Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation and Hot Extrusion
2.2. Microstructural Observations and Phase Composition
2.3. Corrosion Testing
2.4. Cytotoxicity Tests
3. Results
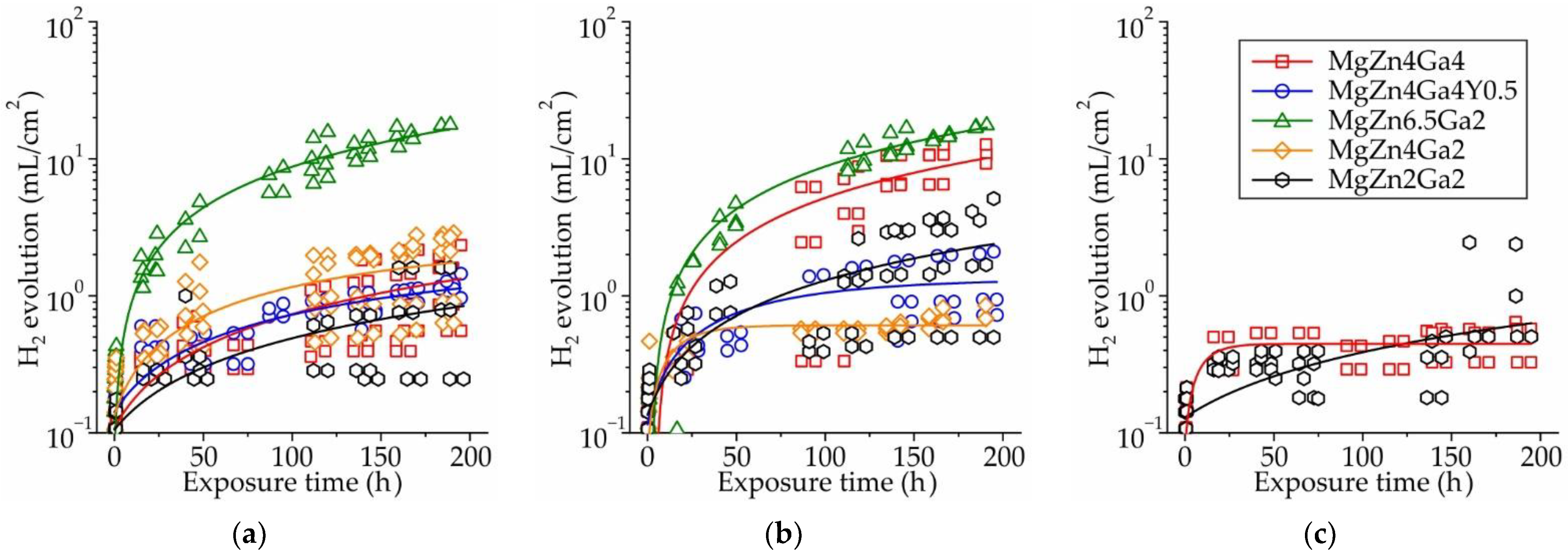
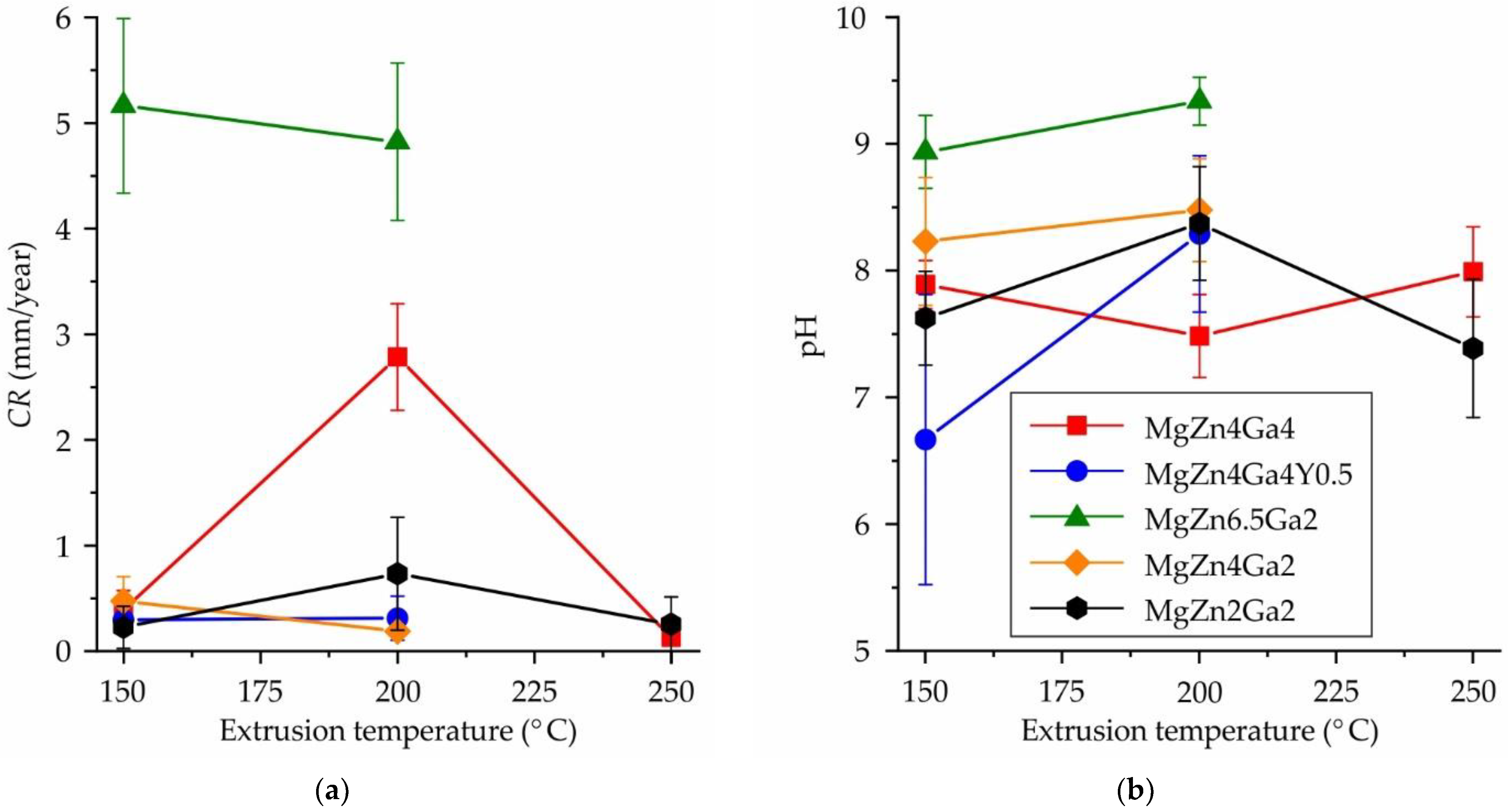
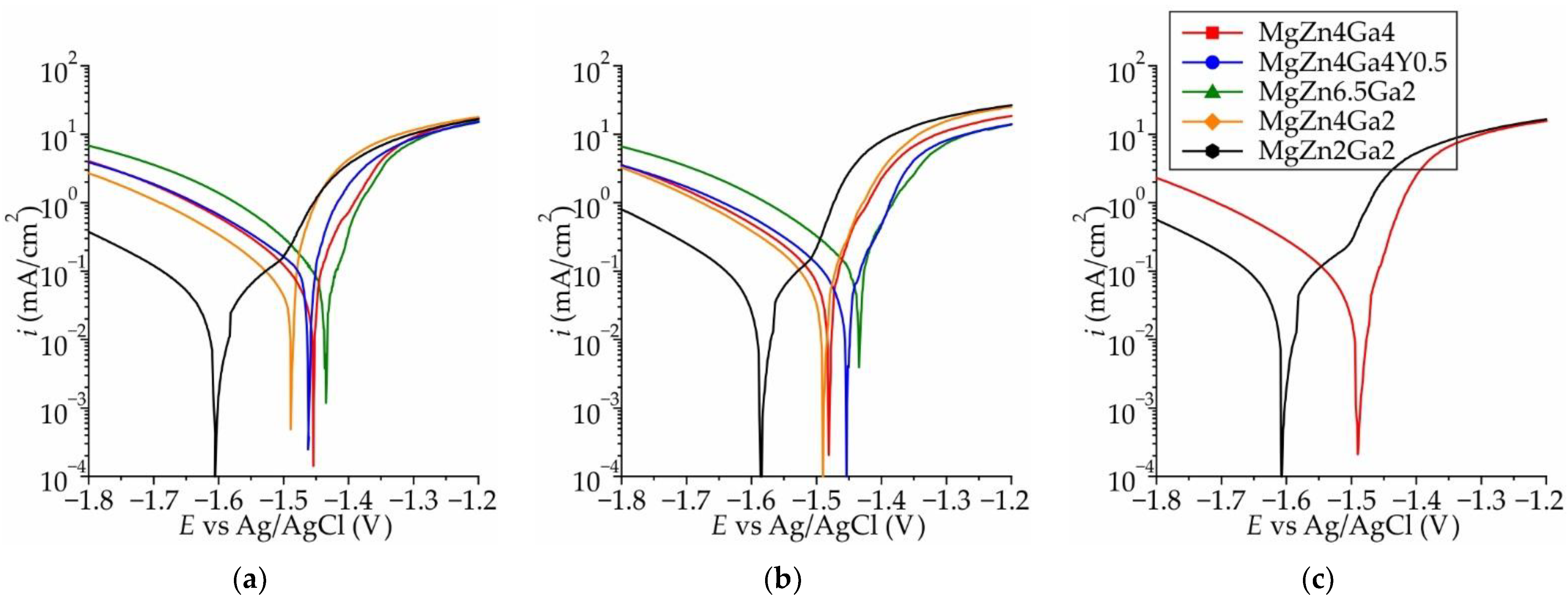
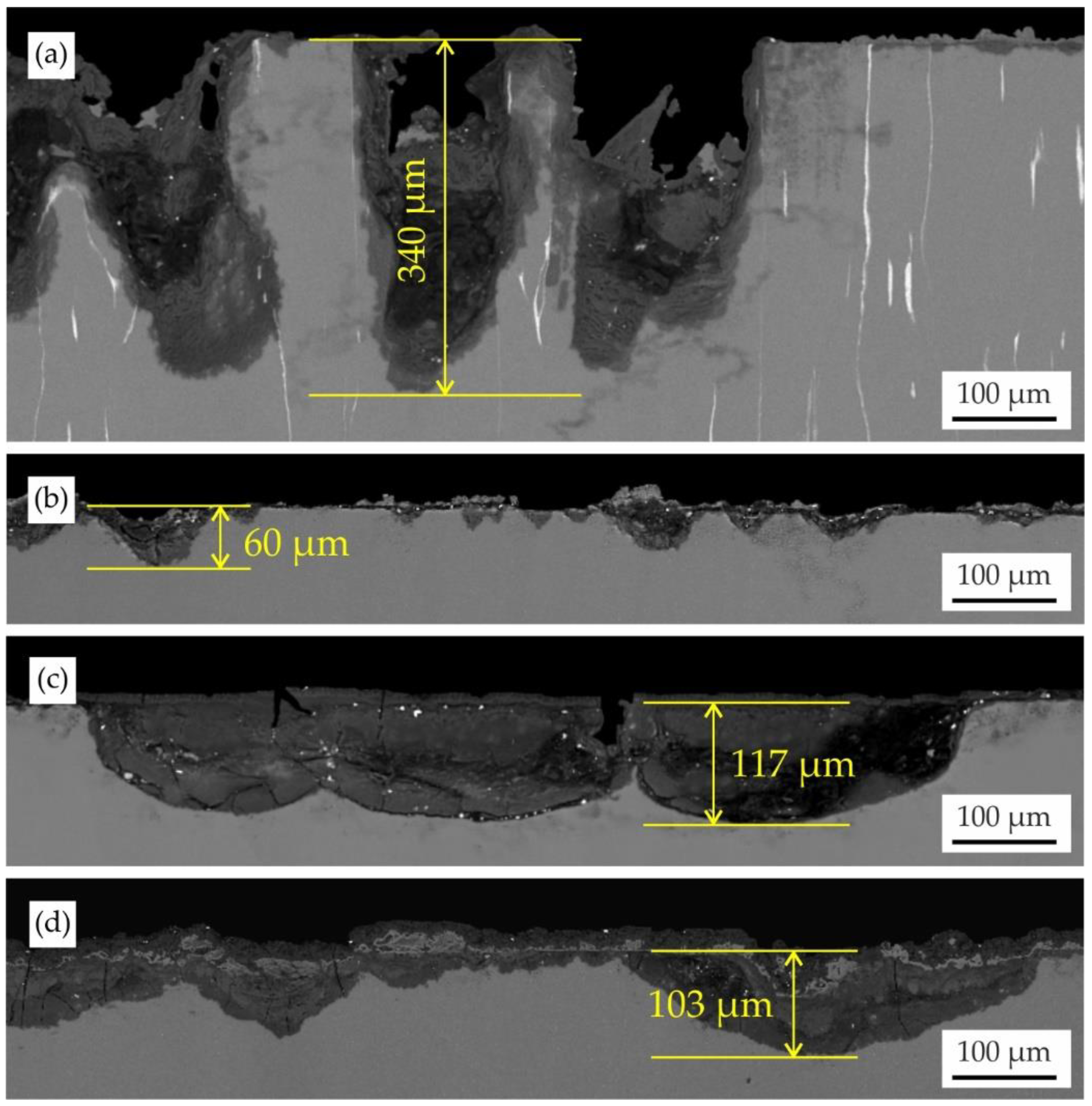
3.1. Corrosion Properties
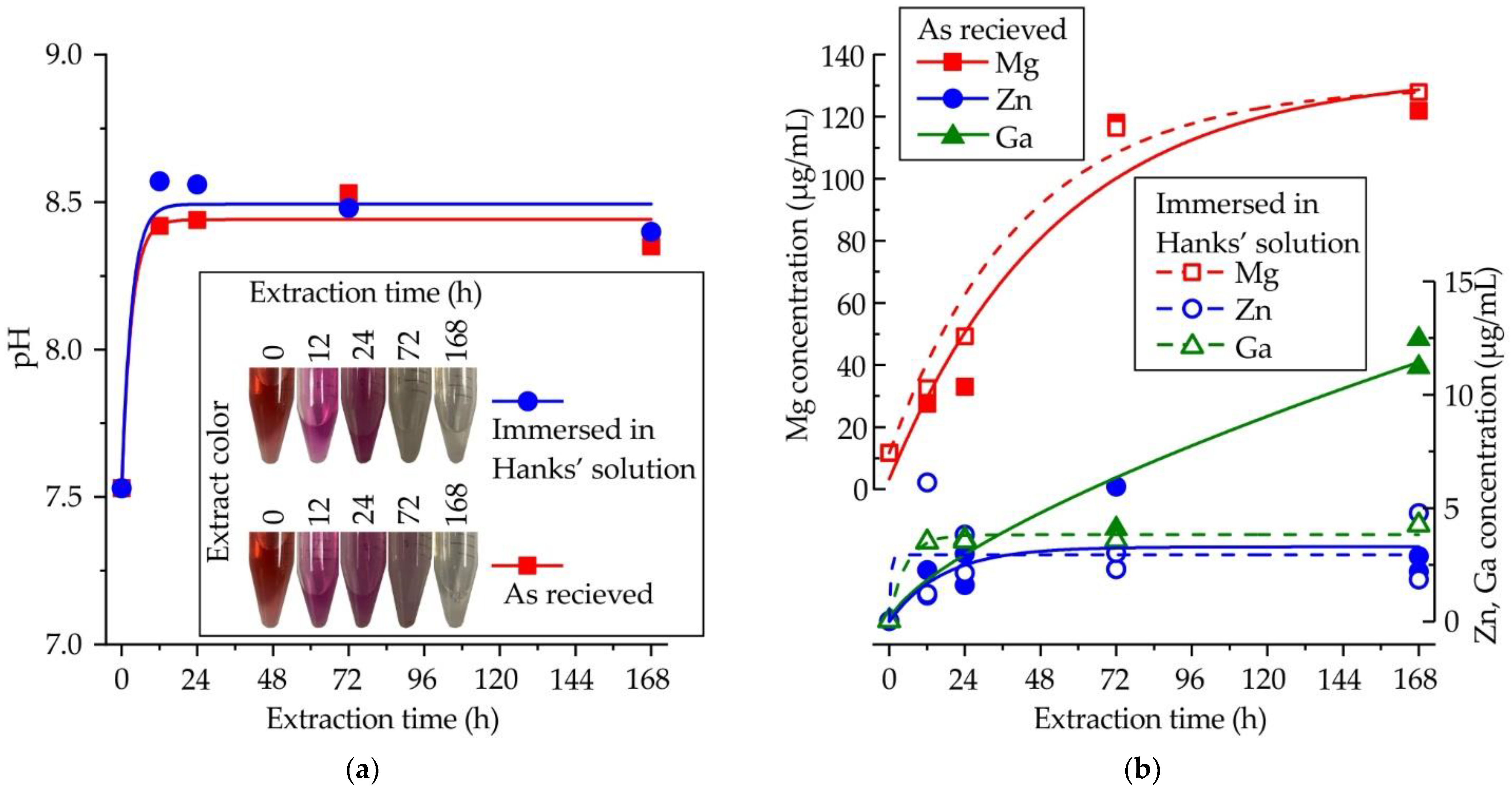
3.2. Cytotoxicity
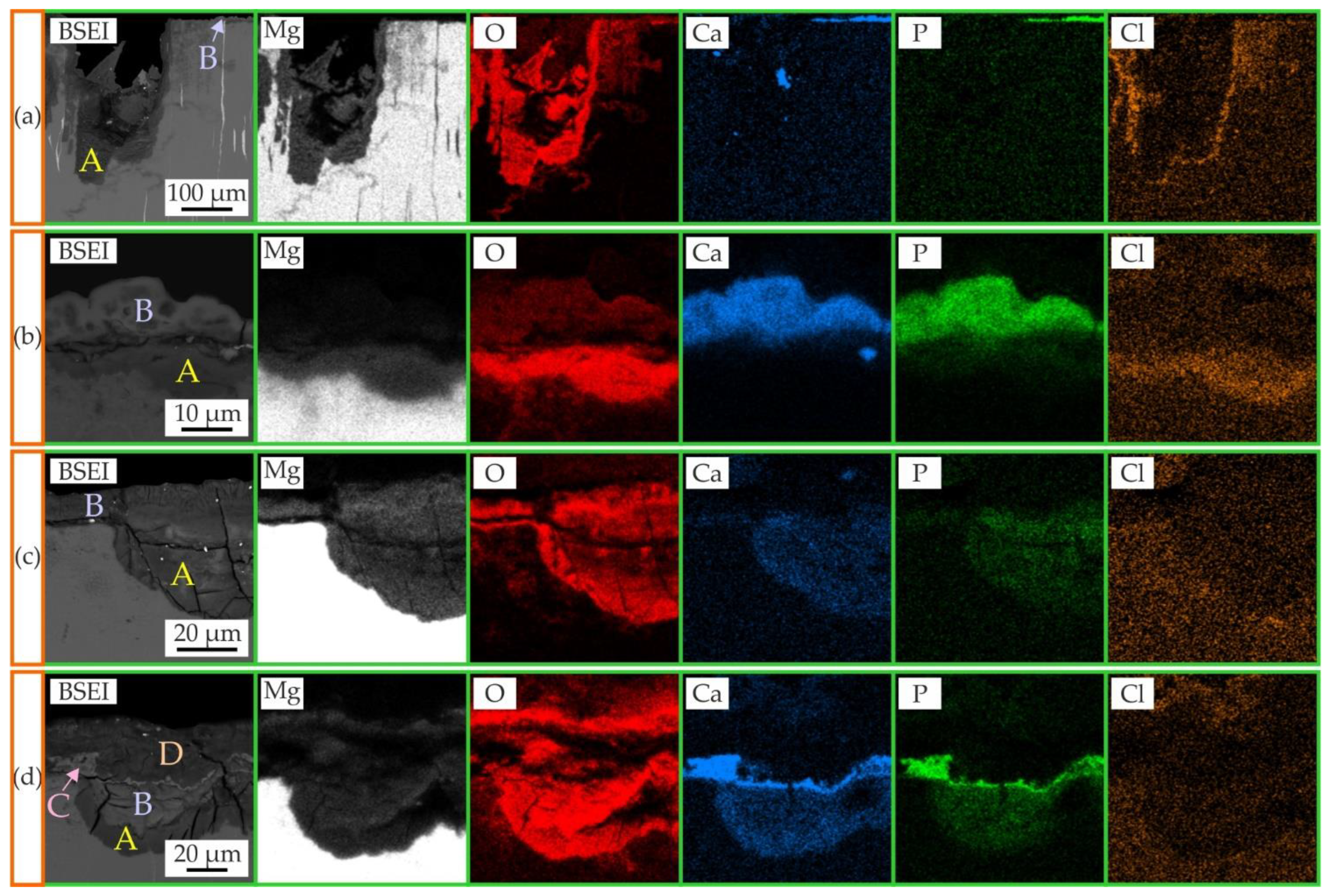
3.3. Surface Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Biodegradation of a Magnesium Alloy Fixation Screw Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 4111. [Google Scholar] [CrossRef] [PubMed]
- Delsmann, M.M.; Stürznickel, J.; Kertai, M.; Stücker, R.; Rolvien, T.; Rupprecht, M. Radiolucent Zones of Biodegradable Magnesium-Based Screws in Children and Adolescents—A Radiographic Analysis. Arch. Orthop. Trauma Surg. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2010, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-N.; Li, S.-S.; Li, X.-M.; Fan, Y.-B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Nguyen, A.; Kunert, M.; Hort, N.; Schrader, C.; Weisser, J.; Schmidt, J. Cytotoxicity of the Ga-containing coatings on biodegradable magnesium alloys. Surf. Innov. 2015, 3, 10–19. [Google Scholar] [CrossRef]
