Cytotoxicity and Efficacy in Debris and Smear Layer Removal of HOCl-Based Irrigating Solution: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Cytotoxity
2.1.1. Cell Culture Preparation
2.1.2. MTT Assay
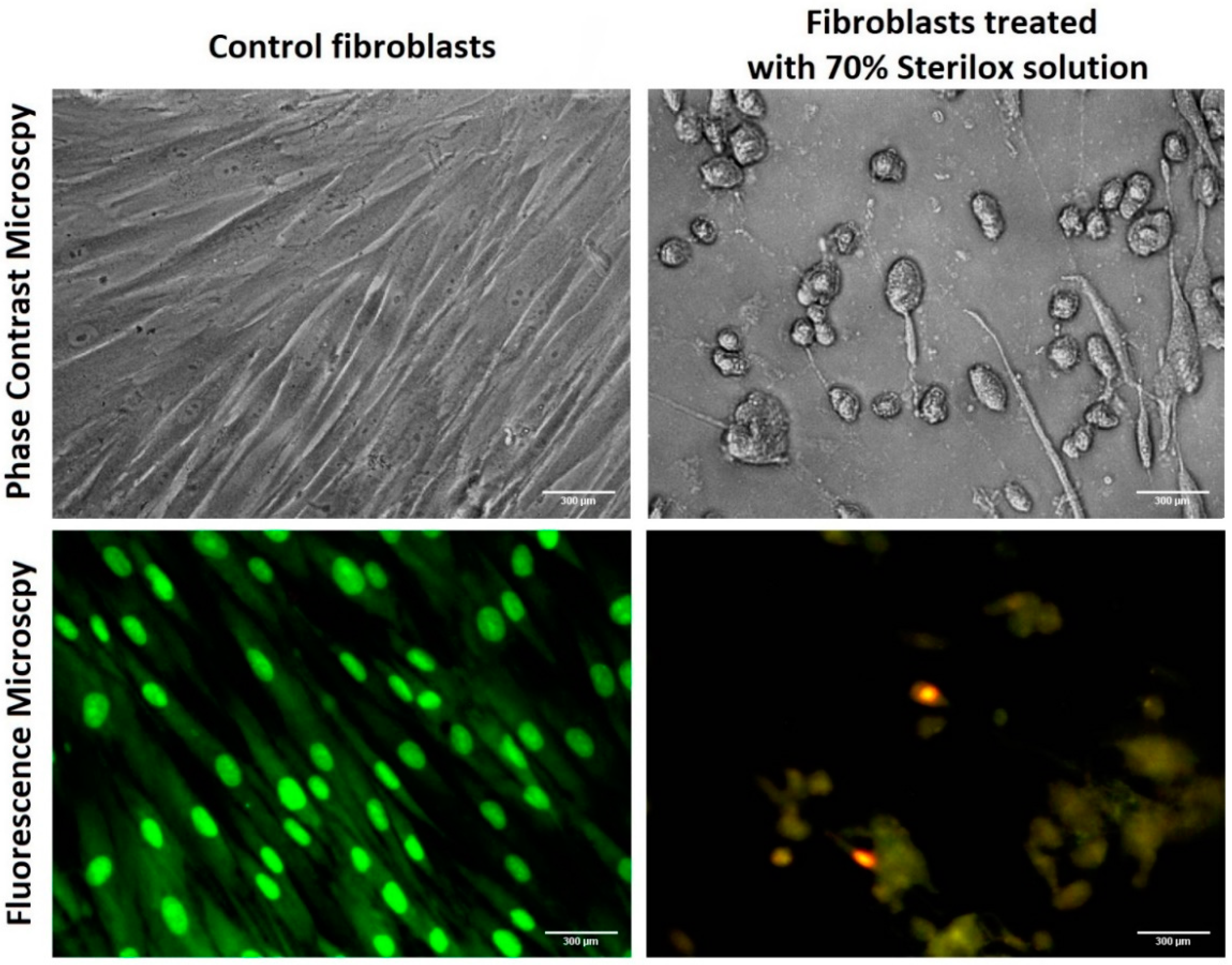
2.1.3. Fluorescence and Phase Contrast Microscopy
2.2. Assessment of Smear Layer and Debris Removal
2.2.1. Specimen Selection and Preparation
- Sterilox group—root canals were repeatedly irrigated with 2 mL Sterilox solution, containing 200 ppm of available free chlorine. At the end of instrumentation, the irrigation needle was placed 1 mm from the WL and a final 1 min rinse with 4 mL Sterilox, followed by 4 mL distilled water, was performed, moving the needle along the root canal in a 5 mm amplitude.
