Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources
Abstract
:1. Introduction
2. Naturally-Derived Bioinks for 3D Bioprinting of Cartilage Tissue
2.1. Three-Dimensional Printing of Principal Vegetable-Derived Hydrogels for CTE
2.1.1. Land Plants: Cellulose and Nanocellulose
2.1.2. Marine Algae: Alginate, Agarose, Carrageenan
Alginate
Agarose
Carrageenan
2.2. Three-Dimensional Printing of Principal Animal-Derived Hydrogels for CTE
2.2.1. Land and Marine-Source Hyaluronic Acid, Collagen, Gelatin
Hyaluronic Acid
Collagen and Gelatin
2.2.2. Chitosan
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GENERAL | |
| RGD | Arginyl-Glycyl-Aspartic acid |
| BMP-2 | Bone morphogenetic protein 2 |
| CTE | Cartilage tissue engineering |
| ECM | Extracellular matrix |
| 4D | Four dimensional |
| GFs | Growth factors |
| O2 | Oxygen |
| 3D | Three dimensional |
| TGF-β1 | Transforming GF beta 1 |
| UV | Ultraviolet |
| MATERIALS | |
| AG | Agarose |
| a-CNC | Aldehyde-functionalized cellulose nanocrystals |
| ALG | Alginate |
| ADA | Alginate-di-aldehyde |
| CAR | Carrageenan |
| CNC | Cellulose nanocrystals |
| CS | Chitosan |
| CS-AEMA | Chondroitin sulfate amino ethyl methacrylate |
| COL | Collagen |
| DC-ALG | Double crosslinked Alginate |
| GEL | Gelatin |
| GELMA | Gelatin methacryloyl or Methacrylamide-modified Gelatin |
| HA | Hyaluronic acid |
| i-CAR | Iota-Carrageenan |
| k-CAR | Kappa-Carrageenan |
| λ-CAR | Lambda-Carrageenan |
| HAMA | Methacrylated Hyaluronic acid |
| CARMA | Methacrylated k-Carrageenan |
| MC | Methylcellulose |
| Mw-CARMA | Microwave-methacrylated k-Carrageenan |
| NC | Nanocellulose |
| NFC | Nanofibrillated cellulose |
| nHAp | Nano-Hydroxyapatite |
| nSi | Nanosilicates |
| QSM | Quince seed mucilage |
| sALG | Sodium Alginate |
| CELLS | |
| AMSCs | Adipose tissue-derived mesenchymal stem cells |
| bPAC | Bovine primary articular chondrocytes |
| hASCs | Human adipose tissue-derived stem cells |
| hBMSCs | Human bone marrow–derived mesenchymal stem cells |
| HepG2 | Human liver cancer cells |
| hMSCs | Human mesenchymal stem cells |
| hNSCs | Human nasoseptal chondrocytes |
| hUCB-MSCs | Human umbilical cord blood-derived mesenchymal stem cells |
| huMSCs | Human umbilical cord mesenchymal stem cells |
| iPSCs | Human-derived induced pluripotent stem cells |
| ATDC5 | Mouse chondrogenic cell line |
| NIH 3T3-GFP | Mouse fibroblasts expressing green fluorescent protein |
| C2C12 | Mouse myoblasts |
| MC3T3-E1 | Mouse preosteoblasts |
| ACPCs | Multipotent articular cartilage-resident chondroprogenitor cells |
| BCs | Primary bovine chondrocytes |
References
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Health Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D printing: Approaches and bioapplications. Biosens. Bioelectron. 2021, 175, 112849. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Wiley Online Libr. 2017, 6, 1700298. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, O.; Henrionnet, C.; Bourge, K.; Loeuille, D.; Gillet, P.; Pinzano, A. Stem cells and extrusion 3D printing for hyaline cartilage engineering. Cells 2020, 10, 2. [Google Scholar] [CrossRef]
- Wu, Y.; Kennedy, P.; Bonazza, N.; Yu, Y.; Cartilage, A.D.-U. Three-dimensional bioprinting of articular cartilage: A systematic review. Cartilage 2018, 12, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Campos, Y.; Almirall, A.; Fuentes, G.; Bloem, H.L.; Kaijzel, E.L.; Cruz, L.J. Tissue engineering: An alternative to repair cartilage. Tissue Eng. Part B Rev. 2019, 25, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue engineering; current status & futuristic scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Dolcimascolo, A.; Castrogiovanni, P.; Fabbi, C.; Puglisi, C.; Lauretta, G.; Di Rosa, M.; Castorina, A. Evaluation of a cell-free collagen type I-based scaffold for articular cartilage regeneration in an orthotopic rat model. Materials 2020, 13, 2369. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; D’Amora, U.; Ravalli, S.; Ambrosio, L.; Di Rosa, M.; Musumeci, G. functional biomolecule delivery systems and bioengineering in cartilage regeneration. Curr. Pharm. Biotechnol. 