Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Modifications
2.2. Analysis of Surface Topography
2.3. Cleaning and Sterilization of the Titanium Discs
2.4. Cell Cultivation
2.5. Crystal Violet Assay (CVA)
2.6. MTT Assay
2.7. Alkaline Phosphatase (ALP) Activity Assay
2.8. Alizarin Red S Staining (ARS Assay)
2.9. LDH Assay
2.10. Statistical Analysis
3. Results
3.1. Surface Characterization
3.1.1. Micro-Roughness
3.1.2. Nano-Roughness
3.1.3. Physico-Chemical Properties
3.2. Osteoblast Attachment and Viability
3.2.1. Crystal Violet Assay
3.2.2. MTT Assay
3.2.3. LDH Assay
3.3. Osteoblast Differentiation
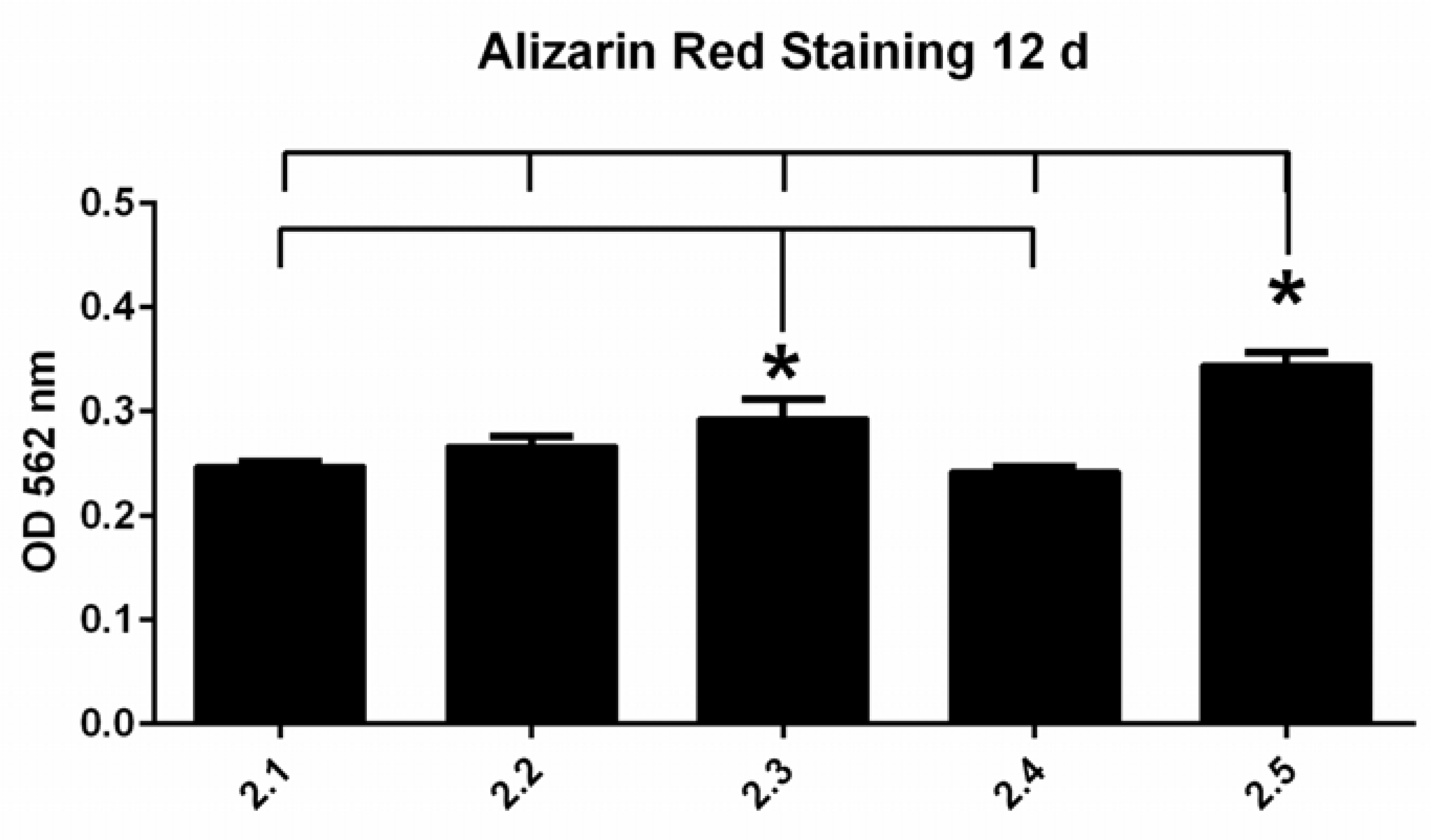
3.3.1. Alizarin Red S Staining
3.3.2. ALP Activity Assay
3.4. Comparison of cpTi and Ti6Al4V
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Scarano, A.; Di Stefano, D.; Arosio, P.; Doi, K.; Ricci, L.; Piattelli, A.; Perrotti, V. Correlation between the Bone Density Recorded by a Computerized Implant Motor and by a Histomorphometric Analysis: A Preliminary In Vitro Study on Bovine Ribs. Clin. Implant Dent. Relat. Res. 2015, 17, e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E.; Thomsen, P.; Lyngstadaas, S.P. Advances in dental implant materials and tissue regeneration. Periodontology 2000 2006, 41, 136–156. [Google Scholar] [CrossRef]
- Quirynen, M.; Al-Nawas, B.; Meijer, H.J.; Razavi, A.; Reichert, T.E.; Schimmel, M.; Storelli, S.; Romeo, E.; Roxolid Study Group. Small-diameter titanium Grade IV and titanium-zirconium implants in edentulous mandibles: Three-year results from a double-blind, randomized controlled trial. Clin. Oral Implants Res. 2015, 26, 831–840. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; Resende, C.R.; Roestel, J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent. Mater. 2015, 31, e1–e13. [Google Scholar] [CrossRef]
- Qiu, K.J.; Liu, Y.; Zhou, F.Y.; Wang, B.L.; Li, L.; Zheng, Y.F.; Liu, Y.H. Microstructure, mechanical properties, castability and in vitro biocompatibility of Ti–Bi alloys developed for dental applications. Acta Biomater. 2015, 15, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Barão, V.A. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a new titanium-zirconium dental implant: A biomechanical and histological comparative study in the mini pig. Clin. Implant. Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Brägger, U.; Meijer, H.J.; Naert, I.; Persson, R.; Perucchi, A.; Quirynen, M.; Raghoebar, G.M.; Reichert, T.E.; Romeo, E.; et al. A double-blind randomized controlled trial (rct) of titanium-13zirconium versus titanium grade iv small-diameter bone level implants in edentulous mandibles—Results from a 1-year observation period. Clin. Implant. Dent. Relat. Res. 2012, 14, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Mishnaevsky, L., Jr.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Sergey Prokoshkin, S.; Solov’yove, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 19, 1197–1215. [Google Scholar] [CrossRef]