- NaOCl/EDTA group—root canals were irrigated with 2 mL 3% NaOCl solution (Ultradent Products Inc., South Jordan, UT, USA) after every change of the instrument. The final 1 min flush was performed in the following sequence: 2 mL 3% NaOCl, 2 mL 18% EDTA (Ultradent Products Inc., South Jordan, UT, USA) and 4 mL distilled water.
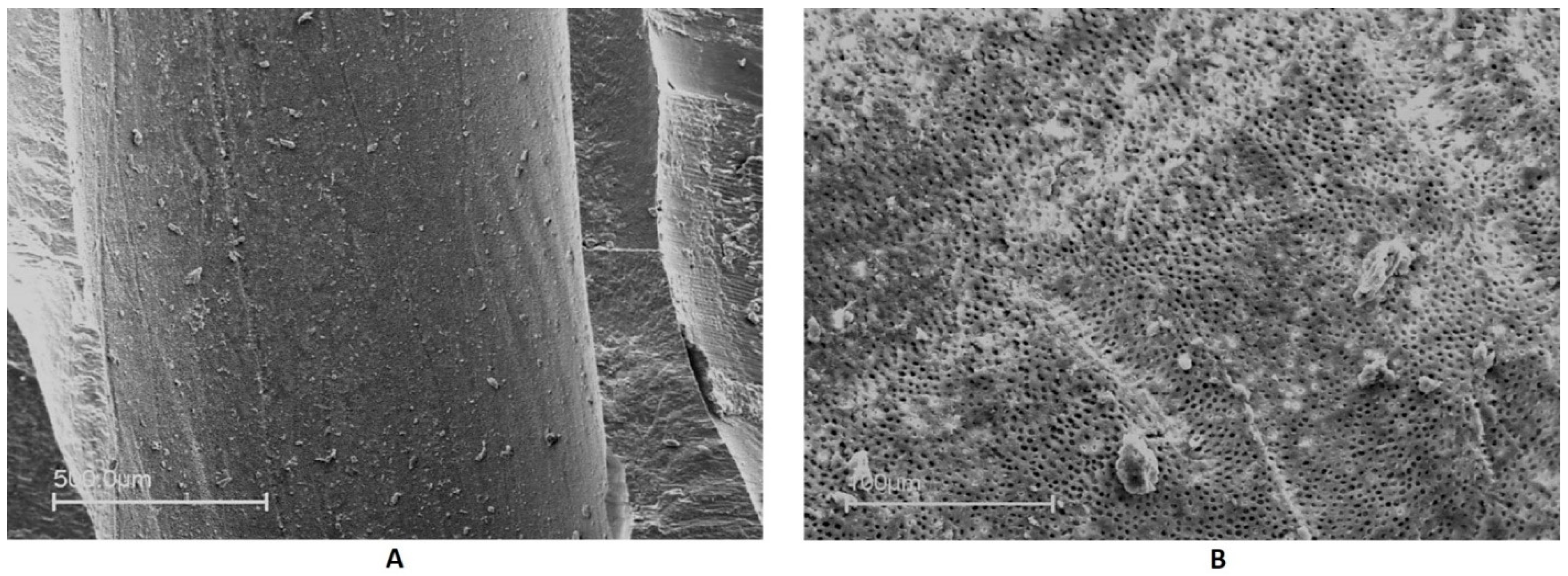
2.2.2. Scanning Electron Microscopy
- Score 1—clean root canal wall, only few small particles of debris;
- Score 2—small agglomerations of debris;
- Score 3—debris covering < 50% of the surface;
- Score 4—debris covering > 50% of the surface;
- Score 5—complete or nearly complete coverage by debris.
- Score 1—no smear layer, dentinal tubules are open;
- Score 2—small amount of smear layer, some dentinal tubules are open;
- Score 3—homogenous smear layer covering the surface, only a few dentinal tubules are open;
- Score 4—complete coverage by homogenous smear layer, no open dentinal tubules;
- Score 5—complete coverage by a heavy, non-homogenous smear layer.