- Vujović, S.; Desnica, J.; Stanišić, D.; Ognjanović, I.; Stevanovic, M.; Rosic, G. Applications of Biodegradable Magnesium-Based Materials in Reconstructive Oral and Maxillofacial Surgery: A Review. Molecules 2022, 27, 5529. [Google Scholar] [CrossRef]
- Kasinath, R.; Ernsberg, C.; Vass, S.; Ginn, S.N.; Qu, H.; Tong, W. Orthopedic Implant Having a Crystalline Gallium-Containing Hydroxyapatite Coating and Methods for Making the Same. U.S. Patent 11,141,505, 21 January 2021. [Google Scholar]
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M.d.B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, Q. Therapeutic Effect of Organic Gallium on Ovariectomized Osteopenic Rats by Decreased Serum Minerals and Increased Bone Mineral Content. Biol. Trace Elem. Res. 2010, 133, 342–349. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Warrell, R.P., Jr.; Bockman, R.S.; Coonley, C.J.; Isaacs, M.; Staszewski, H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J. Clin. Investig. 1984, 73, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P., Jr.; Skelos, A.; Alcock, N.W.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-related Hypercalcemia: Clinicopharmacological and Dose Response Analysis. Cancer Res. 1986, 46, 4208–4212. [Google Scholar] [PubMed]
- Warrell, R.P., Jr.; Israel, R.; Frisone, M.; Snyder, T.; Gaynor, J.J.