2019, 20, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Castrogiovanni, P.; Nsir, H.; Di Rosa, M.; Guglielmino, C.; Parenti, R.; Calabrese, G.; Pricoco, E.; Salvatorelli, L.; Magro, G. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp. Cell Res. 2017, 357, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of human tissues: Biofabrication, bioinks, and bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Gao, G.; Yonezawa, T.; Cui, X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12, 1600734. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Bozchalooi, I.S.; Li, Y.; Han, L.; Hung, H.-H.; Frank, E.; Youcef-Toumi, K.; Ortiz, C.; Grodzinsky, A. High-bandwidth AFM-based rheology reveals that cartilage is most sensitive to high loading rates at early stages of impairment. Biophys. J. 2013, 104, 1529–1537. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 7388. [Google Scholar] [CrossRef]
- Barbour, K.E.; Helmick, C.G.; Boring, M.; Brady, T.J. Vital signs: Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. Morb. Mortal. Wkly. Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef]
- Onoi, Y.; Hiranaka, T.; Hida, Y.; Fujishiro, T.; Okamoto, K.; Matsumoto, T.; Kuroda, R. Second-look Arthroscopic Findings and Clinical Outcomes after Adipose-derived Regenerative Cell Injection in Knee Osteoarthritis. Clin. Orthop. Surg. 2022, 14, 212–216. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Sarkar, K.; Xue, Y.; Sant, S. Host response to synthetic versus natural biomaterials. In The Immune Response to Implanted Materials and Devices; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 81–105. [Google Scholar]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Naghieh, S.; Sardroud, H.A.; Yazdanpanah, Z.; Soltani, Y.A.; Sernaglia, J.; Chen, X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- Ruvinov, E.; Re’em, T.T.; Witte, F.; Cohen, S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: A 6-month study in a mini-pig model of osteochondral defects. J. Orthop. Transl. 2019, 16, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef]
- Apelgren, P.; Amoroso, M.; Lindahl, A.; Brantsing, C.; Rotter, N.; Gatenholm, P.; Kölby, L. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 2017, 12, e0189428. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Kyle, S.; Thomas, B.; Badiei, N.; Hawkins, K.; Whitaker, I.S. Printability of pulp derived crystal, fibril and blend nanocellulose-alginate bioinks for extrusion 3D bioprinting. Biofabrication 2019, 11, 45006. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Health Mater. 2020, 9, e2001410. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Espona-Noguera, A.; Galvez-Martin, P.; López-Ruiz, E.; Marchal, J.A.; López-Donaire, M.L.; Zabala, A.; Ciriza, J.; Saenz-del-Burgo, L. Development, characterization and sterilisation of Nanocellulose-alginate-(hyaluronic acid)-bioinks and 3D bioprinted scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 126, 112160. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Polez, R.T.; Kimiaei, E.; Madani, Z.; Rojas, O.J.; Österberg, M.; Seppälä, J. 3D printing and properties of cellulose nanofibrils-reinforced quince seed mucilage bio-inks. Int. J. Biol. Macromol. 2021, 192, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Hodder, E.; Duin, S.; Kilian, D.; Ahlfeld, T.; Seidel, J.; Nachtigall, C.; Bush, P.; Covill, D.; Gelinsky, M.; Lode, A. Investigating the effect of sterilisation methods on the physical properties and cytocompatibility of methyl cellulose used in combination with alginate for 3D-bioplotting of chondrocytes. J. Mater. Sci. Mater. Med. 2019, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, I.; Gatenholm, P.; Hägg, D.A. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 2017, 9, 015022. [Google Scholar] [CrossRef] [PubMed]