2.3. Statistical Analysis
3. Results
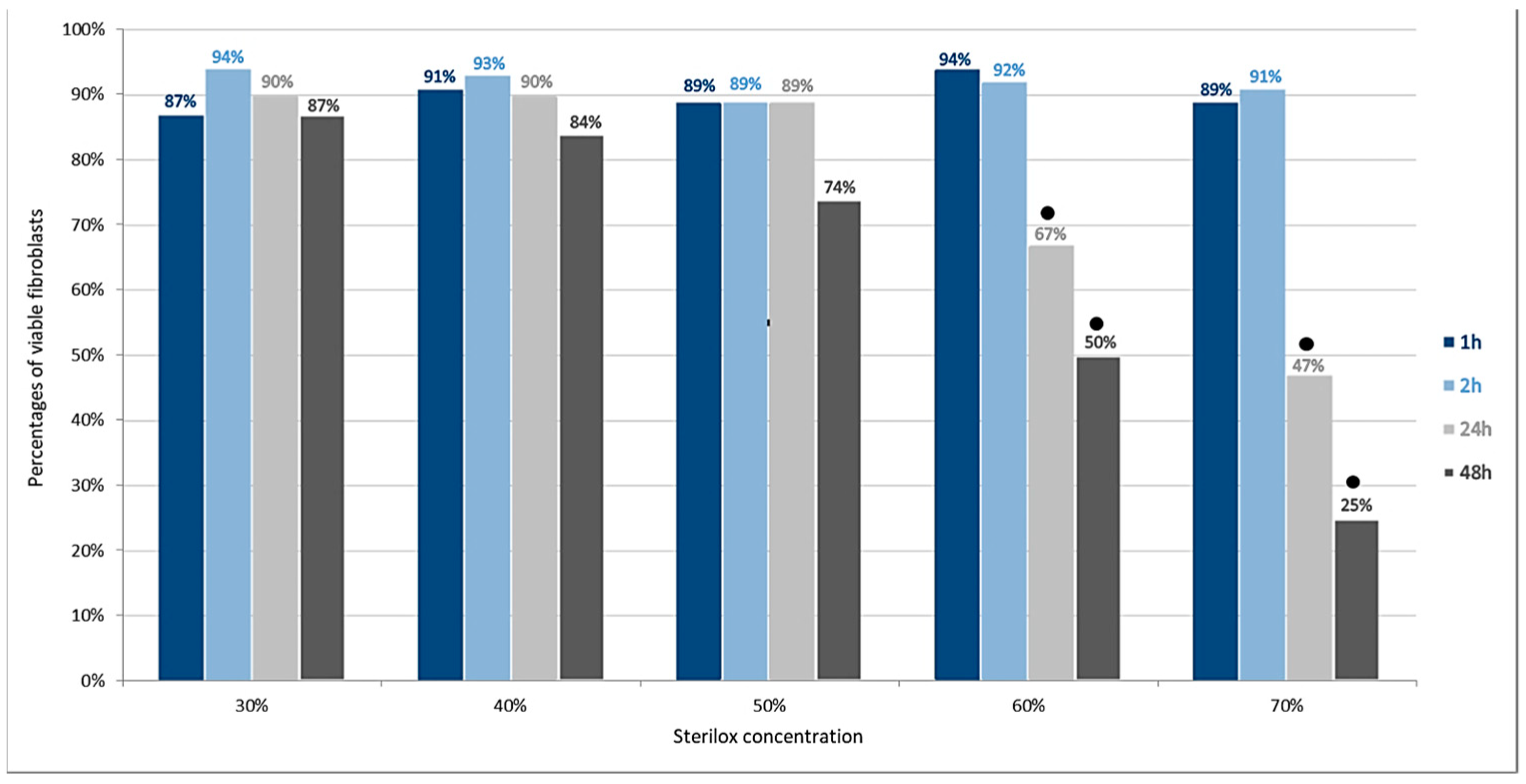
3.1. Cytotoxity
3.2. Smear Layer and Debris Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matos, F.S.; Khoury, R.D.; Carvalho, C.A.T.; Martinho, F.C.; Bresciani, E.; Valera, M.C. Effect of EDTA and QMIX ultrasonic activation on the reduction of microorganisms and endotoxins in ex vivo human root canals. Braz. Dent. J. 2019, 30, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.S.; Falk, W.; Frankenberger, R.; Braun, A. Impact of adjunctive laser irradiation on the bacterial load of dental root canals: A randomized controlled clinical trial. Antibiotics 2021, 10, 1557. [Google Scholar] [CrossRef]
- Versiani, M.A.; Alves, F.R.F.; Andrade-Junior, C.V.; Marceliano-Alves, M.F.; Provenzano, J.C.; Rôças, I.N.; Sousa-Neto, M.D.; Siqueira Jr, J.F. Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. Int. Endod. J. 2016, 49, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of ultrasonically activated irrigation on root canal disinfection: A systematic review of in vitro studies. Clin. Oral. Investig. 2018, 22, 655–670. [Google Scholar] [CrossRef]
- Tavares, S.; Pintor, A.; de Almeida Barros Mourão, C.F.; Magno, M.; Montemezzi, P.; Sacco, R.; Alves, G.; Scelza, M.Z. Effect of different root canal irrigant solutions on the release of dentin-growth factors: A systematic review and meta-analysis. Materials 2021, 14, 5829. [Google Scholar] [CrossRef]
- Shabahang, S. Treatment options: Apexogenesis and apexification. J. Endod. 2013, 39, 26–29. [Google Scholar] [CrossRef]
- Guivarc, M.; Ordioni, U.; Mohamed Aly Ahmed, H.; Cohen, S.; Catherine, J.H.; Bukiet, F. Sodium hypochlorite accident: A systematic review. J. Endod. 2017, 43, 16–24. [Google Scholar] [CrossRef]
- Iandolo, A.; Amato, A.; Pantaleo, G.; Dagna, A.; Ivaldi, L.; di Spirito, F.; Abdellatif, D. An innovative technique to safely perform active cleaning in teeth with open apices: CAB technique. J. Conserv. Dent. 2021, 24, 153–157. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Irrigating Solutions, Devices, and Techniques. In Endodontic Materials in Clinical Practice, 1st ed.; Camilleri, J., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 133–180. [Google Scholar]
- Fernandes Zancan, R.; Hadis, M.; Burgess, D.; Zhang, Z.J.; Di Maio, A.; Tomson, P.; Hungaro Duarte, M.A.; Camilleri, J. A matched irrigation and obturation strategy for root canal therapy. Sci. Rep. 2021, 11, 4666. [Google Scholar] [CrossRef]
- Ghisi, A.C.; Kopper, P.M.P.; Baldasso, F.E.R.; Stürmer, C.P.; Rossi-Fedele, G.; Steier, L.; de Figueiredo, J.A.P.; Morgental, R.D.; Vier-Pelisser, F.V. Effect of superoxidized water and sodium hypochlorite, associated or not with EDTA, on organic and inorganic components of bovine root dentin. J. Endod. 2015, 41, 925–930. [Google Scholar] [CrossRef]