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-Related Hypercalcemia: A Randomized, Double-Blind Comparison to Calcitonin. Ann. Intern. Med. 1988, 108, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P., Jr.; Bosco, B.; Weinerman, S.; Levine, B.; Lane, J.; Bockman, R.S. Gallium Nitrate for Advanced Paget Disease of Bone: Effectiveness and Dose-Response Analysis. Ann. Intern. Med. 1990, 113, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, V.; Apseloff, G.; Shepard, D.R.; Gerber, N. Use of gallium to treat Paget’s disease of bone: A pilot study. Lancet 1990, 335, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Niesvizky, R. Gallium nitrate in multiple myeloma: Prolonged survival in a cohort of patients with advanced-stage disease. Semin. Oncol. 2003, 30, 20–24. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Yin, H.; Huang, W. Effects of Ga addition on the corrosion behaviours of high strain rate rolled Mg–Ga alloy sheets. Corros. Eng. Sci. Technol. 2021, 56, 530–542. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Yasakau, K.A.; Arrabal, R.; Matykina, E.; Mingo, B.; Scharnagl, N.; Ferreira, M.G.S.; Zheludkevich, M.L. Characterization and corrosion behavior of binary Mg-Ga alloys. Mater. Charact. 2017, 128, 85–99. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg–X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C 2013, 33, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Almanza-Robles, J.M. Effect of Gallium Content and Heat Treatment on the Microstructure and Corrosion Rate of Magnesium Binary Alloys. Metals 2019, 9, 990. