A Novel Hydrophilic, Antibacterial Chitosan-Based Coating Prepared by Ultrasonic Atomization Assisted LbL Assembly Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Antibacterial PLA Film
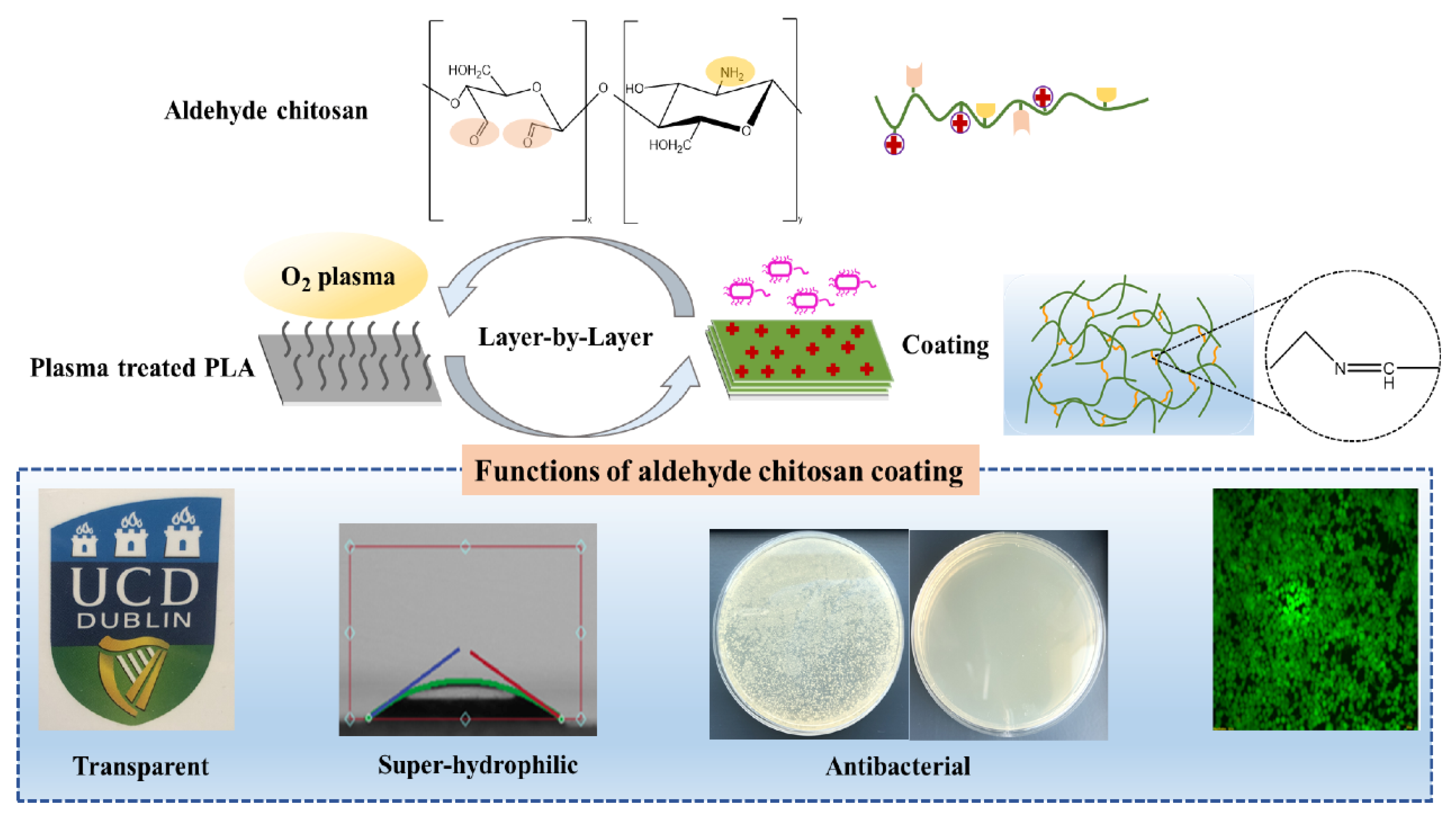
2.2.1. Preparation of CTS-CHO
2.2.2. Bioactive Ink Formulation
2.2.3. Preparation of Coatings
2.3. Characterizations
2.3.1. CTS-CHO
2.3.2. Bioactive Ink
2.3.3. Coating
3. Results and Discussion
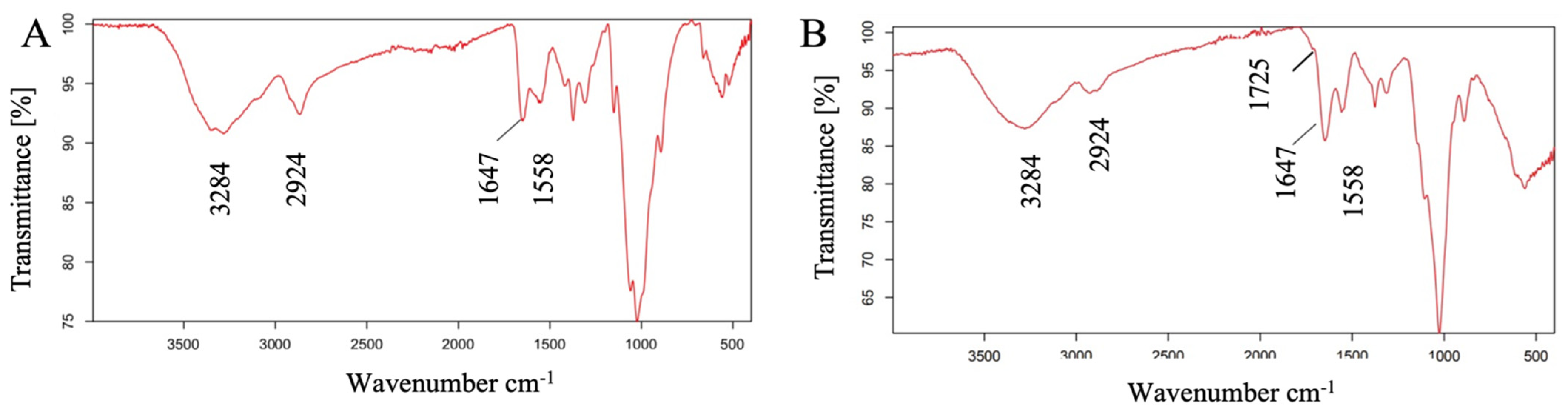
3.1. Characterization of CTS-CHO
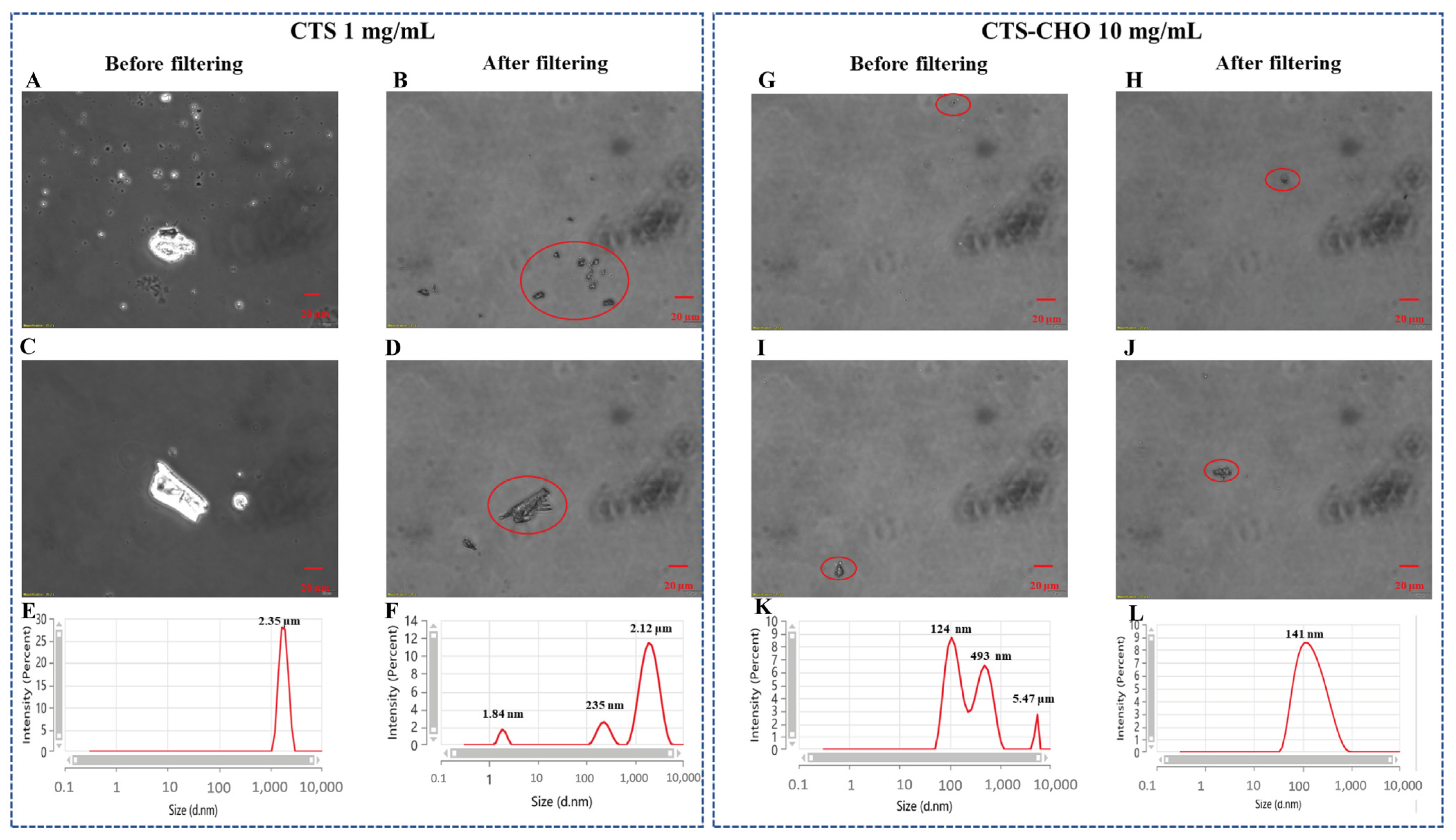
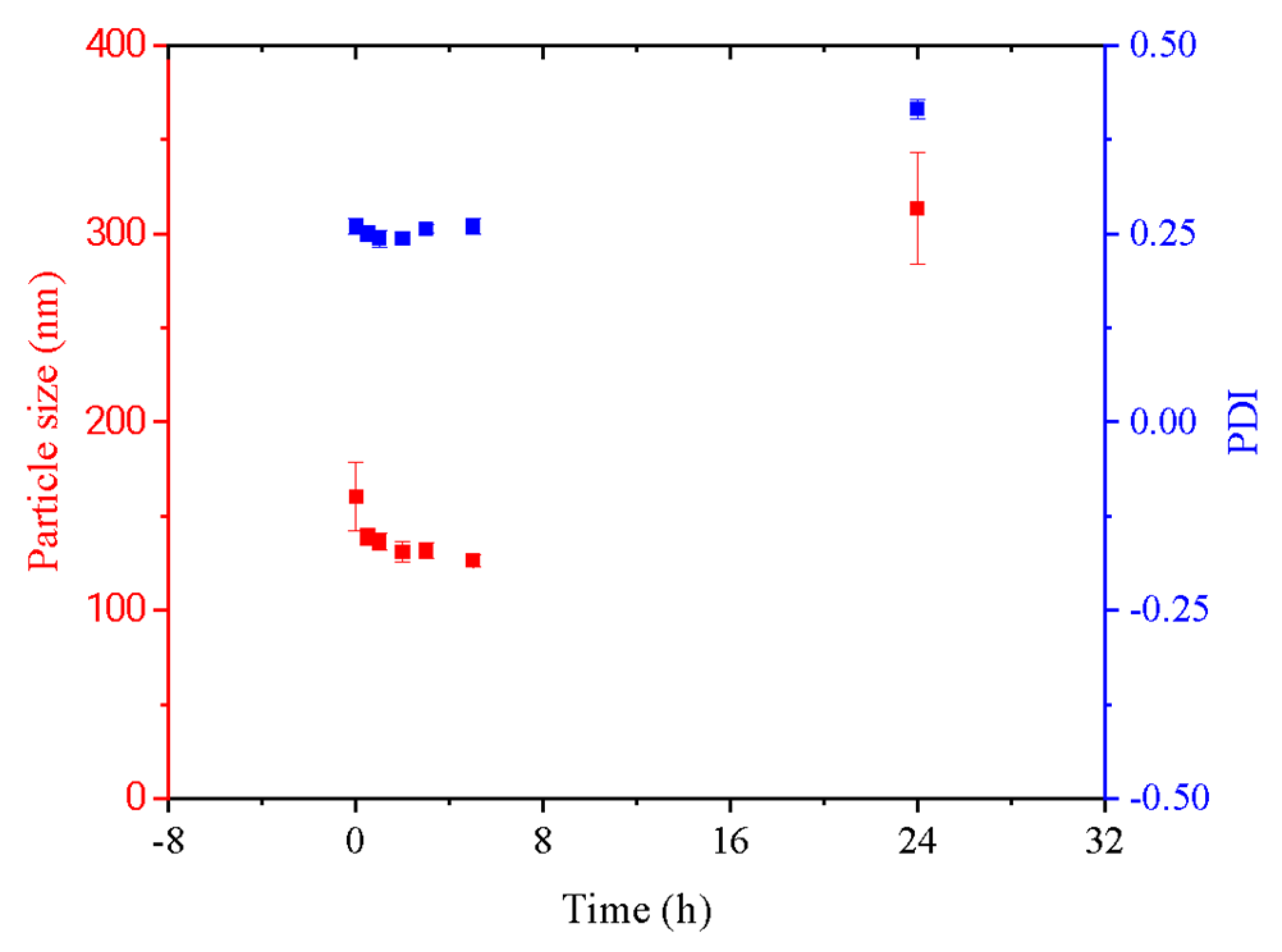
3.2. Characterization of Bioactive Inks
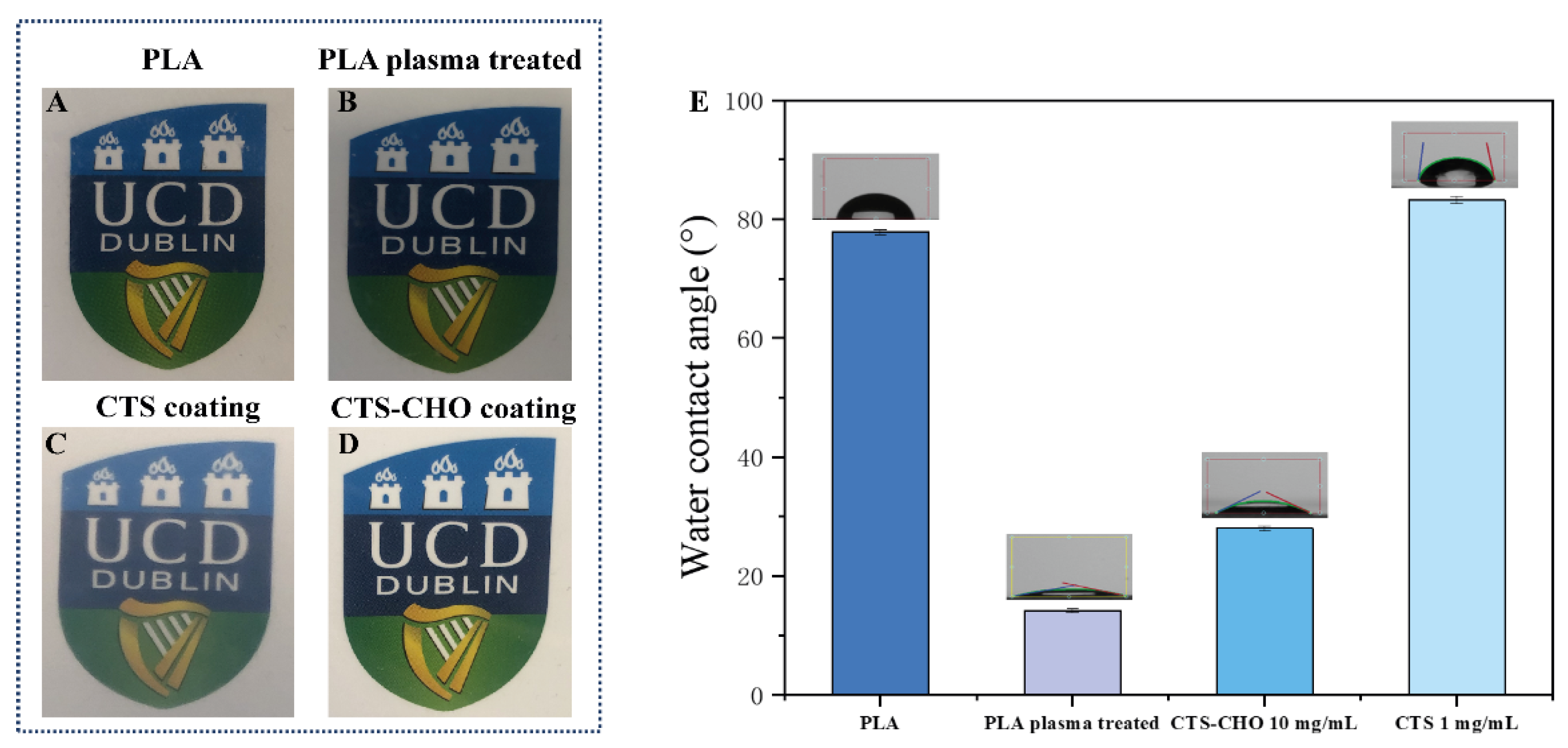
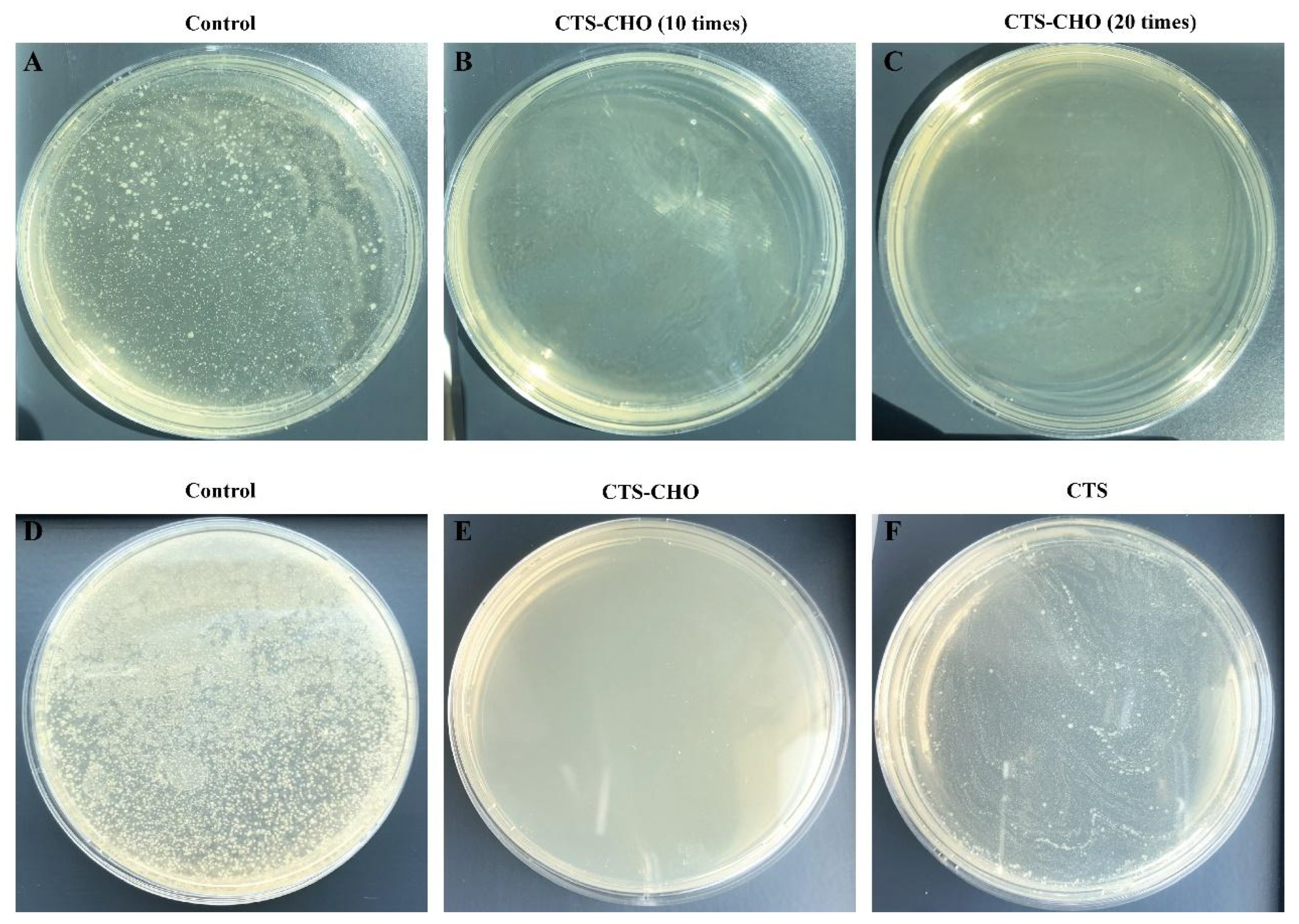
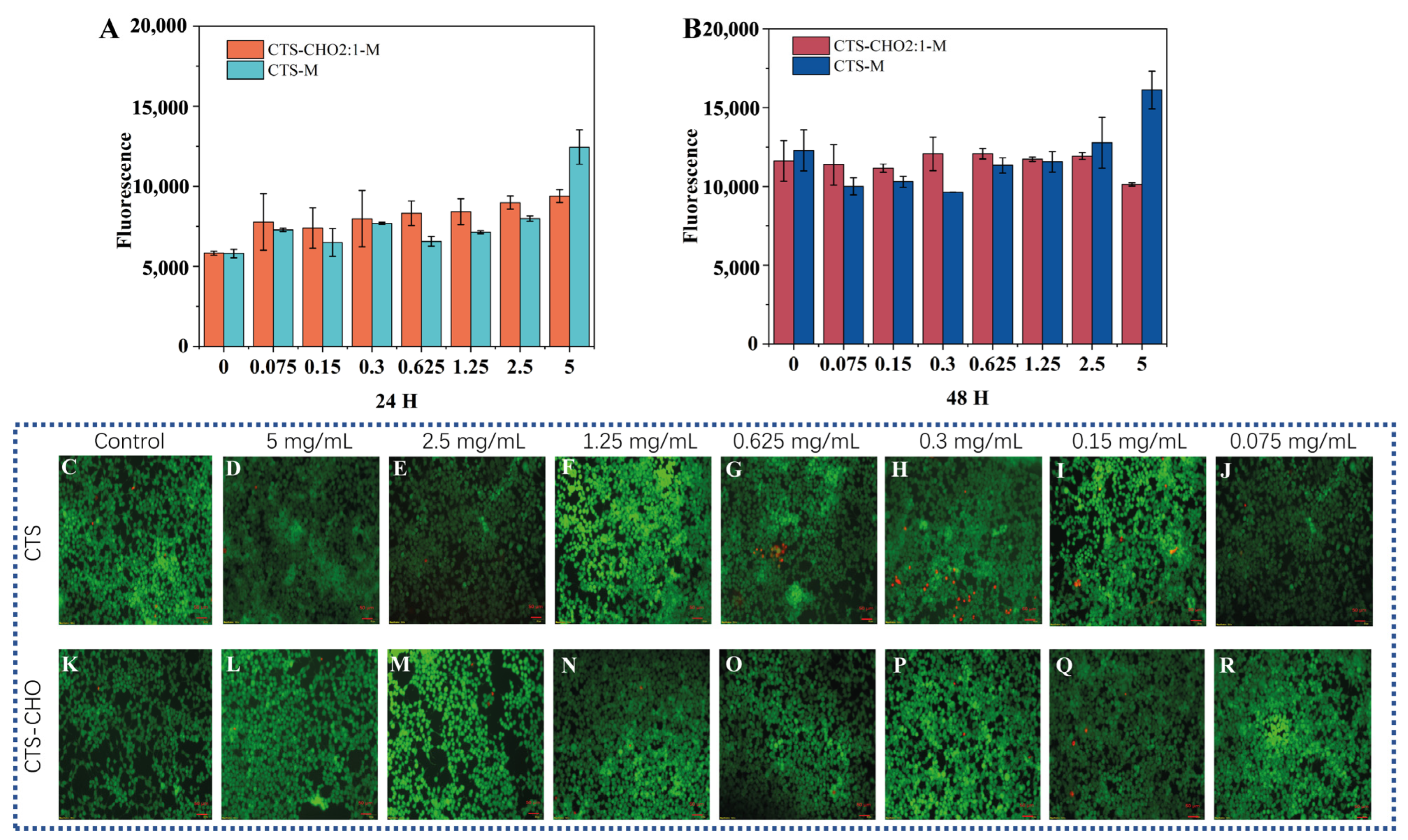
3.3. Characterization of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, H.L.; Du, Z.H.; Yang, B.; Wu, J.; Wang, R.; Zhang, X.Q. Hydrophilic and Antibacterial Modification of Poly(lactic acid) Films by gamma-ray Irradiation. ACS Omega 2019, 4, 21439–21445. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Chu, C.C. Materials for absorbable and nonabsorbable surgical sutures. In Biotextiles as Medical Implants; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–334. [Google