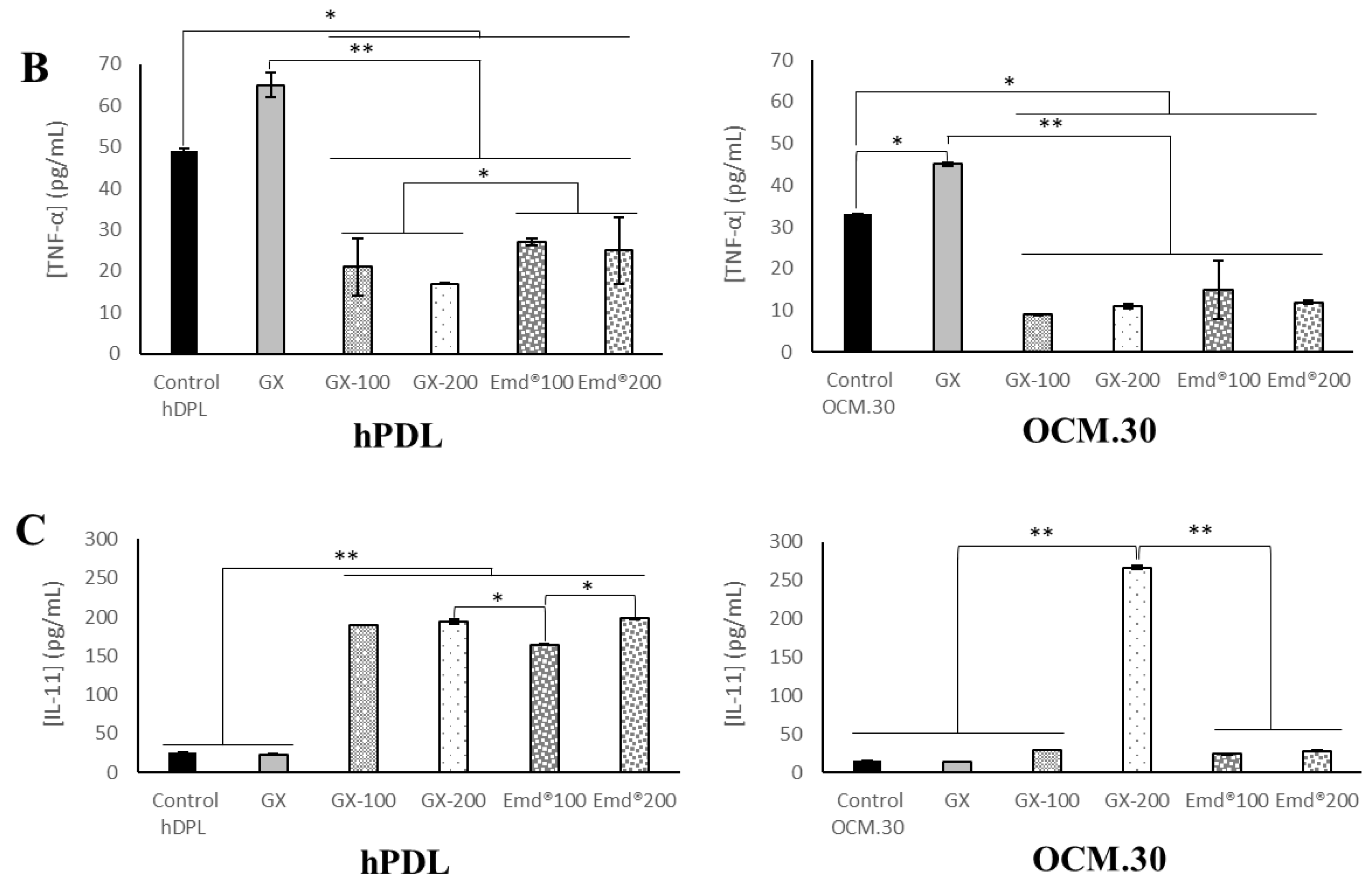
3.5. hPDL and OCM.30 Cells Inflammatory Mediator Quantification by ELISA Assay
4. Discussion
5. Conclusions
- -
- Enhanced proliferation, adhesion, and spreading of all periodontal cell types, and especially periodontal ligament cells;
- -
- Slight inhibition of human gingival fibroblasts proliferation;
- -
- Enhancement in periodontal cell mineralization potential;
- -
- Improvement of key inflammatory mediators.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primer 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Mariotti, A. The Future of Periodontal-Systemic Associations: Raising the Standards. Curr. Oral Health Rep. 2017, 4, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Minervini, G.; Basili, M.; Franco, R.; Bollero, P.; Mancini, M.; Gozzo, L.; Romano, G.L.; Marrapodi, M.M.; Gorassini, F.; D’Amico, C.; et al. Periodontal Disease and Pregnancy: Correlation with Underweight Birth. Eur. J. Dent. 2022, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.A.; Akhter, R.; Coulton, K.M.; Nhu Vo, N.T.; Duong, L.T.Y.; Nong, H.V.; Yaacoub, A.; Condous, G.; Eberhard, J.; Nanan, R. Periodontitis and preeclampsia in pregnancy: A systematic revieuw and meta-analysis. Matern. Child Health J. 2022, 26, 2418–2443. [Google Scholar] [CrossRef]
- Delbove, T.; Gueyffier, F.; Juillard, L.; Kalbacher, E.; Maucort-Boulch, D.; Nony, P.; Grosgogeat, B.; Gritsch, K. Effect of periodontal treatment on the glomerular filtration rate, reduction of inflammatory markers and mortality in patients with chronic kidney disease: A systematic review. PLoS ONE 2021, 16, e0245619. [Google Scholar] [CrossRef]
- Bi, W.G.; Emami, E.; Luo, Z.C.; Santamaria, C.; Wei, S.Q. Effect of periodontal treatment in pregnancy on perinatal outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 3259–3268. [Google Scholar] [CrossRef]
- Sallum, E.A.; Ribeiro, F.V.; Ruiz, K.S.; Sallum, A.W. Experimental and clinical studies on regenerative periodontal therapy. Periodontol. 2000 2019, 79, 22–55. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Stadlinger, B.; Terheyden, H. Cell-to-cell communication-periodontal regeneration. Clin. Oral Impancts Res. 2015, 3, 229–2239. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000 2015, 68, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Huck, O.; Stutz, C.; Gegout, P.Y.; Özçelik, H.; Benkirane-Jessel, N.; Petit, C.; Batool, F. Nanomedicine and Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 9, 658–668. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef]
- Nagano, T.; Iwata, T.; Ogata, Y.; Tanabe, T.; Gomi, K.; Fukae, M.; Arai, T.; Oida, S. Effect of heat treatment on bioactivities of enamel matrix derivatives in human periodontal ligament (HPDL) cells. J. Periodontal Res. 2004, 39, 249–256. [Google Scholar] [CrossRef]
- Gungormus, M.; Oren, E.E.; Horst, J.A.; Fong, H.; Hnilova, M.; Somerman, M.J.; Snead, M.; Samudrala, R.; Tamerler, C.; Sarikaya, M. Cementomimetics—Constructing a cementum-like biomineralized microlayer via amelogenin-derived peptides. Int. J. Oral Sci. 2012, 4, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Amin, D.; Olson, I.; Knowles, J.; Dard, M.; Donos, N. Interaction of enamel matrix proteins with human periodontal ligament cells. Clin. Oral Investig. 2016, 20, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Feng, X.; Guo, H.; Zhang, T.; Zhao, H.; Zhang, Q. Remineralization Efficacy of an Amelogenin-Based Synthetic Peptide on Carious Lesions. Front. Physiol. 2018, 9, 842. [Google Scholar] [CrossRef]