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Neel, E.A.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial Gallium-Doped Phosphate-Based Glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; O’Dell, L.A.; Chrzanowski, W.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; et al. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta Biomater. 2009, 5, 1198–1210. [Google Scholar] [CrossRef]
- Gao, Z.; Song, M.; Liu, R.L.; Shen, Y.; Ward, L.; Cole, I.; Chen, X.B.; Liu, X. Improving in vitro and in vivo antibacterial functionality of Mg alloys through micro-alloying with Sr and Ga. Mater. Sci. Eng. C 2019, 104, 109926. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloys 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Bazhenov, V.; Li, A.; Tavolzhanskii, S.; Bazlov, A.; Tabachkova, N.; Koltygin, A.; Komissarov, A.; Shin, K.S. Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials 2022, 15, 6849. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Kolawole, S.K.; Wang, J.; Su, X.; Tan, L.; Yang, K. Systems, Properties, Surface Modification and Applications of Biodegradable Magnesium-Based Alloys: A Review. Materials 2022, 15, 5031. [Google Scholar] [CrossRef]
- ASTM Standard G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Anishchenko, A.; Khasenova, R.; Komissarova, J.; Bautin, V.; Seferyan, A.; Fozilov, B. Microstructure, Mechanical and Corrosion Properties of Biodegradable Mg-Ga-Zn-X (X = Ca, Y, Nd) Alloys. In Proceedings of the 27th Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2018; TANGER Ltd.: Ostrava, Czech Republic, 2018; pp. 1375–1380. [Google Scholar]
- ASTM Standard G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Zander, D.; Zumdick, N.A. Influence of Ca and Zn on the microstructure and corrosion of biodegradable Mg–Ca–Zn alloys. Corros. Sci. 2015, 93, 222–233. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Li, A.V.; Komissarov, A.A.; Koltygin, A.V.; Tavolzhanskii, S.A.; Bautin, V.A.; Voropaeva, O.O.; Mukhametshina, A.M.; Tokar, A.A. Microstructure and mechanical and corrosion properties of hot-extruded Mg–Zn–Ca–(Mn) biodegradable alloys. J. Magnes. Alloys 2021, 9, 1428–1442. [Google Scholar] [CrossRef]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. IOP Conf. Ser.-Mater. Sci. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Bockman, R.S.; Boskey, A.L.; Blumenthal, N.C.; Alcock, N.W.; Warrell, R.P. Gallium increases bone calcium and crystallite perfection of hydroxyapatite. Calcif. Tissue Int. 1986, 39, 376–381. [Google Scholar] [CrossRef] [PubMed]
- de Haas, W.G.; Watson, J.; Morrison, D.M. Non-invasive Treatment of Ununited Fractures of the Tibia using Electrical Stimulation. J. Bone Jt. Surg. Br. 1980, 62-B, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of Tibial