Scholar]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnova, V.; Lukasova, V.; Bartos, M.; Necas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically biomimetic bone scaffold materials: Nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef]
- Singh, D.; Babbar, A.; Jain, V.; Gupta, D.; Saxena, S.; Dwibedi, V. Synthesis, characterization, and bioactivity investigation of biomimetic biodegradable PLA scaffold fabricated by fused filament fabrication process. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 121. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef]
- Rendeki, S.; Nagy, B.; Bene, M.; Pentek, A.; Toth, L.; Szanto, Z.; Told, R.; Maroti, P. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers 2020, 12, 2703. [Google Scholar] [CrossRef]
- Viera-Artiles, J.; Valdiande, J.J. 3D-printable headlight face shield adapter. Personal protective equipment in the COVID-19 era. Am. J. Otolaryngol. 2020, 41, 102576. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M. Antimicrobial Polymer-Based Materials for Food Packaging Applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Frine, V.C.; Hector, A.P.; Manuel, N.S.; Estrella, N.D.; Antonio, G.J. Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene-Cyclodextrin Complexes against Alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef]
- Yang, B.; Wang, R.; Ma, H.L.; Li, X.; Brunig, H.; Dong, Z.; Qi, Y.; Zhang, X. Structure Mediation and Properties of Poly(l-lactide)/Poly(d-lactide) Blend Fibers. Polymers 2018, 10, 1353. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films. Materials 2018, 11, 1973. [Google Scholar] [CrossRef] [PubMed]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Poly(D,L-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, E.; Frey, M. Increasing Surface Hydrophilicity in Poly(Lactic Acid) Electrospun Fibers by Addition of Pla-b-Peg Co-Polymers. J. Eng. Fibers Fabr. 2014, 9, 153–164. [Google Scholar] [CrossRef]
- Suganya, A.; Shanmugavelayutham, G.; Rodríguez, C.S. Study on plasma pre-functionalized PVC film grafted with TiO2/PVP to improve blood compatible and antibacterial properties. J. Phys. D Appl. Phys. 2017, 50, 145402. [Google Scholar] [CrossRef]
- Minati, L.; Migliaresi, C.; Lunelli, L.; Viero, G.; Dalla Serra, M.; Speranza, G. Plasma assisted surface treatments of biomaterials. Biophys. Chem. 2017, 229, 151–164. [Google Scholar] [CrossRef]