- Coaguila-Llerena, H.; Denegri-Hacking, A.; Lucano-Tinoco, L.; Mendiola-Aquino, C.; Faria, G. Accidental extrusion of sodium hypochlorite in a patient taking alendronate: A case report with an 8-year follow-up. J. Endod. 2021, 47, 1947–1952. [Google Scholar] [CrossRef]
- Scott, M.B.; Zilinski, G.S.; Kirkpatrick, T.C.; Himel, V.T.; Sabey, K.A.; Lallier, T.E. The effects of irrigants on the survival of human stem cells of the apical papilla, including endocyn. J. Endod. 2018, 44, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Gawdat, S.I.; Bedier, M.M. Influence of dual rinse irrigation on dentinal penetration of a bioceramic root canal sealer: A Conofocal microscopic Analysis. Aust. Endod. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G.; Chugal, N.; Lin, L.M. An evidence-based review of the efficacy of treatment approaches for immature permanent teeth with pulp necrosis. J. Endod. 2017, 43, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.C.; Teng, N.C.; Chu, C.C.; Chu, Y.T.; Chen, C.H.; Chang, L.Y.; Hsu, C.H.; Huang, C.S.; Hsiao, G.Y.W.; Yang, J.C. The antibacterial efficacy and in vivo toxicity of sodium hypochlorite and electrolyzed oxidizing (EO) water-based endodontic irrigating solutions. Materials 2020, 13, 260. [Google Scholar] [CrossRef]
- Yan, P.; Daliri, E.B.M.; Oh, D.H. New clinical applications of electrolyzed water: A review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Gold, M.H.; Andriessen, A.; Bhatia, A.C.; Bitter, P.; Chilukuri, S.; Cohen, J.L.; Robb, C.W. Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures. J. Cosmet. Dermatol. 2020, 19, 270–277. [Google Scholar] [CrossRef]
- Giles, L.H.; Chan, S.N.; Jarad, F.; Hope, C. The efficacy of different endodontic irrigants against Enterococcus faecalis in an extracted human tooth model. Int. Endod. J. 2010, 43, 353. [Google Scholar] [CrossRef]
- Weigelt, M.A.; McNamara, S.A.; Sanchez, D.; Hirt, P.A.; Kirsner, R.S. Evidence-based review of antibiofilm agents for wound care. Adv. Wound Care 2021, 10, 13. [Google Scholar] [CrossRef]
- Rathakrishnan, M.; Sukumaran, V.G.; Subbiya, A. To evaluate the efficacy of an innovative irrigant on smear layer removal—SEM analysis. J. Clin. Diagnostic. Res. 2016, 10, 104–106. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Psimma, Z.; Van der Sluis, L.W.M. Factors affecting irrigant extrusion during root canal irrigation: A systematic review. Int. Endod. J. 2013, 46, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Nocca, G.; Ahmed, H.M.A.; Martorana, G.E.; Callà, C.; Gambarini, G.; Rengo, S.; Spagnuolo, G. Chromographic analysis and cytotoxic effects of chlorhexidine and sodium hypochlorite reaction mixtures. J. Endod. 