Fracture-Healing by Non-Invasive, Low-Intensity Pulsed Ultrasound. J. Bone Jt. Surg. Am. 1994, 76-A, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Poitevin, A.A.; Viezzer, C.; Machado, D.C.; da Costa, B.E.P.; Figueiredo, A.E.; d’Avila, D.; Poli-de-Figueiredo, C.E. Effect of standard and neutral-pH peritoneal dialysis solutions upon fibroblasts proliferation. J. Brasileiro de Nefrologia 2014, 36, 150–154. [Google Scholar] [CrossRef]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Han, H.-S.; Kim, H.-K.; Kim, Y.-C.; Seok, H.-K.; Kim, Y.-Y. Conventional and improved cytotoxicity test methods of newly developed biodegradable magnesium alloys. Met. Mater. Int. 2015, 21, 1108–1117. [Google Scholar] [CrossRef]
- Marco, I.; Feyerabend, F.; Willumeit-Römer, R.; Van der Biest, O. Influence of Testing Environment on the Degradation Behavior of Magnesium Alloys for Bioabsorbable Implants. In Proceedings of the Annual Meeting & Exhibition (TMS2015), Orlando, FL, USA, 15–19 March 2015; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 497–506. [Google Scholar] [CrossRef]
- Maradze, D.; Musson, D.; Zheng, Y.; Cornish, J.; Lewis, M.; Liu, Y. High Magnesium Corrosion Rate has an Effect on Osteoclast and Mesenchymal Stem Cell Role During Bone Remodelling. Sci. Rep. 2018, 8, 10003. [Google Scholar] [CrossRef] [PubMed]