- Demina, T.S.; Piskarev, M.S.; Romanova, O.A.; Gatin, A.K.; Senatulin, B.R.; Skryleva, E.A.; Zharikova, T.M.; Gilman, A.B.; Kuznetsov, A.A.; Akopova, T.A.; et al. Plasma Treatment of Poly(ethylene terephthalate) Films and Chitosan Deposition: DC- vs. AC-Discharge. Materials 2020, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Mozetic, M.; Stana-Kleinschek, K.; Frohlich, M.; Turk, B.; Vesel, A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials 2015, 8, 1526–1544. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M.; Drobota, M.; Dimitriu, D.G.; Stoica, I.; Simionescu, B.; Harabagiu, V. Collagen immobilization on polyethylene terephthalate surface after helium plasma treatment. Mater. Sci. Eng. B 2013, 178, 1303–1310. [Google Scholar] [CrossRef]
- Recek, N.; Jaganjac, M.; Kolar, M.; Milkovic, L.; Mozetic, M.; Stana-Kleinschek, K.; Vesel, A. Protein adsorption on various plasma-treated polyethylene terephthalate substrates. Molecules 2013, 18, 12441–12463. [Google Scholar] [CrossRef]
- Sophonvachiraporn, P.; Rujiravanit, R.; Sreethawong, T.; Tokura, S.; Chavadej, S. Surface Characterization and Antimicrobial Activity of Chitosan-Deposited DBD Plasma-Modified Woven PET Surface. Plasma Chem. Plasma Process. 2010, 31, 233–249. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E. Chitosan hydrophobic domains are favoured at low degree of acetylation and molecular weight. Polymer 2013, 54, 2081–2087. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Strnad, S. Improvement of adhesion of fucoidan on polyethylene terephthalate surface using gas plasma treatments. Vacuum 2011, 85, 1083–1086. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Gutierrez-Villarreal, M.H.; Ulloa-Hinojosa, M.G.; Gaona-Lozano, J.G. Surface functionalization of poly(lactic acid) film by UV-photografting ofN-vinylpyrrolidone. J. Appl. Polym. Sci. 2008, 110, 163–169. [Google Scholar] [CrossRef]
- Carosio, F.; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-Based Layer by Layer Assembly: Efficient and Sustainable Approach to Cotton Fire Protection. ACS Appl. Mater. Interfaces 2015, 7, 12158–12167. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Ultrasonic nebulization-assisted layer-by-layer assembly for spray coating of multilayered, multicomponent, bioactive nanostructures. J. Coat. Technol. Res. 2010, 8, 275–281. [Google Scholar] [CrossRef]
- Doshi, B.; Repo, E.; Heiskanen, J.P.; Sirvio, J.A.; Sillanpaa, M. Effectiveness of N,O-carboxymethyl chitosan on destabilization of Marine Diesel, Diesel and Marine-2T oil for oil spill treatment. Carbohydr. Polym. 2017, 167, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Sangsuwan, J.; Rattanapanone, N.; Rachtanapun, P. Effect of chitosan/methyl cellulose films on microbial and quality characteristics of fresh-cut cantaloupe and pineapple. Postharvest Biol. Technol. 2008, 49, 403–410. [Google Scholar] [CrossRef]
- Wang, X.; Song, R.; Johnson, M.; Asigen, S.; He, Z.; Milne, C.; Wang, X.; Lara-Saez, I.; Xu, Q.; Wang, W. An Injectable Chitosan-Based Self-Healable Hydrogel System as an Antibacterial Wound Dressing. Materials 2021, 14, 5956. [Google Scholar] [CrossRef]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of biostable scaffold based on collagen crosslinked by dialdehyde chitosan with presence of gallic acid. Int. J. Biol. Macromol. 2019, 130, 836–844. [Google Scholar] [CrossRef]
- Vold, I.M.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym Sci Ser A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Portnov, I.V.; Rudov, A.A.; Blagodatskikh, I.V.; Grigoriev, T.E.; Gallyamov, M.O.; Potemkin, I.I. Stabilization of Chitosan Aggregates at the Nanoscale in Solutions in Carbonic Acid. Macromolecules 2014, 47, 5749–5758. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kwon, I.C.; Kim, Y.H.; Jo, W.H.; Jeong, S.Y. Preparation of chitosan self-aggregates as a gene delivery system. J. Control. Release 1998, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Pichot, C.; Delair, T.; Viton, C.; Domard, A. Static Light Scattering Studies on Chitosan Solutions: From Macromolecular Chains to Colloidal Dispersions. Langmuir 2003, 19, 9896–9903. [Google Scholar] [CrossRef]
- You, J.; Hu, F.Q.; Du, Y.Z.; Yuan, H. Polymeric Micelles with Glycolipid-like Structure and Multiple Hydrophobic Domains for Mediating Molecular Target Delivery of Paclitaxel. Biomacromolecules 2007, 8, 2450–2456. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kato, Y.; Yoshida, Y.; Isogai, A. SEC-MALS study on aggregates of chitosan molecules in aqueous solvents: Influence of residual N-acetyl groups. Carbohydr. Polym. 2006, 66, 192–198. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2019; pp. 457–489. [Google Scholar] [CrossRef]
| Sample | Concentration (mg/mL) | Viscosity (cp) |
|---|---|---|
| CTS | 1 | 47.64 |
| 5 | 269.6 | |
| 10 | 399.1 | |
| CTS-CHO | 1 | 7.64 |
| 5 | 7.73 | |
