Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
2.2. Cryoprecipitate Isolation
2.3. Fibrin Addition
2.4. Homogenization
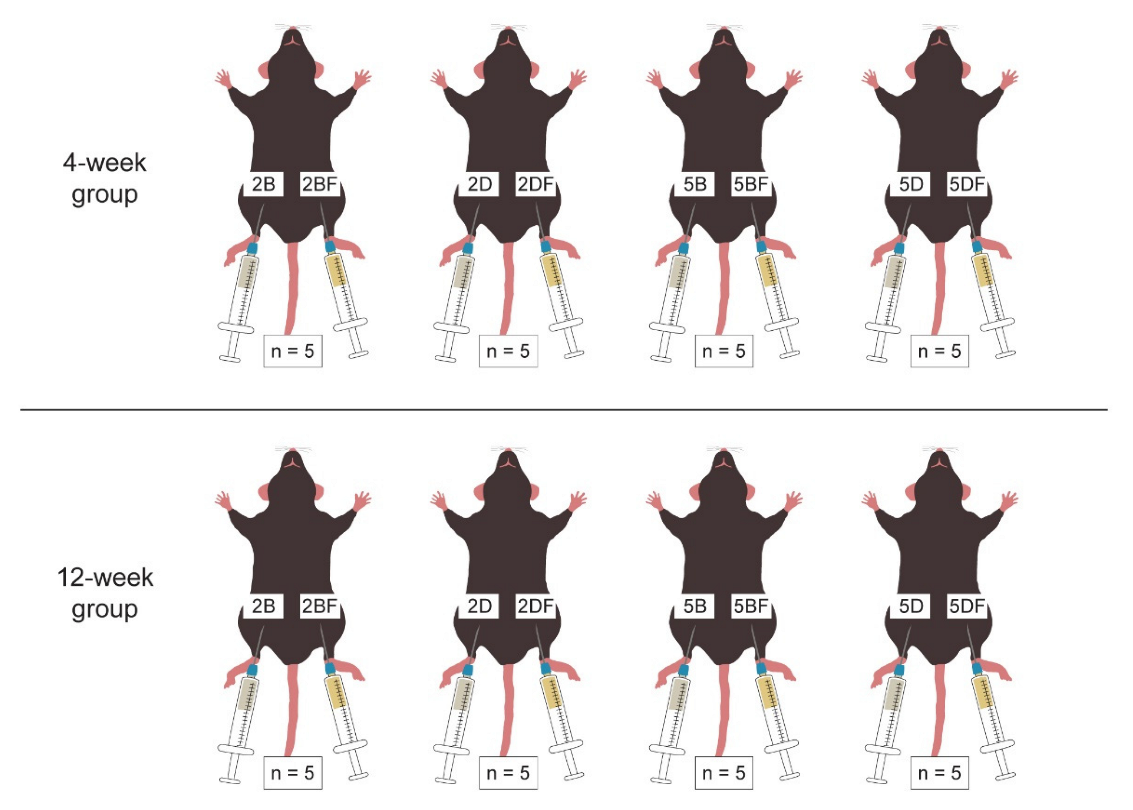
2.5. HA Injection to Mice
2.6. Harvesting the Scaffolds
2.7. Hematoxylin-Eosin (H-E) Staining
2.8. Statistical Analysis
3. Results
3.1. Weight Measurement
3.2. Relative Cross-Section Area of the Implants
3.3. Microscopic Images of the Implants
3.4. Evaluation of Vascularization
3.5. Histological Analysis
3.6. The Relative Area of HA and Cells + Extracellular Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janoušková, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef] [PubMed]
- Terzic, A.; Pfenning, M.A.; Gores, G.J.; Harper, C.M., Jr. Regenerative Medicine Build-Out. Stem Cells Transl. Med. 2015, 4, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Almici, E.; Caballero, D.; Montero Boronat, J.; Samitier Martí, J. Engineering cell-derived matrices with controlled 3D architectures for pathophysiological studies. Methods Cell Biol. 2020, 156, 161–183. [Google Scholar] [PubMed]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Elkhoury, K.; Russell, C.S.; Sanchez-Gonzalez, L.; Mostafavi, A.; Williams, T.J.; Kahn, C.; Peppas, N.A.; Arab-Tehrany, E.; Tamayol, A. Soft-Nanoparticle Functionalization of Natural Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2019, 8, e1900506. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Valero, C.; Amaveda, H.; Mora, M.; García-Aznar, J.M. Combined experimental and computational characterization of crosslinked collagen-based hydrogels. PLoS ONE 2018, 13, e0195820. [Google Scholar] [CrossRef]