- Fazel Anvari-Yazdi, A.; Tahermanesh, K.; Hadavi, S.M.; Talaei-Khozani, T.; Razmkhah, M.; Abed, S.M.; Mohtasebi, M.S. Cytotoxicity assessment of adipose-derived mesenchymal stem cells on synthesized biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2016, 69, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2019, 8, 305–319. [Google Scholar] [CrossRef]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg Based Alloys for Biomedical Implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Ufelle, A.C.; Barchowsky, A. Toxic effects of metals. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; Klaassen, C.D., Ed.; McGraw Hill: New York, NY, USA, 2019; pp. 811–867. [Google Scholar]


| Alloy | Element Content (wt.%) | |||
|---|---|---|---|---|
| Mg | Zn | Ga | Y | |
| MgZn4Ga4 | Bal. | 4.2 | 4.1 | - |
| MgZn4Ga4Y0.5 | Bal. | 4.2 | 4.1 | 0.4 |
| MgZn6.5Ga2 | Bal. | 6.5 | 2.0 | - |
| MgZn4Ga2 | Bal. | 4.2 | 2.2 | - |
| MgZn2Ga2 | Bal. | 2.3 | 2.3 | - |
| Sample | Area | Element Content (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Mg | Zn | Ga | P | Ca | Cl | ||
| MgZn6.5Ga2 Hanks’ solution | A | 62.5 | 35.5 | 0.7 | 0.2 | 0 | 0 | 0.1 |
| B | 71.4 | 6.0 | 0.1 | 0 | 10.3 | 11.3 | 0 | |
| MgZn2Ga2 Hanks’ solution | A | 70.6 | 24.4 | 0.5 | 0.5 | 1.4 | 0.4 | 0.9 |
| B | 71.2 | 4.9 | 0 | 0 | 10.8 | 12.3 | 0.1 | |
| MgZn2Ga2 DMEM/F12 incubation | A | 64.5 | 28.8 | 1.0 | 0.3 | 1.8 | 1.9 | 0.1 |
| B | 66.2 | 31.8 | 0.2 | 0.1 | 0.1 | 0.1 | 0 | |