- Ding, L.; Han, S.; Wanf, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 3, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Fan, Y.; Zhou, Z.; Tu, H.; Li, D.; Lv, X.; Ding, L.; Zhang, L. Promotion of enamel caries remineralization by an amelogenin-derived peptide in a rat model. Arch. Oral Biol. 2017, 73, 66–71. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993; Biological Evaluation of Dental Devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Prapulla, D.V.; Sujatha, P.B.; Pradeep, A.R. Gingival Crevicular Fluid VEGF Levels in Periodontal Health and Disease. J. Periodontol. 2007, 78, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ö.Ö.; Berker, E.; Gariboğlu, S.; Otlu, H. Interleukin-11, interleukin-1β, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J. Clin. Periodontol. 2008, 35, 365–370. [Google Scholar] [CrossRef]
- Schroeder, H.E. Biological problems of regenerative cementogenesis: Synthesis and attachment of collagenous matrices on growing and established root surfaces. Int. Rev. Cytol. 1992, 142, 1–59. [Google Scholar]
- MacNeil, R.L.; Somerman, M.J. Development and regeneration of the periodontium: Parallels and contrasts. Periodontol. 2000 1999, 19, 8–20. [Google Scholar] [CrossRef]
- Wang, Y.L.; He, H.; Liu, Z.J.; Cao, Z.G.; Wang, X.Y.; Yang, K.; Huo, F.Y. Effects of TNF-α on Cementoblast Differentiation, Mineralization, and Apoptosis. J. Dent. Res. 2015, 94, 1225–1232. [Google Scholar] [CrossRef]
- Liao, F.; Chen, Y.; Li, Z.; Wang, Y.; Shi, B.; Gong, Z.; Cheng, X. A novel bioactive three-dimensional β-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 489–496. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J. Periodontal Res. 2014, 49, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Salètes, M.; Vartin, M.; Mocquot, C.; Chevalier, C.; Grosgogeat, B.; Colon, P.; Colon, P.; Attik, N. Mesoporous Bioactive Glasses Cytocompatibility Assessment: A Review of In Vitro Studies. Biomimetics 2021, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.; Yan, W.; Yan, J.; Tong, W.; Chen, W.; Lin, Y. LPCGF and EDTA conditioning of the root surface promotes the adhesion, growth, migration and differentiation of periodontal ligament cells. J. Periodontol. 2021, 92, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kitagawa, M.; Sakamoto, K.; Iizuka, S.; Kudo, Y.; Ogawa, I.; Miyauchi, M.; Chu, E.Y.; Foster, B.L.; Somerman, M.J.; et al. Enamel Matrix Derivative Exhibits Anti-Inflammatory Properties in Monocytes. J. Periodontol. 2008, 79, 535–540. [Google Scholar] [CrossRef]