- Bettadapur, A.; Suh, G.C.; Geisse, N.A.; Wang, E.R.; Hua, C.; Huber, H.A.; Viscio, A.A.; Kim, J.Y.; Strickland, J.B.; McCain, M.L. Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels. Sci. Rep. 2016, 6, 28855. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Silva-Correia, J.; Gonçalves, C.; Pina, S.; Radhouani, H.; Montonen, T.; Hyttinen, J.; Roy, A.; Oliveira, A.L.; Reis, R.L.; et al. Rapidly responsive silk fibroin hydrogels as an artificial matrix for the programmed tumor cells death. PLoS ONE 2018, 13, e0194441. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.T.; Stilhano, R.S.; Silva, E.A. Enzymatically degradable alginate hydrogel systems to deliver endothelial progenitor cells for potential revasculature applications. Biomaterials 2018, 179, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels—Definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, A.; Ézsiás, B.; Pál, É.; Hricisák, L.; Fülöp, Á.; Besztercei, B.; Somkuti, J.; Smeller, L.; Pinke, B.; Kardos, D.; et al. Crosslinked Hyaluronic Acid Gels with Blood-Derived Protein Components for Soft Tissue Regeneration. Tissue Eng. Part A 2021, 27, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Kang, Q.K.; Ramamurthi, A. The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels. J. Biomed. Mater. Res. A 2010, 94, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, A.; Vesely, I. Smooth muscle cell adhesion on crosslinked hyaluronan gels. J. Biomed. Mater. Res. 2002, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; França, C.G.; Dávila, J.L.; Batista, N.A.; Caliari-Oliveira, C.; d’Ávila, M.A.; Luzo, Â.C.M.; Lana, J.; Santana, M.H.A. Hyaluronic acid and fibrin from L-PRP form semi-IPNs with tunable properties suitable for use in regenerative medicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110547. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005, 70, 247–299. [Google Scholar]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, A.; Kun, K.; Gajnut, F.; Majer, A.; Lacza, Z.; Hornyák, I. Cell Attachment Capacity and Compounds of Fibrin Membranes Isolated from Fresh Frozen Plasma and Cryoprecipitate. Membranes 2021, 11, 783. [Google Scholar] [CrossRef]
- Nascimento, B.; Goodnough, L.T.; Levy, J.H. Cryoprecipitate therapy. Br. J. Anaesth. 2014, 113, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Stanworth, S.; Baglin, T. Cryoprecipitate: An outmoded treatment? Transfus. Med. 2012, 22, 315–320. [Google Scholar] [CrossRef]
- Kruse, R.L.; Neally, M.; Cho, B.C.; Bloch, E.M.; Lokhandwala, P.M.; Ness, P.M.; Frank, S.M.; Tobian, A.A.R.; Gehrie, E.A. Cryoprecipitate Utilization Patterns Observed With a Required Prospective Approval Process vs Electronic Dosing Guidance. Am. J. Clin. Pathol. 2020, 154, 362–368. [Google Scholar] [CrossRef] [PubMed]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Biomed. Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef] [PubMed]
- Bogdan Allemann, I.; Baumann, L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin. Interv. Aging 2008, 3, 629–634. [Google Scholar] [PubMed]
- Brandt, F.S.; Cazzaniga, A. Hyaluronic acid gel fillers in the management of facial aging. Clin. Interv. Aging 2008, 3, 153–159. [Google Scholar] [PubMed]
- Hinsenkamp, A.; Kardos, D.; Lacza, Z.; Hornyák, I. A Practical Guide to Class IIa Medical Device Development. Appl. Sci. 2020, 10, 3638. [Google Scholar] [CrossRef]
- Hinsenkamp, A.; Benyó, Z.; Hornyák, I. Overview of Tissue Engineering Patent Strategies and Patents from 2010 to 2020, Including Outcomes. Tissue Eng. Part B Rev. 2022, 28, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Zhang, Y.; Yin, Y.; Zhao, H.; Han, X. Fabrication of an injectable iron (III) crosslinked alginate-hyaluronic acid hydrogel with shear-thinning and antimicrobial activities. Carbohydr. Polym. 2021, 260, 117777. [Google Scholar] [CrossRef]