2017, 43, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Hope, C.K.; Garton, S.G.; Wang, Q.; Burnside, G.; Farrelly, P.J. A direct comparison between extracted tooth and filter-membrane biofilm models of endodontic irrigation using Enterococcus faecalis. Arch. Microbiol. 2010, 192, 775–781. [Google Scholar] [CrossRef][Green Version]
- Gray, N.F. Free and combined chlorine. In Microbiology of Waterborne Diseases: Microbiological Aspects and Risks, 2nd ed.; Percival, S.T., Yates, M.V., Williams, D.D., Chalmers, R., Gray, N., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 571–590. [Google Scholar]
- Yang, Y.T.; Whiteman, M.; Gieseg, S.P. HOCl causes necrotic cell death in human monocyte derived macrophages through calcium dependent calpain activation. Biochim. Biophys. Acta 2012, 1823, 420–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carmona-Gutierrez, D.; Alavian-Ghavanini, A.; Habernig, L.; Bauer, M.A.; Hammer, A.; Rossmann, C.; Zimmermann, A.; Ruckenstuhl, C.; Buttner, S.; Eisenberg, T.; et al. The cell death protease Kex1p is essential for hypochlorite-induced apoptosis in yeast. Cell Cycle 2013, 12, 1704–1712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakarya, S.; Gunay, N.; Karakulak, M.; Ozturk, B.; Ertugrul, B. Hypochlorous Acid: An ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds Compend. Clin. Res. Pract. 2014, 26, 342–350. [Google Scholar]
- Zhang, L.; Wang, T.; Lu, Y.; Zheng, Q.; Gao, Y.; Zhou, X.; Huang, D. Tumor necrosis factor receptor-associated factor 6 plays a role in the inflammatory responses of human periodontal ligament fibroblasts to Enterococcus faecalis. J. Endod. 2015, 41, 1997–2001. [Google Scholar] [CrossRef]
- Cheng, R.; Choudhury, D.; Liu, C.; Billet, S.; Hu, T.; Bhowmick, N. Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov. 2015, 1, 1504. [Google Scholar] [CrossRef]
- Lopes, R.M.V.; Marins, F.C.; Belladonna, F.G.; Souza, E.M.; De-Deus, G.; Lopes, R.T.; Silva, E.J.N.L. Untouched canal areas and debris accumulation after root canal preparation with rotary and adaptive systems. Aust. Endod. J. 2018, 44, 260–266. [Google Scholar] [CrossRef]
- Macedo, R.G.; Herrero, N.P.; Wesselink, P.; Versluis, M.; Van Der Sluis, L. Influence of the dentinal wall on the pH of sodium hypochlorite during root canal irrigation. J. Endod. 2014, 40, 1005–1008. [Google Scholar] [CrossRef]