- Gatenholm, P.; Martinez, H.; Karabulut, E.; Amoroso, M.; Kölby, L.; Markstedt, K.; Gatenholm, E.; Henriksson, I. Development of Nanocellulose-Based Bioinks for 3D Bioprinting of Soft Tissue. In 3D Printing and Biofabrication; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–23. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of mesenchymal stem cells delivered from methacrylated hyaluronic acid patch improve the regenerative properties of endothelial and dermal cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, D.; Lin, H.; Xiao, Y.; Zhang, X. Cellulose nanocrystal reinforced collagen-based nanocomposite hydrogel with self-healing and stress-relaxation properties for cell delivery. Biomacromolecules 2020, 21, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Naranda, J.; Bračič, M.; Vogrin, M.; Maver, U. Recent advancements in 3D printing of polysaccharide hydrogels in cartilage tissue engineering. Materials 2021, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Guduric, V.; Duin, S.; Akkineni, A.R.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo-Mateo, N.; Dani, S.; Witzleben, M. v Methylcellulose–a versatile printing material that enables biofabrication of tissue equivalents with high shape fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Köhler, T.; Czichy, C.; Lode, A.; Gelinsky, M. A methylcellulose hydrogel as support for 3D plotting of complex shaped calcium phosphate scaffolds. Gels 2018, 4, 68. [Google Scholar] [CrossRef]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef]
- Maity, C.; Das, N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top. Curr. Chem. 2022, 380, 1–67. [Google Scholar] [CrossRef]
- Andrade, L.R.; Arcanjo, K.D.S.; Martins, H.S.H.L.; dos Reis, J.S.N.; Farina, M.; Borojevic, R.; Duarte, M.E.L. Fine structure and molecular content of human chondrocytes encapsulated in alginate beads. Cell Biol. Int. 2011, 35, 293–297. [Google Scholar] [CrossRef]
- Liu, H.; Lee, Y.-W.; Dean, M. Re-expression of differentiated proteoglycan phenotype by dedifferentiated human chondrocytes during culture in alginate beads. Biochim. Biophys. Acta (BBA)-General Subj. 1998, 1425, 505–515. [Google Scholar] [CrossRef]
- Hsieh-Bonassera, N.D.; Wu, I.; Lin, J.K.; Schumacher, B.L.; Chen, A.C.; Masuda, K.; Bugbee, W.D.; Sah, R.L. Expansion and redifferentiation of chondrocytes from osteoarthritic cartilage: Cells for human cartilage tissue engineering. Tissue Eng. Part A 2009, 15, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Fragonas, E.; Valente, M.; Pozzi-Mucelli, M.; Toffanin, R.; Rizzo, R.; Silvestri, F.; Vittur, F. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials 2000, 21, 795–801. [Google Scholar] [CrossRef]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Burke, M.; Carter, B.M.; Davis, S.A.; Perriman, A.W. 3D bioprinting using a templated porous bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Placht, A.-M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2015, 11, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kuth, S.; Distler, T.; Gögele, C.; Stölzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. C 2020, 116, 111189. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huang, L.; Hao, W.; Zhao, T.; Zhao, H.; Yang, W.; Xie, X.; Qian, L.; Chen, Y.; Dai, J. Long-term stability, high strength, and 3D printable alginate hydrogel for cartilage tissue engineering application. Biomed. Mater. 2021, 16, 64102. [Google Scholar] [CrossRef]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Muthusamy, S.; Narmadha, R.; Krishnakumar, G.S.; Selvakumar, R. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Sardroud, H.A.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Gu, Y.; Schwarz, B.; Forget, A.; Barbero, A.; Martin, I.; Shastri, V.P. Advanced bioink for 3D bioprinting of complex free-standing structures with high stiffness. Bioengineering 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-containing agarose hydrogel for wound and burn care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Hafezi, M.; Qin, L.; Mahmoodi, P.; Dong, G. Osmosis effect on protein sustained release of Agarose hydrogel for anti-friction performance. Tribol. Int. 2019, 132, 108–117. [Google Scholar] [CrossRef]