- Oh, E.J.; Kang, S.W.; Kim, B.S.; Jiang, G.; Cho, I.H.; Hahn, S.K. Control of the molecular degradation of hyaluronic acid hydrogels for tissue augmentation. J. Biomed. Mater. Res. A 2008, 86, 685–693. [Google Scholar] [CrossRef]
- Yang, R.; Tan, L.; Cen, L.; Zhang, Z. An injectable scaffold based on crosslinked hyaluronic acid gel for tissue regeneration. RSC Adv. 2016, 6, 16838–16850. [Google Scholar] [CrossRef]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Effect of hyaluronic acid initial concentration on cross-linking efficiency of hyaluronic acid—Based hydrogels used in biomedical and cosmetic applications. Pharmazie 2017, 72, 81–86. [Google Scholar] [PubMed]
- Shmidov, Y.; Zhu, Y.; Matson, J.B.; Bitton, R. Effect of Crosslinker Topology on Enzymatic Degradation of Hydrogels. Biomacromolecules 2020, 21, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, S.; Medved, L. Effect of fibrinogen, fibrin, and fibrin degradation products on transendothelial migration of leukocytes. Thromb. Res. 2018, 162, 93–100. [Google Scholar] [CrossRef] [PubMed]
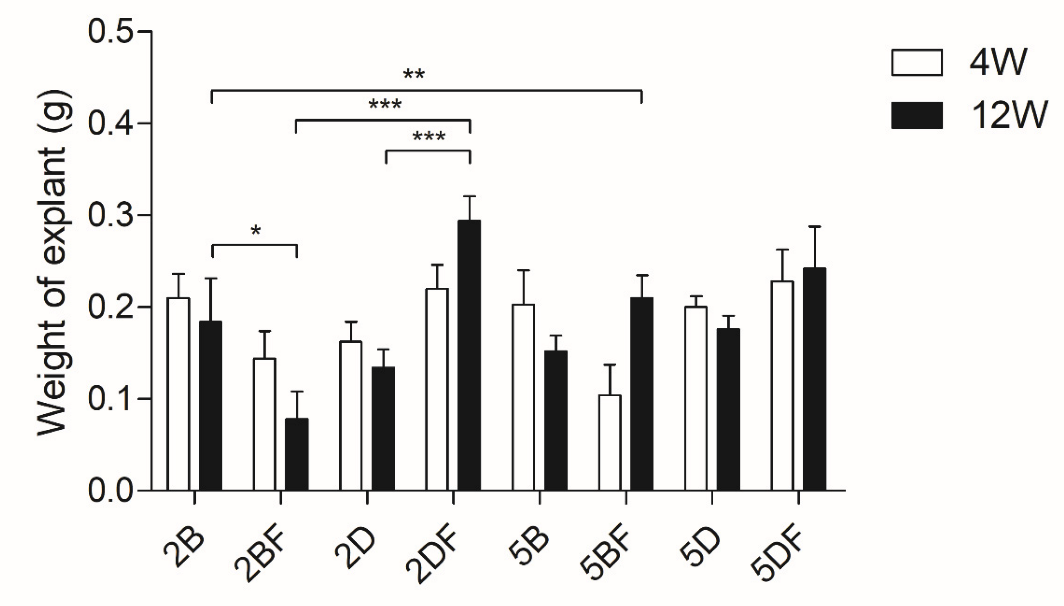



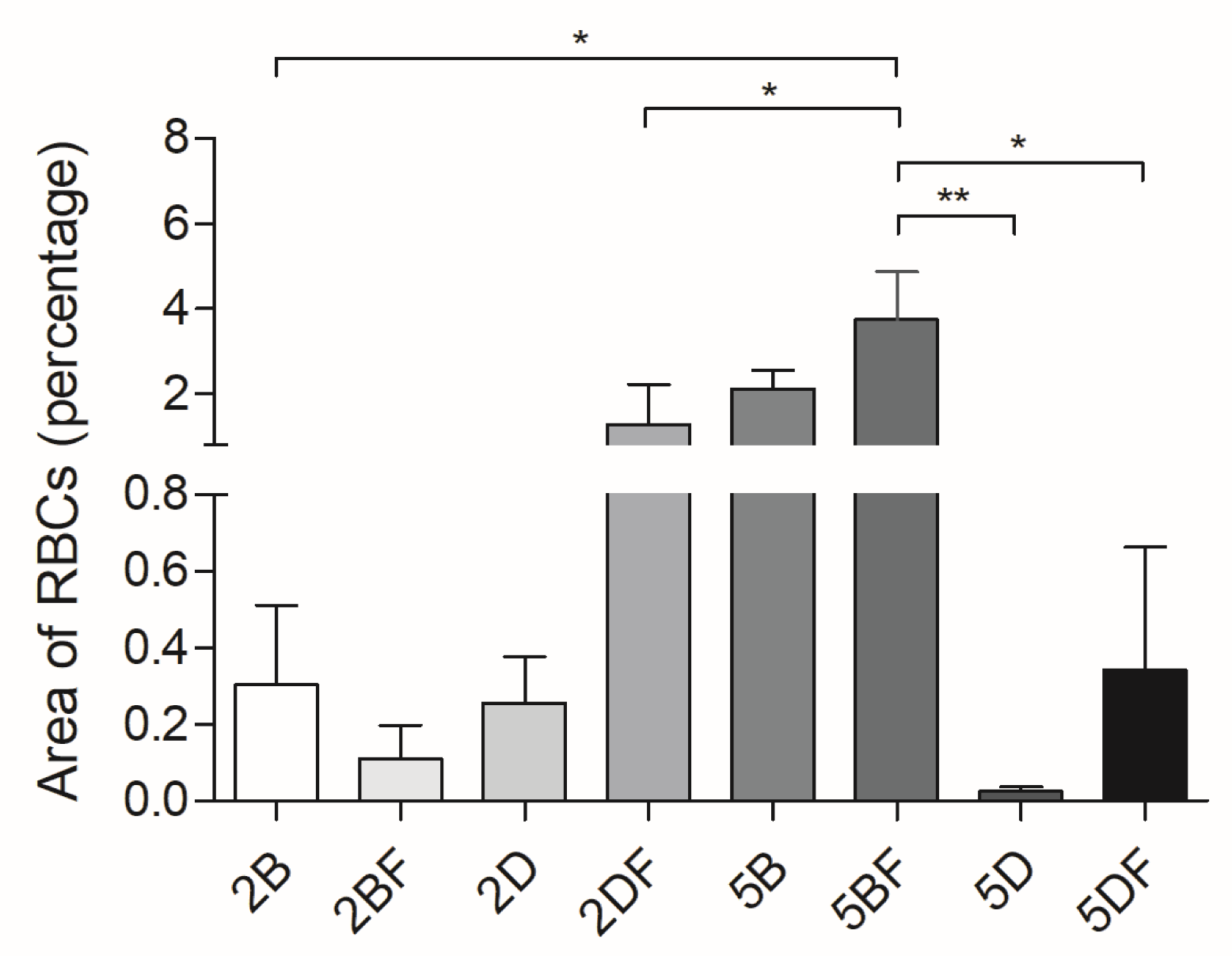

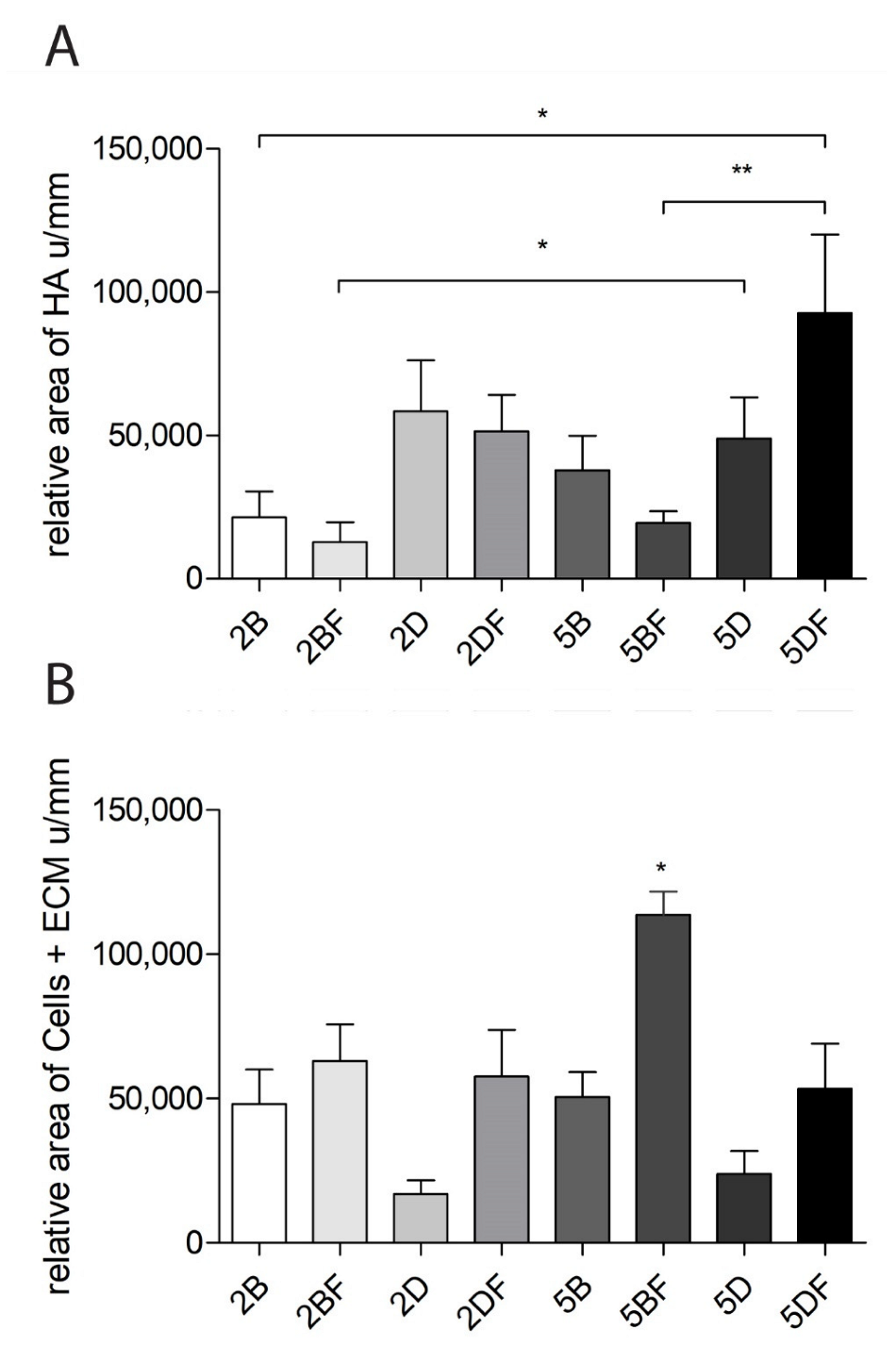
| Scaffold Type | Scaffold Type | Time Group (Week) | Weight Difference | Scaffold Type | Scaffold Type | Time Group (Week) | Weight Difference |
|---|---|---|---|---|---|---|---|
| 2B | 2BF | 12 | * | 2D | 2DF | 12 | *** |
| 2B | 2DF | 12 | * | 2D | 5DF | 12 | * |
| 2B | 5BF | 4 | * | 2DF | 5B | 12 | ** |
| 2BF | 2DF | 12 | *** | 2DF | 5BF | 4 | * |
| 2BF | 5BF | 12 | ** | 2DF | 5D | 12 | * |