| 10 | 7.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, Y.; Johnson, M.; Milne, C.; A, S.; Li, Y.; Wang, W.; Zhang, N.; Xu, Q. A Novel Hydrophilic, Antibacterial Chitosan-Based Coating Prepared by Ultrasonic Atomization Assisted LbL Assembly Technique. J. Funct. Biomater. 2023, 14, 43. https://doi.org/10.3390/jfb14010043
Wang X, Zhou Y, Johnson M, Milne C, A S, Li Y, Wang W, Zhang N, Xu Q. A Novel Hydrophilic, Antibacterial Chitosan-Based Coating Prepared by Ultrasonic Atomization Assisted LbL Assembly Technique. Journal of Functional Biomaterials. 2023; 14(1):43. https://doi.org/10.3390/jfb14010043
Chicago/Turabian StyleWang, Xiaoyu, Yuyang Zhou, Melissa Johnson, Cameron Milne, Sigen A, Yening Li, Wenxin Wang, Nan Zhang, and Qian Xu. 2023. "A Novel Hydrophilic, Antibacterial Chitosan-Based Coating Prepared by Ultrasonic Atomization Assisted LbL Assembly Technique" Journal of Functional Biomaterials 14, no. 1: 43. https://doi.org/10.3390/jfb14010043
APA StyleWang, X., Zhou, Y., Johnson, M., Milne, C., A, S., Li, Y., Wang, W., Zhang, N., & Xu, Q. (2023). A Novel Hydrophilic, Antibacterial Chitosan-Based Coating Prepared by Ultrasonic Atomization Assisted LbL Assembly Technique. Journal of Functional Biomaterials, 14(1), 43. https://doi.org/10.3390/jfb14010043