- Hasan, M.L.; Padalhin, A.R.; Kim, B.; Lee, B. Preparation and evaluation of BCP-CSD-agarose composite microsphere for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Vizcaíno-López, G.; Chato-Astrain, J.; Durand-Herrera, D.; Alaminos, M.; Campos, A.; Sánchez-Montesinos, I.; Campos, F. Scleral surgical repair through the use of nanostructured fibrin/agarose-based films in rabbits. Exp. Eye Res. 2019, 186, 107717. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, X.; Jiang, Y.; Xing, L.; Xu, Y.; Lu, Y.; Ding, P.; Ma, J.; Xu, Y.; Gui, J. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: An in vitro study. J. Biomater. Appl. 2014, 28, 1039–1050. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of agarose/silk fibroin blended hydrogel for in vitro cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.S.; Kim, W.K.; Lee, W.; Kim, N.; Song, C.U.; Jung, J.J.; Song, J.E.; Khang, G. Evaluation of hyaluronic acid/agarose hydrogel for cartilage tissue engineering biomaterial. Macromol. Res. 2020, 28, 979–985. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Balding, P.; Gorkin III, R.; Spinks, G.M. Printed ionic-covalent entanglement hydrogels from carrageenan and an epoxy amine. Rsc Adv. 2014, 4, 38088–38092. [Google Scholar] [CrossRef]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.; Kaunas, R.; Gaharwar, A.K. Nanoengineered ionic–covalent entanglement (NICE) bioinks for 3D bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-thinning and thermo-reversible nanoengineered inks for 3D bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef] [PubMed]
- In, E.; Naguib, H.E.; Haider, M. Mechanical stability analysis of carrageenan-based polymer gel for magnetic resonance imaging liver phantom with lesion particles. J. Med. Imaging 2014, 1, 035502. [Google Scholar] [CrossRef]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2013, 9, 550–563. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva Jr, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug delivery systems and other biomedical applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Raveendran, S.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 605–626. [Google Scholar] [CrossRef]
- Tytgat, L.; Vagenende, M.; Declercq, H.; Martins, J.C.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Van Vlierberghe, S. Synergistic effect of κ-carrageenan and gelatin blends towards adipose tissue engineering. Carbohydr. Polym. 2018, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Li, L. A strategy for strong interface bonding by 3D bioprinting of oppositely charged κ-carrageenan and gelatin hydrogels. Carbohydr. Polym. 2018, 198, 261–269. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Arevalo, M.D.P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- İlhan, G.T.; Irmak, G.; Gümüşderelioğlu, M. Microwave assisted methacrylation of Kappa carrageenan: A bioink for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3523–3534. [Google Scholar] [CrossRef]
- Du, C.; Hu, J.; Wu, X.; Shi, H.; Yu, H.C.; Qian, J.; Yin, J.; Gao, C.; Wu, Z.L.; Zheng, Q. 3D printing of a tough double-network hydrogel and its use as a scaffold to construct a tissue-like hydrogel composite. J. Mater. Chem. B 2022, 10, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ye, L.; Liang, H. Extremely stretchable and tough hybrid hydrogels based on gelatin, κ-carrageenan and polyacrylamide. Soft Matter 2021, 17, 9708–9715. [Google Scholar] [CrossRef]
- Laurent, T.C.; Laurent, U.B.; Fraser, J. Functions of hyaluronan. Ann. Rheum. Dis. 1995, 54, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Groll, J.; Teßmar, J.; Blunk, T. Tethered TGF-β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. Int. J. Mol. Sci. 2022, 23, 924. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T. Double printing of hyaluronic acid/poly (glycidol) hybrid hydrogels with poly (ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Pan, H.M.; Chen, S.; Jang, T.-S.; Han, W.T.; Li, Y.; Song, J. Plant seed-inspired cell protection, dormancy, and growth for large-scale biofabrication. Biofabrication 2019, 11, 25008. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Grijpma, D.W.; Alini, M.; Eglin, D.; D’Este, M. Three-dimensional printing of a tyramine hyaluronan derivative with double gelation mechanism for independent tuning of shear thinning and postprinting curing. ACS Biomater. Sci. Eng. 2018, 4, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, J.H.; Locke, R.C.; Witherel, C.E.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, J.A. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 2021, 14, 14106. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 32001. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Dozio, S.M.