- Martins, L.; Amorim, B.R.; Salmon, C.R.; Leme, A.F.P.; Kantovitz, K.R.; Nociti, F.H. Novel LRAP-binding partner revealing the plasminogen activation system as a regulator of cementoblast differentiation and mineral nodule formation in vitro. J. Cell. Physiol. 2020, 235, 4545–4558. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Du, M.; Liu, Z.; Cao, Z.; Hao, Y.; He, H. Inhibition of Stat3 signaling pathway decreases TNF-α-induced autophagy in cementoblasts. Cell Tissue Res. 2018, 374, 567–575. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Liao, H.; Wang, C.; Jiang, C.; Zhang, Y.; Cao, Z. MicroRNA-155-3p Mediates TNF-α-Inhibited Cementoblast Differentiation. J. Dent. Res. 2017, 96, 1430–1437. [Google Scholar] [CrossRef]
- Gestrelius, S.; Andersson, C.; Lidstrom, D.; Hammarstrom, L.; Somerman, M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 1997, 24, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Okuda, K.; Yoshie, H.; Burns, D.M. Cytostatic action of enamel matrix derivative (EMDOGAIN) on human oral squamous cell carcinoma-derived SCC25 epithelial cells. J. Periodontal Res. 2000, 35, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.M.; Oates, T.W.; Cochran, D.L. In Vitro Wound Healing Responses to Enamel Matrix Derivative. J. Periodontol. 2000, 71, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, C.; Colon, P.; Fernando, D.; Jackson, P.; Pradelle-Plasse, N.; Grosgogeat, B.; Attik, N. The influence of experimental bioactive glasses on pulp cells behavior in vitro. Dent. Mater. 2020, 36, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Zhou, Y.; Li, J.; Hu, M.; Li, X.; Wang, P.; Jia, Z.; Li, L.; Liu, D. Azithromycin Promotes the Osteogenic Differentiation of Human Periodontal Ligament Stem Cells after Stimulation with TNF-α. Stem Cells Int. 2018, 2018, 7961962. [Google Scholar] [CrossRef] [Green Version]
- Pontremoli, C.; Izquierdo-Barba, I.; Montalbano, G.; Vallet-Regí, M.; Vitale-Brovarone, C.; Fiorilli, S. Strontium-releasing mesoporous bioactive glasses with anti-adhesive zwitterionic surface as advanced biomaterials for bone tissue regeneration. J. Colloid Interface Sci. 2020, 563, 92–103. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Cao, Z.; Fang, Y.; Du, M.; Liu, Z. Regulatory effects of bone morphogenetic protein-4 on tumour necrosis factor-α-suppressed Runx2 and osteoprotegerin expression in cementoblasts. Cell Prolif. 2017, 50, e12344. [Google Scholar] [CrossRef] [Green Version]
- Yamamichi, K.; Fukuda, T.; Sanui, T.; Toyoda, K.; Tanaka, U.; Nakao, Y.; Yotsumoto, K.; Yamato, H.; Taketomi, T.; Uchiumi, T.; et al. Amelogenin induces M2 macrophage polarisation via PGE2/cAMP signalling pathway. Arch. Oral Biol. 2017, 83, 241–251. [Google Scholar] [CrossRef]
- Gölz, L.; Memmert, S.; Rath-Deschner, B.; Jäger, A.; Appel, T.; Baumgarten, G.; Götz, W.; Frede, S. Hypoxia and P. gingivalis Synergistically Induce HIF-1 and NFκB Activation in PDL Cells and Periodontal Diseases. Mediat. Inflamm. 2015, 2015, 438085. [Google Scholar] [CrossRef] [Green Version]
- Teramatsu, Y.; Maeda, H.; Sugii, H.; Tomokiyo, A.; Hamano, S.; Wada, N.; Yuda, A.; Yamamoto, N.; Koori, K.; Akamine, A. Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell Tissue Res. 2014, 357, 633–643. [Google Scholar] [CrossRef]
- Agis, H.; Watzek, G.; Gruber, R. Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor by periodontal fibroblasts: Fibroblasts and PHD inhibitors. J. Periodontal Res. 2012, 47, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for IL-11 in inflammatory diseases. Cy. Emerging roles for IL-11 in inflammatory diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr. Med. Chem. 2021, 28, 3032–3058. [Google Scholar] [CrossRef]