- Smith, D.C.; Lugowski, S.; McHugh, A.; DePorter, D.; Watson, P.A.; Chipman, M. Systemic metal ion levels in dental implant patients. Int. J. Oral Maxillofac. Implant. 1997, 12, 828–834. [Google Scholar]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; dos Santos Monteiro, E.; de Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2018, 8, 1060–1069. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Toyoda, K.; Kobayashi, E.; Doi, H.; Yoneyama, T.; Hamanaka, H.; Tsuchiya, T. Improved biocompatibility of titanium–zirconium (Ti–Zr) alloy: Tissue reaction and sensitization to Ti–Zr alloy compared with pure Ti and Zr in rat implantation study. Mater. Trans. 2005, 46, 2260–2267. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part, I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Bierbaum, S.; Sewing, A. Effect of modifications of dual acid-etched implant surfaces on peri-implant boneformation. Part I: Organic coatings. Clin. Oral Implants Res. 2009, 20, 31–37. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; de Souza, S.L.S.; de Barros, R.R.M.; Pereira, K.K.Y.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef]
- Webster, T.J.; Ross, A.P. Anodizing color coded anodized Ti6Al4V medical devices for increasing bone cell functions. Int. J. Nanomed. 2013, 8, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Lopez-Heredia, M.-A.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Cochran, D.L. A Comparison of Endosseous Dental Implant Surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Fernandes, D.J.; Marques, R.G.; Elias, C.N. Influence of acid treatment on surface properties and in vivo performance of Ti6Al4V alloy for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 164. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Deschner, J.; Jäger, A.; Wenghoefer, M.; Bayer, S.; Jepsen, S.; Allam, J.P.; Novak, N.; Meyer, R.; Winter, J. Human β-defensins differently affect proliferation, differentiation, and mineralization of osteoblast-like MG63 cells. J. Cell. Physiol. 2012, 227, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Duyck, J.; Slaets, E.; Sasaguri, K.; Vandamme, K.; Naert, I. Effect of intermittent loading and surface roughness on peri-implant bone formation in a bone chamber model. J. Clin. Periodontol. 2007, 34, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Rønold, H.J.; Ellingsen, J.E. Effect of micro-roughness produced by TiO2 blasting—Tensile testing of bone attachment by using coin-shaped implants. Biomaterials 2002, 23, 4211–4219. [Google Scholar] [CrossRef]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Kournetas, N.; Spintzyk, S.; Schweizer, E.; Sawada, T.; Said, F.; Schmid, P.; Geis-Gerstorfer, J.; Eliades, G.; Rupp, F. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent. Mater. 2017, 33, e317–e327. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef]
- Zhao, G.; Raines, A.L.; Wieland, M.; Schwartz, Z.; Boyan, B. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Dean, D.D.; Boyan, B.D. The Role of Implant Surface Characteristics in the Healing of Bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef]
- Li, D.; Ferguson, S.J.; Beutler, T.; Cochran, D.L.; Sittig, C.; Hirt, H.P.; Buser, D. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J. Biomed. Mater. Res. 2002, 60, 325–332. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Almas, K.; Smith, S.; Kutkut, A. What is the best micro and macro dental implant topography? Dent. Clin. N. Am. 2019, 63, 447–460. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Perrin, D.; Ahossi, V.; Magnin, G.; Bernard, J.P. Biological properties of acid etched titanium implants: Effect of sandblasting on bone anchorage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68B, 149–159. [Google Scholar] [CrossRef]