| 2BF | 5D | 12 | * | 5BF | 5D | 4 | * |
| 2BF | 5DF | 12 | *** | 5BF | 5DF | 4 | ** |
| Scaffold Type | Scaffold Type | Time Group (Week) | Area Difference | Scaffold Type | Scaffold Type | Time Group (Week) | Area Difference |
|---|---|---|---|---|---|---|---|
| 2B | 2BF | 12 | *** | 2BF | 5DF | 4 | ** |
| 2B | 5B | 4 | * | 12 | ** | ||
| 12 | *** | 2D | 5B | 4 | * | ||
| 2B | 5BF | 4 | *** | 2D | 5BF | 4 | *** |
| 2B | 5D | 4 | ** | 2D | 5D | 4 | * |
| 2B | 5DF | 4 | *** | 2D | 5DF | 4 | *** |
| 2BF | 2D | 12 | * | 2DF | 5B | 4 | * |
| 2BF | 2DF | 12 | *** | 12 | * | ||
| 2BF | 5BF | 4 | ** | 2DF | 5BF | 4 | *** |
| 12 | * | 2DF | 5D | 4 | *** | ||
| 2BF | 5D | 12 | *** | 5B | 5D | 12 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinsenkamp, A.; Fülöp, Á.; Hricisák, L.; Pál, É.; Kun, K.; Majer, A.; Varga, V.; Lacza, Z.; Hornyák, I. Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. J. Funct. Biomater. 2022, 13, 119. https://doi.org/10.3390/jfb13030119
Hinsenkamp A, Fülöp Á, Hricisák L, Pál É, Kun K, Majer A, Varga V, Lacza Z, Hornyák I. Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. Journal of Functional Biomaterials. 2022; 13(3):119. https://doi.org/10.3390/jfb13030119
Chicago/Turabian StyleHinsenkamp, Adél, Ágnes Fülöp, László Hricisák, Éva Pál, Kiara Kun, Aliz Majer, Viktória Varga, Zsombor Lacza, and István Hornyák. 2022. "Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling" Journal of Functional Biomaterials 13, no. 3: 119. https://doi.org/10.3390/jfb13030119
APA StyleHinsenkamp, A., Fülöp, Á., Hricisák, L., Pál, É., Kun, K., Majer, A., Varga, V., Lacza, Z., & Hornyák, I. (2022). Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. Journal of Functional Biomaterials, 13(3), 119. https://doi.org/10.3390/jfb13030119