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. In situ sol-gel synthesis of hyaluronan derivatives bio-nanocomposite hydrogels. Regen. Biomater. 2019, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; D’Amora, U.; Raucci, M.G.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. A combined approach of double network hydrogel and nanocomposites based on hyaluronic acid and poly(ethylene glycol) diacrylate blend. Materials 2018, 11, 2454. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Lin, H.; Soriente, A.; Fan, Y.; Zhang, X.; Ambrosio, L. Bioactive composites based on double network approach with tailored mechanical, physico-chemical, and biological features. J. Biomed. Mater. Res. Part A 2018, 106, 3079–3089. [Google Scholar] [CrossRef]
- Zhang, L.; D’Amora, U.; Ronca, A.; Li, Y.; Mo, X.; Zhou, F.; Yuan, M.; Ambrosio, L.; Wu, J.; Raucci, M.G. In vitro and in vivo biocompatibility and inflammation response of methacrylated and maleated hyaluronic acid for wound healing. RSC Adv. 2020, 10, 32183–32192. [Google Scholar] [CrossRef]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Horder, H.; Stahlhut, P.; Groll, J.; Blunk, T.; Teßmar, J. Bioink Platform Utilizing Dual-Stage Crosslinking of Hyaluronic Acid Tailored for Chondrogenic Differentiation of Mesenchymal Stromal Cells. Macromol. Biosci. 2022, 22, 2100331. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; del Pilar González, M.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural biomaterials and their use as bioinks for printing tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. An Off. J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 1–12. [Google Scholar] [CrossRef]
- Diamantides, N.; Dugopolski, C.; Blahut, E.; Kennedy, S.; Bonassar, L.J. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication 2019, 11, 045016. [Google Scholar] [CrossRef]
- Henrionnet, C.; Pourchet, L.; Neybecker, P.; Messaoudi, O.; Gillet, P.; Loeuille, D.; Mainard, D.; Marquette, C.; Pinzano, A. Combining innovative bioink and low cell density for the production of 3D-Bioprinted cartilage substitutes: A pilot study. Stem Cells Int. 2020, 2020, 2487072. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, T.; Yue, X.; Sun, F.; Yao, D. 3D Composite Cell Printing Gelatin/Sodium Alginate/n-HAP Bioscaffold. J. Phys. Conf. Ser. 2019, 1213, 042020. [Google Scholar] [CrossRef]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D bioprinting using cross-linker-free silk–gelatin bioink for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef]
- Trucco, D.; Sharma, A.; Manferdini, C.; Gabusi, E.; Petretta, M.; Desando, G.; Ricotti, L.; Chakraborty, J.; Ghosh, S.; Lisignoli, G. Modeling and Fabrication of Silk Fibroin–Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater. Sci. Eng. 2021, 7, 3306–3320. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Lungu, A.; Dragusin, D.M.; Stancu, I.C.; Dinescu, S.; Balahura, L.R.; Mereuta, P.; Costache, M.; Iovu, H. 3D Bioprinting of Biosynthetic Nanocellulose-Filled GelMA Inks Highly Reliable for Soft Tissue-Oriented Constructs. Materials 2021, 14, 4891. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 15003. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef]
- Han, X.; Chang, S.; Zhang, M.; Bian, X.; Li, C.; Li, D. Advances of hydrogel-based bioprinting for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 746564. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Prelipcean, A.-M.; Iosageanu, A.; Gaspar-Pintiliescu, A.; Moldovan, L.; Craciunescu, O.; Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Mitran, R.-A.; Marin, M.; D’Amora, U. Marine and Agro-Industrial By-Products Valorization Intended for Topical Formulations in Wound Healing Applications. Materials 2022, 15, 3507. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of collagen: Considerations, potentials, and applications. Macromol. Biosci. 2021, 21, 2000280. [Google Scholar] [CrossRef]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Wu, W.; Zhang, A.; Lu, B.; Zhang, T.; Kong, M. Dual cure (thermal/photo) composite hydrogel derived from chitosan/collagen for in situ 3D bioprinting. Int. J. Biol. Macromol. 2021, 182, 689–700. [Google Scholar] [CrossRef]