- Bachle, M.; Kohal, R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implant. Res. 2004, 15, 683–692. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, C.-W.; Lim, Y.-J.; Heo, S.-J. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J. Biomed. Mater. Res. Part A 2006, 79A, 1023–1032. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Tandy, E.M.; Sylvia, V.L.; Hell-Vocke, A.K.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Response of normal female human osteoblasts (NHOst) to 17β-estradiol is modulated by implant surface morphology. J. Biomed. Mater. Res. 2002, 62, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Schwartz, Z.; Olivares-Navarrete, R.; Wieland, M.; Cochran, D.L.; Boyan, B.D. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials 2009, 30, 3390–3396. [Google Scholar] [CrossRef]
- Bannister, S.R.; Lohmann, C.H.; Liu, Y.; Sylvia, V.L.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Shear force modulates osteoblast response to surface roughness. J. Biomed. Mater. Res. 2002, 60, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ponsonnet, L.; Comte, V.; Othmane, A.; Lagneau, C.; Charbonnier, M.; Lissac, M.; Jaffrezic, N. Effect of surface topography and chemistry on adhesion, orientation and growth of fibroblasts on nickel–titanium substrates. Mater. Sci. Eng. C 2002, 21, 157–165. [Google Scholar] [CrossRef]
- Linez-Bataillon, P.; Monchau, F.; Bigerelle, M.; Hildebrand, H.F. In vitro MC3T3 osteoblast adhesion with respect to surface roughness of Ti6Al4V substrates. Biomol. Eng. 2002, 19, 133–141. [Google Scholar] [CrossRef]
- Rosa, A.L.; Beloti, M.M. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz. Dent. J. 2003, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capillaa, M.; Ruiz, C.; Cabrerizo-Vílchez, M.A. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2010, 32, 951–960. [Google Scholar] [CrossRef]
- Xavier, S.P.; Carvalho, P.S.; Beloti, M.M.; Rosa, A.L. Response of rat bone marrow cells to commercially pure titanium submitted to different surface treatments. J. Dent. 2003, 31, 173–180. [Google Scholar] [CrossRef]
- Nishimura, N.; Kawai, T. Effect of microstructure of titanium surface on the behaviour of osteogenic cell line MC3T3-E1. J. Mater. Sci. Mater. Med. 1998, 9, 99–102. [Google Scholar] [CrossRef]
- Borsari, V.; Giavaresi, G.; Fini, M.; Torricelli, P.; Salito, A.; Chiesa, R.; Chiusoli, L.; Volpert, A.; Rimondini, L.; Giardino, R. Physical characterization of different-roughness titanium surfaces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75B, 359–368. [Google Scholar] [CrossRef]
- Lauer, G.; Wiedmann-Al-Ahmad, M.; Otten, J.E.; Hübner, U.; Schmelzeisen, R.; Schilli, W. The titanium surface texture effects adherence and growth of human gingival keratinocytes and human maxillar osteoblast-like cells in vitro. Biomaterials 2001, 22, 2799–2809. [Google Scholar] [CrossRef]
- Conserva, E.; Menini, M.; Ravera, G.; Pera, P. The role of surface implant treatments on the biological behavior of SaOS-2 osteoblast-like cells. An in vitro comparative study. Clin. Oral Implant. Res. 2013, 24, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Martin, J.Y.; Dean, D.D.; Simpson, J.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J. Biomed. Mater. Res. 1996, 30, 145–155. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choi, M.-U.; Kim, C.-W. Activation of phospholipase D1 by surface roughness of titanium in MG63 osteoblast-like cell. Biomaterials 2006, 27, 5502–5511. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewska-Kuska, M.; Wirstlein, P.; Majchrowski, R.; Dorocka-Bobkowska, B. Osteoblastic cell behaviour on modified titanium surfaces. Micron 2018, 105, 55–63. [Google Scholar] [CrossRef]