- Aspriello, S.D.; Zizzi, A.; Spazzafumo, L.; Rubini, C.; Lorenzi, T.; Marzioni, D.; Bullon, P.; Piemontese, M. Effects of Enamel Matrix Derivative on Vascular Endothelial Growth Factor Expression and Microvessel Density in Gingival Tissues of Periodontal Pocket: A Comparative Study. J. Periodontol. 2011, 82, 606–612. [Google Scholar] [CrossRef]
- Cetinkaya, B.O.; Keles, G.C.; Ayas, B.; Sakallioglu, E.E.; Acikgoz, G. The expression of vascular endothelial growth factor in a rat model at destruction and healing stages of periodontal disease. J. Periodontol. 2007, 78, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, S.; Werthén, M.; Lyngstadaas, S.P.; Gretzer, C.; Thomsen, P. Amelogenins modulate cytokine expression in LPS-challenged cultured human macrophages. Cytokine 2012, 58, 274–279. [Google Scholar] [PubMed]
- Villa, O.; Wohlfahrt, J.C.; Koldsland, O.C.; Brookes, S.J.; Lyngstadaas, S.P.; Aass, A.M.; Reseland, J.E. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 2016, 6, 23060. [Google Scholar] [CrossRef]

| First investigation | ||
| Cell types | Conditions (ADP-5 concentration (µg/mL)) | |
| Human gingival fibroblasts (HGF) Human periodontal ligament (hPDL) Osteoblasts (MG63) Cementoblasts (OCM.30) | Control cells (glass substrate, no ADP-5) | |
| 5 | ||
| 10 | ||
| 50 | ||
| 100 | ||
| 200 | ||
| Second investigation | ||
| Cell types | Groups | Conditions |
| Human gingival fibroblasts (HGF) Human periodontal ligament (hPDL) Osteoblasts (MG63) Cementoblasts (OCM.30) | Control cells | Glass substrate, no hydrogel |
| GX | 3% GX hydrogel | |
| GX-100 | 3% GX hydrogel + 100 µg/mL ADP-5 | |
| GX-200 | 3% GX hydrogel + 200 µg/mL ADP-5 | |
| Emd®100 | Emdogain® 100 µg/mL (dilution compared to culture medium) | |
| Emd®200 | Emdogain® 200 µg/mL (dilution compared to culture medium) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attik, N.; Garric, X.; Bethry, A.; Subra, G.; Chevalier, C.; Bouzouma, B.; Verdié, P.; Grosgogeat, B.; Gritsch, K. Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile. J. Funct. Biomater. 2023, 14, 53. https://doi.org/10.3390/jfb14020053
Attik N, Garric X, Bethry A, Subra G, Chevalier C, Bouzouma B, Verdié P, Grosgogeat B, Gritsch K. Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile. Journal of Functional Biomaterials. 2023; 14(2):53. https://doi.org/10.3390/jfb14020053
Chicago/Turabian StyleAttik, Nina, Xavier Garric, Audrey Bethry, Gilles Subra, Charlène Chevalier, Brahim Bouzouma, Pascal Verdié, Brigitte Grosgogeat, and Kerstin Gritsch. 2023. "Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile" Journal of Functional Biomaterials 14, no. 2: 53. https://doi.org/10.3390/jfb14020053
APA StyleAttik, N., Garric, X., Bethry, A., Subra, G., Chevalier, C., Bouzouma, B., Verdié, P., Grosgogeat, B., & Gritsch, K. (2023). Amelogenin-Derived Peptide (ADP-5) Hydrogel for Periodontal Regeneration: An In Vitro Study on Periodontal Cells Cytocompatibility, Remineralization and Inflammatory Profile. Journal of Functional Biomaterials, 14(2), 53. https://doi.org/10.3390/jfb14020053