- Sanz, B.; Albillos Sanchez, A.; Tangey, B.; Gilmore, K.; Yue, Z.; Liu, X.; Wallace, G. Light cross-linkable marine collagen for coaxial printing of a 3D model of neuromuscular junction formation. Biomedicines 2020, 9, 16. [Google Scholar] [CrossRef]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Suitability of Marine-and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and properties of chitosan inks for 3D printing of hydrogel microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.M.; Myers, D.E. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef]
- He, Y.; Derakhshanfar, S.; Zhong, W.; Li, B.; Lu, F.; Xing, M.; Li, X. Characterization and application of carboxymethyl chitosan-based bioink in cartilage tissue engineering. J. Nanomater. 2020, 2020, 2057097. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication 2018, 10, 045006. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Wu, W.; Wu, Z.; Yuan, Y.; Wu, J.; Liu, C. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small 2022, 2106824. [Google Scholar] [CrossRef]
- Almeida, H.V.; Sathy, B.N.; Dudurych, I.; Buckley, C.T.; O’Brien, F.J.; Kelly, D.J. Anisotropic shape-memory alginate scaffolds functionalized with either type I or type II collagen for cartilage tissue engineering. Tissue Eng. Part A 2017, 23, 55–68. [Google Scholar] [CrossRef]
- Betsch, M.; Cristian, C.; Lin, Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4D into bioprinting: Real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv. Healthc. Mater. 2018, 7, e1800894. [Google Scholar] [CrossRef]
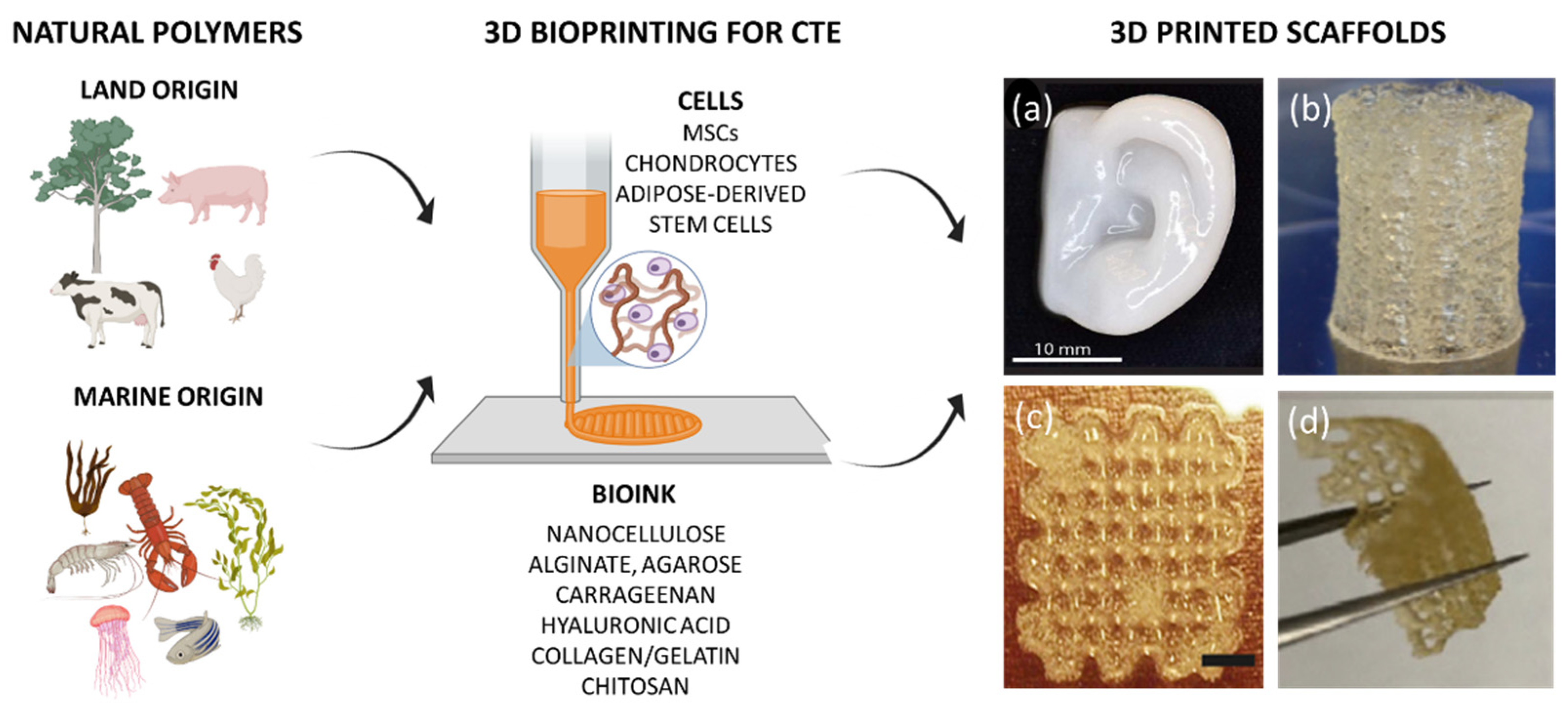


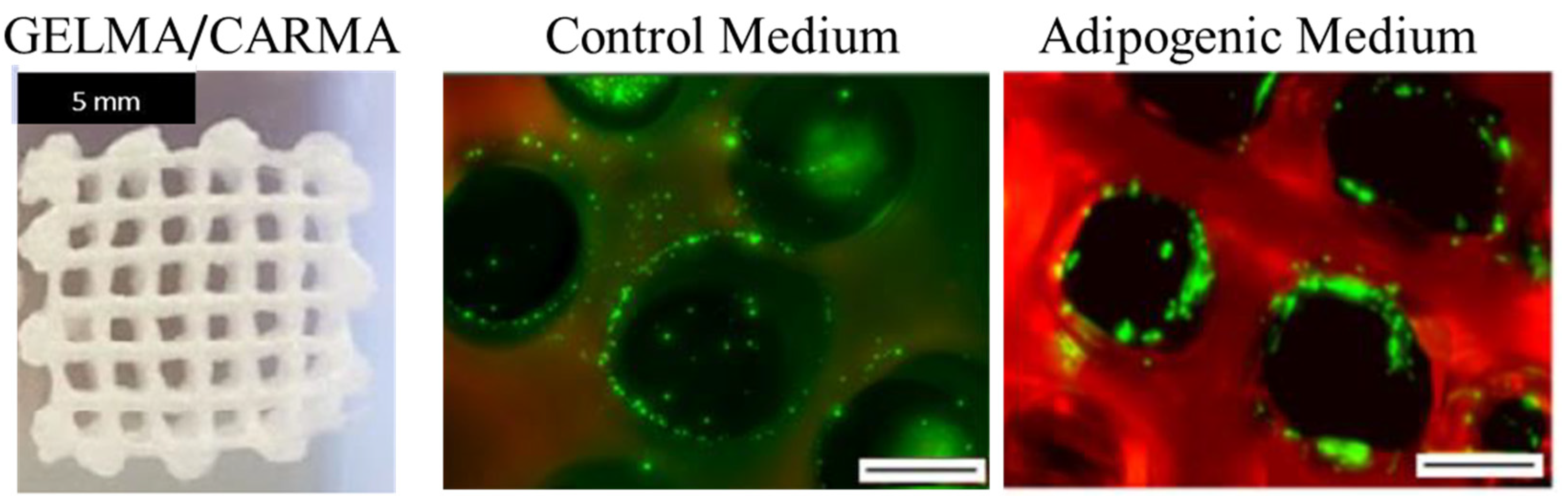
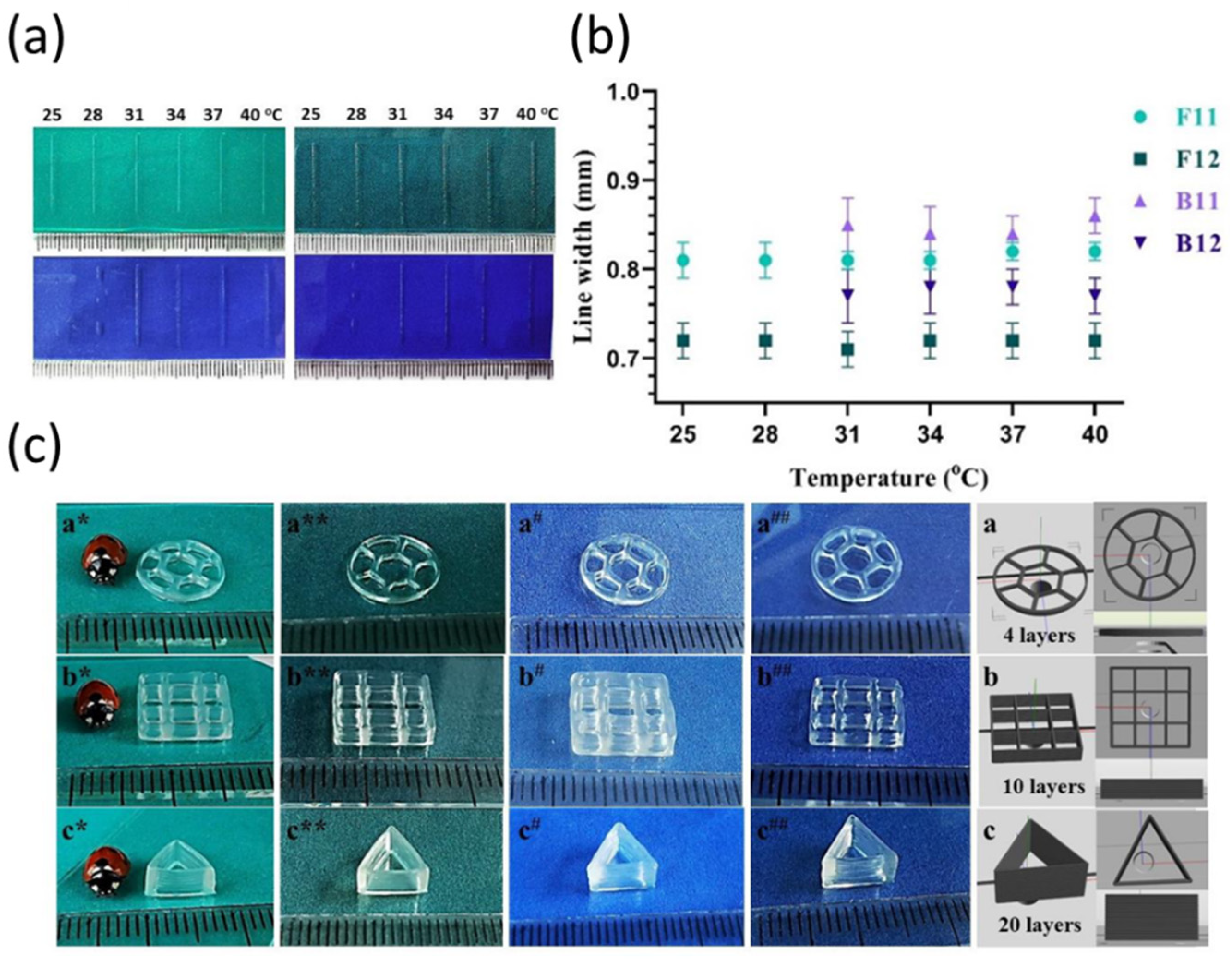
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| Nanofibrillated Cellulose (NFC)/Alginate (ALG) | Human nasoseptal chondrocytes (hNSCs, 15 × 106 cells/mL) | High shape fidelity; decrease of cell viability due to shear forces during mixing and crosslinking | Markstedt et al. (2015), [25] |
| NFC/ALG | hNSCs (20 × 106 cells/mL) | Optimized shape and stability at 28 days; neo-synthesis of cartilage-specific extracellular matrix | Ávila et al. (2016), [31] |
| NFC/ALG/ Hyaluronic acid (HA) | Human-derived induced pluripotent stem cells (iPSCs); Human chondrocytes (20 × 106 cells/mL) | Maintaining of pluripotency of stem cells; cartilage formation; collagen expression | Nguyen et al. (2017), [32] |
| NFC/ALG sulfate | Chondrocytes from old calves (6 × 106 cells/mL) | High viability of chondrocytes; deposition of collagen II; wide diameter, conical needles preserved cell function | Müller et al. (2016), [33] |
| NFC/ALG | Human bone marrow–derived mesenchymal stem cells (hBMSCs) and hNSCs (10 × 106 cells/mL) | Good printability and dimensional stability; good mechanical properties; chondro-permissive; glycosaminoglycan (GAG)-positive cell proliferation | Möller et al. (2017), [34]; Apelgren et al. (2017), [35] |
| NC/ALG | hNSCs (2 × 106 cells/mL) | Shear thinning behavior; favorable swelling features; high metabolic activity of hNSCs; limited mechanical properties | Jessop et al. (2019), [36] |
| Cellulose nanocrystals/Gelatin methacryloyl/ methacrylated hyaluronic acid (CNC/GELMA/HAMA) | Mouse chondrogenic cell line (ATDC5, 1 × 106 cells/mL) | Good printability; shear thinning behavior; high structural support and integration; good cell viability | Fan et al. (2020), [37] |
| NC/ALG/HA | Murine D1-MSCs (2.5 × 106 and 5 × 106 cells/mL) | HA induced a more fibrous structure; less rounded morphology; earlier water swelling in 3 to 4 h; slower degradation; better biological behavior | Lafuente-Merchan et al. (2021), [38] |
| Quince seed mucilage (QSM)/NFC | Human liver cancer cells (HepG2, 5 × 106 cells/mL) | Precise control on printing fidelity; suitable water uptake capacity and mechanical properties; good cell attachment and proliferation | Baniasadi et al. (2021), [39] |
| Methylcellulose (MC)/ALG | Primary bovine chondrocytes (BCs, 0.5 × 106 cells/mL) | Good viscosity and stability; high cell survival and proteoglycan matrix production | Hodder et al. (2019), [40] |
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| Pure Alginate (ALG) Pure Agarose (AG) | Human bone marrow stromal cells (hBMSCs, 2 × 106 cells/mL) | Development of hyaline-like cartilage tissue | Daly et al. (2016), [55] |