- Orsini, G.; Assenza, B.; Scarano, A.; Piattelli, M.; Piattelli, A. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int. J. Oral Maxillofac. Implant. 2000, 15, 779–784. [Google Scholar]
- Vu, N.B.; Truong, N.H.; Dang, L.T.; Phi, L.T.; Ho, N.T.T.; Pham, T.N.; Phan, T.P.; Pham, P.V. In vitro and in vivo bio-compatibility of Ti-6Al-4V titanium alloy and UHMWPE polymer for total hip replacement. Biomed. Res. Ther. 2016, 3, 14. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Role of materials surface topography on mammalian cell response. Int. Mater. Rev. 2011, 56, 243–266. [Google Scholar] [CrossRef]
- Keller, J.C.; Schneider, G.B.; Stanford, C.M.; Kellogg, B. Effects of implant microtopography on osteoblast cell attachment. Implant. Dent. 2003, 12, 175–181. [Google Scholar] [CrossRef]
- Hansson, S.; Hansson, K.N. The effect of limited lateral resolution in the measurement of implant surface roughness: A computer simulation. J. Biomed. Mater. Res. Part A 2005, 75, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part I: Review of titanium alloys, surface characteristics and treatments. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e514–e525. [Google Scholar] [CrossRef] [PubMed]



| Group No. | Abbr. | Material | Blasting Grain Size | Pressure |
|---|---|---|---|---|
| 1 | 4-F70S | cpTi | F70 [180–250 µm] | Std. |
| 2.1 | 5-F70S | Ti6Al4V | F70 [180–250 µm] | Std. |
| 2.2 | 5-F120S | Ti6Al4V | F120 [90–125 µm] | Std. |
| 2.3 | 5-F180S | Ti6Al4V | F180 [63–90 µm] | Std. |
| 2.4 | 5-F70R | Ti6Al4V | F70 [180–250 µm] | 2/3 × Std. |
| 2.5 | 5--- | Ti6Al4V | - | - |
| Abbr. | Group No. | Sa [µm] | Sz [µm] | Sq [µm] | Sdr [%] | Sds [103*mm−2] | San [nm] | ϒ [mN×m−1] |
|---|---|---|---|---|---|---|---|---|
| 5-F70S | 2.1 | 1.4 ± 0.04 | 17.9 ± 1.4 | 1.8 ± 0.08 | 55.5 ±2.8 | 150 ± 0.07 | 11 ± 0.3 | 21.85 ± 0.7 |
| 5-F120S | 2.2 | 1.0 ± 0.05 | 10.7 ± 0.9 | 1.3 ± 0.06 | 34.4 ± 1.4 | 165 ± 0.14 | 12 ± 2.1 | 18.45 ± 0.7 |
| 5-F180S | 2.3 | 0.8 ± 0.02 | 8.2 ± 0.2 | 1.1 ± 0.02 | 29.7 ± 0.6 | 173 ± 0.99 | 9 ± 2.2 | 22.4 ± 0.7 |
| 5-F70R | 2.4 | 1.2 ± 0.01 | 14.7 ± 0.5 | 1.5 ± 0.04 | 37.2 ± 1.7 | 150 ± 0.35 | 11 ± 3.7 | 22.8 ± 0.7 |
| 5--- | 2.5 | 0.3 ± 0.01 | 3.4 ± 0.6 | 0.4 ± 0.01 | 18.4 ± 0.5 | 183 ± 0.92 | 12 ± 3.9 | 18.95 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoilov, M.; Stoilov, L.; Enkling, N.; Stark, H.; Winter, J.; Marder, M.; Kraus, D. Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 143. https://doi.org/10.3390/jfb13030143
Stoilov M, Stoilov L, Enkling N, Stark H, Winter J, Marder M, Kraus D. Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study. Journal of Functional Biomaterials. 2022; 13(3):143. https://doi.org/10.3390/jfb13030143
Chicago/Turabian StyleStoilov, Milan, Lea Stoilov, Norbert Enkling, Helmut Stark, Jochen Winter, Michael Marder, and Dominik Kraus. 2022. "Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study" Journal of Functional Biomaterials 13, no. 3: 143. https://doi.org/10.3390/jfb13030143
APA StyleStoilov, M., Stoilov, L., Enkling, N., Stark, H., Winter, J., Marder, M., & Kraus, D. (2022). Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study. Journal of Functional Biomaterials, 13(3), 143. https://doi.org/10.3390/jfb13030143