- Jungbluth, H.; Marending, M.; De-Deus, G.; Sener, B.; Zehnder, M. Stabilizing sodium hypochlorite at high pH: Effects on soft tissue and dentin. J. Endod. 2011, 37, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Tonini, R.; Giovarruscio, M.; Gorni, F.; Ionescu, A.; Brambilla, E.; Irina, M.M.; Luzi, A.; Pires, P.M.; Sauro, S. In vitro evaluation of antibacterial properties and smear layer removal/sealer penetration of a novel silver-citrate root canal irrigant. Materials 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Colangeli, M.; Özyürek, T.; DeDeus, G.; Panzetta, C.; Castagnola, R.; Grande, N.M.; Marigo, L. Evaluation of smear layer and debris removal by stepwise intraoperative activation (SIA) of sodium hypochlorite. Clin. Oral. Investig. 2021, 25, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Miguéns-Vila, R.; Martín-Biedma, B.; Aboy-Pazos, S.; Uroz-Torres, D.; Álvarez-Nóvoa, P.; Dablanca-Blanco, A.B.; Varela-Aneiros, I.; Castelo-Baz, P. Effectiveness of different irrigant activation systems on smear layer removal: A scanning electron microscopic study. J. Clin. Med. 2022, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fedele, G.; Guastalli, A.R.; Doǧramaci, E.J.; Steier, L.; de Figueiredo, J.A.P. Influence of pH changes on chlorine-containing endodontic irrigating solutions. Int. Endod. J. 2011, 44, 792–799. [Google Scholar] [CrossRef]
| Group | Debris (n = 200) | Smear Layer (n = 300) | ||||
|---|---|---|---|---|---|---|
| Coronal Third | Middle Third | Apical Third | Coronal Third | Middle Third | Apical Third | |
| Sterilox | 1.42 ± 0.42 A | 1.99 ± 0.84 | 2.17 ± 1.06 A | 2.55 ± 1.19 B | 3.36 ± 1.22 | 3.67 ± 0.84 B |
| NaOCl/EDTA | 1.41 ± 0.24 C | 1.67 ± 0.72 | 1.83 ± 0.54 C | 2.08 ± 1.11 D | 2.53 ± 1.22 | 2.75 ± 0.93 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilvinaite, G.; Zongolaviciute, R.; Drukteinis, S.; Bukelskiene, V.; Cotti, E. Cytotoxicity and Efficacy in Debris and Smear Layer Removal of HOCl-Based Irrigating Solution: An In Vitro Study. J. Funct. Biomater. 2022, 13, 95. https://doi.org/10.3390/jfb13030095
Bilvinaite G, Zongolaviciute R, Drukteinis S, Bukelskiene V, Cotti E. Cytotoxicity and Efficacy in Debris and Smear Layer Removal of HOCl-Based Irrigating Solution: An In Vitro Study. Journal of Functional Biomaterials. 2022; 13(3):95. https://doi.org/10.3390/jfb13030095
Chicago/Turabian StyleBilvinaite, Goda, Ruta Zongolaviciute, Saulius Drukteinis, Virginija Bukelskiene, and Elisabetta Cotti. 2022. "Cytotoxicity and Efficacy in Debris and Smear Layer Removal of HOCl-Based Irrigating Solution: An In Vitro Study" Journal of Functional Biomaterials 13, no. 3: 95. https://doi.org/10.3390/jfb13030095
APA StyleBilvinaite, G., Zongolaviciute, R., Drukteinis, S., Bukelskiene, V., & Cotti, E. (2022). Cytotoxicity and Efficacy in Debris and Smear Layer Removal of HOCl-Based Irrigating Solution: An In Vitro Study. Journal of Functional Biomaterials, 13(3), 95. https://doi.org/10.3390/jfb13030095