| Pluronic F127/ALG | hMSCs (3 × 106 cells/mL) | Increased shear thinning; good compressive modulus; good cell viability over 10 days and chondrogenic properties over five weeks | Armstrong et al. (2016), [56] |
| ALG/Methylcellulose (MC) | hBMSCs (5 × 106 cells/g) | Enhanced viscosity; high elasticity and stability; enhanced microporosity; high viability; maintenance of differentiation potential | Schutz et al. (2017), [57] |
| Collagen (COL)/ALG, AG/ALG | Chondrocytes (1 × 107 cells/mL) | Improved mechanical strength; better cell adhesion; increased cell proliferation; increased cartilage genes expression; lower expression of Col1a1 | Yang et al. (2018), [58] |
| Oxidized alginate-di-aldehyde (ADA)/ Gelatin (GEL) | Human nasoseptal chondrocytes (hNSCs, 4 × 106 cells/mL) | Open inner structure; high viscosity and shear thinning behavior; promotion of collagen type II and cartilage proteoglycans Enhanced printability; high shape stability and fidelity without use of chemical additives or crosslinkers | Schwarz et al. (2020), [59] Kreller et al. (2021), [26] |
| Double crosslinked ALG (DC-ALG) | Human umbilical cord MSCs (huMSCs, 1 × 105 cells/mL) | Strong mechanical properties; better stability; good cell viability; high printing accuracy (∼200 µm); expression of chondrogenic genes | Chu et al. (2021), [60] |
| GEL/Carboxymethyl cellulose/ALG | Osteosarcoma cells, MG63 | Increased collagen deposition; improved cell proliferation | Satish et al. (2022), [61] |
| ALG/CS/ Hydroxyapatite (nHAp) | Chondrocytes (ATDC5, 2 × 105 cells/mL) (top seeding) | Increased elastic modulus; improved cell attachment and viability; antibacterial ability of CS | Sadeghianmaryan et al. (2022), [62] |
| Carboxylated AG/neat AG | hNCs (3 × 107 cells/mL) | High print reproducibility and size fidelity; high stability over a wide temperature range (4–37 °C); high cell density without impact on printability | Gu et al. (2020), [63] |
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| kappa Carrageenan/Nanosilicates (k-CAR/nSi) | Mouse preosteoblasts (MC3T3-E1) | High shape and structural fidelity; enhanced mechanical properties | Wilson et al. (2017), [81] |
| k-CAR/Gelatin (GEL) | Mouse myoblasts (C2C12, 2.8 × 105 cells/mL | Good multilayered structural stability at 37 °C and a high cell viability | Li et al. (2019), [92] |
| Methacrylamide-modified gelatin (GELMA)/methacrylated k-CAR (CARMA) | Human adipose tissue-derived stem cells (hASCs, 1 × 105 cells) (top seeding) | Good stability; high water swelling; mechanical properties comparable to those of native tissue | Tytgat et al. (2019), [93] |
| k-CAR/Alginate (ALG) | Rabbit adipose mesenchymal stem cells (AMSCs, 5 × 105 cells/mL) | Excellent structural strength and printability without significant negative effects on cell viability | Kim et al. (2019), [94] |
| CARMA | Embryonal carcinoma-derived chondrogenic cells (ATDC5, 2 × 107 cells/mL) | Improved mechanical behavior and degradation time; improved cell migration, proliferation and differentiation | Ilhan et al. (2020), [95] |
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| Hyaluronic acid (HA) | Chondrocytes (2 × 106 cells/mL) | High viability and function of cells maintained up to 14 days of culture; cell migration | Park et al. (2014), [101] |
| Methacrylated hyaluronic acid (HAMA) | Bone marrow stromal cells (BMSCs, 1 × 107 cells/mL) | Enhanced viability; cell chondrogenic differentiation potential; high mechanical properties; high resolution of the deposition method; resistant to degradation; good biocompatibility | Costantini et al. (2016), [102] |
| HAMA | hBMSCs (2 × 106 cells/mL) | Increased mechanical stiffness; long-term stability; high cell viability; spontaneous osteogenic potential | Poldervaart et al. (2017), [27] |
| HA/Alginate (ALG) | Chondrocytes (1 × 107 cells/mL) | Good printability; gelling abilities; stiffness and good degradability; high cell viability | Antich et al. (2020), [103] |
| Covalently tethered TGF-β1/HA | hBMSCs (2 × 106 cells/mL) | High shape fidelity; highly porous network with low polymer content (2% (w)); high chondrogenisis; homogeneous ECM distribution | Hauptstein et al. (2021), [104] |