| MgZn2Ga2 Hanks’ solution & DMEM/F12 incubation | A | 64.3 | 24.9 | 2.4 | 1.5 | 1.7 | 4.5 | 0 |
| B | 65.3 | 25.2 | 1.1 | 1.0 | 3.6 | 2.3 | 0.2 | |
| C | 69.6 | 12.6 | 0.1 | 0.1 | 9.3 | 7.6 | 0 | |
| D | 54.3 | 36.8 | 0.9 | 0.7 | 0.9 | 1.2 | 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenov, V.; Li, A.; Iliasov, A.; Bautin, V.; Plegunova, S.; Koltygin, A.; Komissarov, A.; Abakumov, M.; Redko, N.; Shin, K.S. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. J. Funct. Biomater. 2022, 13, 294. https://doi.org/10.3390/jfb13040294
Bazhenov V, Li A, Iliasov A, Bautin V, Plegunova S, Koltygin A, Komissarov A, Abakumov M, Redko N, Shin KS. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Journal of Functional Biomaterials. 2022; 13(4):294. https://doi.org/10.3390/jfb13040294
Chicago/Turabian StyleBazhenov, Viacheslav, Anna Li, Artem Iliasov, Vasily Bautin, Sofia Plegunova, Andrey Koltygin, Alexander Komissarov, Maxim Abakumov, Nikolay Redko, and Kwang Seon Shin. 2022. "Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys" Journal of Functional Biomaterials 13, no. 4: 294. https://doi.org/10.3390/jfb13040294
APA StyleBazhenov, V., Li, A., Iliasov, A., Bautin, V., Plegunova, S., Koltygin, A., Komissarov, A., Abakumov, M., Redko, N., & Shin, K. S. (2022). Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Journal of Functional Biomaterials, 13(4), 294. https://doi.org/10.3390/jfb13040294