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| Collagen (COL) | Bovine primary articular chondrocytes (bPAC, 107 cells/mL) | Increased storage modulus and improved printability by blue-light-activated riboflavin crosslinker; gelation kinetics and storage moduli pH dependent | Diamantides et al. (2017), [122] |
| COL branded Viscoll (Imtek Ltd., Russia) | Mouse fibroblasts expressing green fluorescent protein (NIH 3T3-GFP, 0.5 × 106 cells/mL) | Increased storage modulus; improved printability of collagen; appropriate support of spatial distributuin of tissue spheroids into rigid patterns with resolution of 0.5 mm; sufficient cell viability | Osidak et al. (2019), [124] |
| COL | bPAC, up to 108 cells/mL | Increased storage modulus and viscosity before gelation; storage modulus after gelation and gelation rate decreased along with increasing cell density | Diamantides et al. (2019), [125] |
| Alginate (ALG)/Gelatin (GEL)/Fibrinogen | Mesenchymal stem cells (MSCs, 1–2 × 106 cells/mL) | Hypoxia prevention of calcifications by hypoxia; enhanced chondrogenesis by TGF-β1/3 combined with BMP-2 | Henrionnet et al. (2020), [126] |
| Gelatin methacryloyl (GELMA)/Gellan gum | Equine primary chondrocytes (1–2 × 107 cells/mL) | Improved filament deposition; increased construct stiffness; chondrogenic potential | Mouser et al. (2016), [127] |
| GEL/ALG/nano-hydroxyapatite (nHAp) | Mouse chondrocytes (2 × 105 cells/mL) | Improved surface roughness and biodegradability; no cytotoxicity; enhanced cell adhesion and growth; high cell viability | Fan et al. (2019), [128] |
| GELMA | Multipotent articular cartilage-resident chondroprogenitor cells (ACPCs), MSCs (1.5 × 107 cells/mL) | MSCs-laden GELMA printable in a zonal-like architecture; biomimetic GAG distribution | Levato et al. (2017), [129] |
| Silk/GEL | Chondrocytes (106 cells/mL) | Suitable swelling behavior; optimal rheology; supportive structure; cartilage ECM formation; chondrogenic phenotype maintenance | Singh et al. (2019), [130] |
| Silk Fibroin/GEL | hMSCs (0.6 × 107 cells/mL) | Printing parameters optimized by the model; good chondrogenicity | Trucco et al. (2021), [131] |
| GEL/HAp | Human umbilical cord blood-derived MSCs (hUCB-MSCs, 105 cells) (top seeding) | Cell adhesion and proliferation support; chondrogenic differentiation induction; increased hydrogel fluidity; improved gelation kinetics and rheological properties | Huang et al. (2021), [132] |
| Nanofibrillated Cellulose (NFC)/Fish GELMA NFC/Bovine GELMA | Human adipose tissue-derived MSCs (hAMSCs, 106 cells/mL) | Good printability; high shape fidelity and well-defined internal structure; Fish GEL exhibited a broader bioprintability window; NFC/GELMA allowed cell growth and proliferation | Cernencu et al. (2021), [133] |
| Bioink | Cell Population | Main Outcomes | Reference |
|---|---|---|---|
| Chitosan (CS) | Infrapatellar fat pad AMSCs (7.5 × 105 cells/mL) | Cartilage-like tissue formation in 4 weeks of culture | Ye et al. (2014), [148] |
| Carboxymethyl CS | Rabbit chondrocytes (1 × 105 cells/mL) | Higher storage and loss moduli; low cytotoxicity; good cell proliferation rate; fast gelation; high printability | He et al. (2020), [149] |
| CS | Mouse chondrogenic cell line (ATDC5, 106 cells/mL) | Higher elastic modulus for scaffolds with smaller pore sizes; high cell adhesion | Sadeghianmaryan et al. (2020), [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. https://doi.org/10.3390/jfb13030118
Szychlinska MA, Bucchieri F, Fucarino A, Ronca A, D’Amora U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. Journal of Functional Biomaterials. 2022; 13(3):118. https://doi.org/10.3390/jfb13030118
Chicago/Turabian StyleSzychlinska, Marta Anna, Fabio Bucchieri, Alberto Fucarino, Alfredo Ronca, and Ugo D’Amora. 2022. "Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources" Journal of Functional Biomaterials 13, no. 3: 118. https://doi.org/10.3390/jfb13030118
APA StyleSzychlinska, M. A., Bucchieri, F., Fucarino, A., Ronca, A., & D’Amora, U. (2022). Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. Journal of Functional Biomaterials, 13(3), 118. https://doi.org/10.3390/jfb13030118










