Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
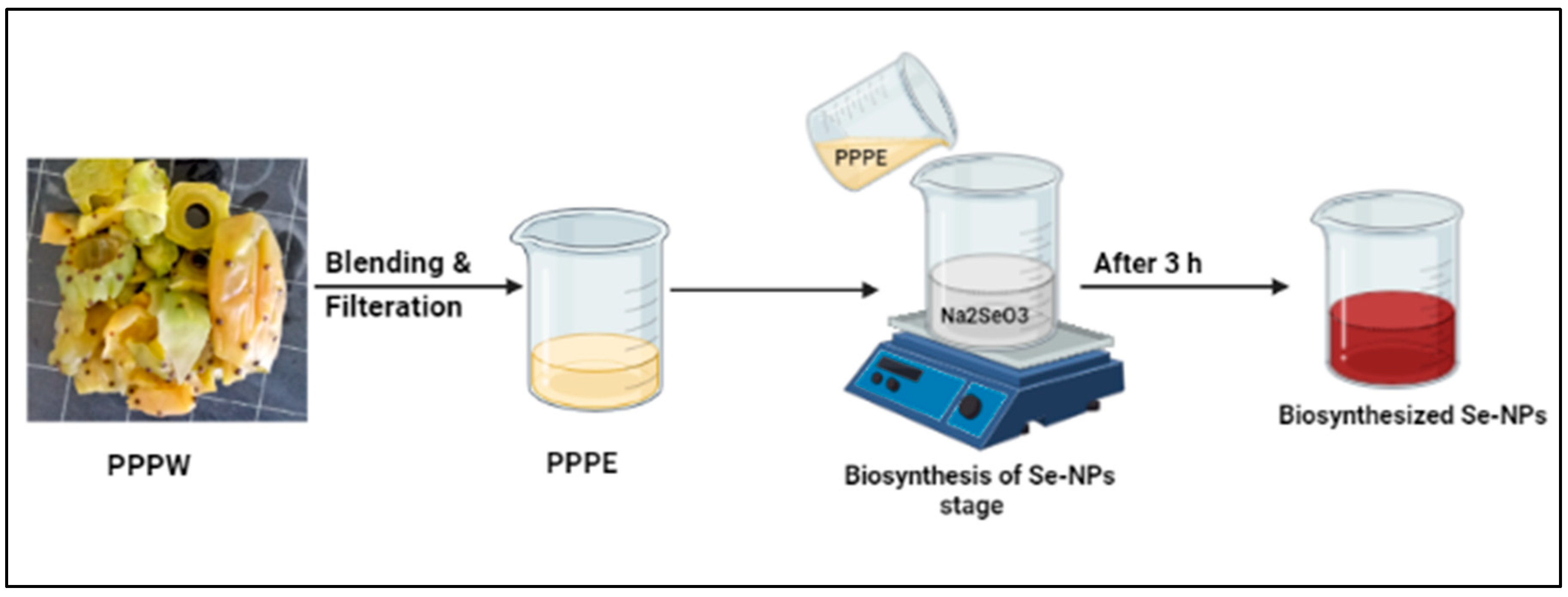
2.2.1. Preparation of Prickly Pear Peel Waste (PPPW) Extract
2.2.2. Biosynthesis of SeNPs
2.2.3. Characterization of SeNPs
UV–Vis Spectroscopy
Fourier-Transform Infrared (FT-IR) Spectroscopy
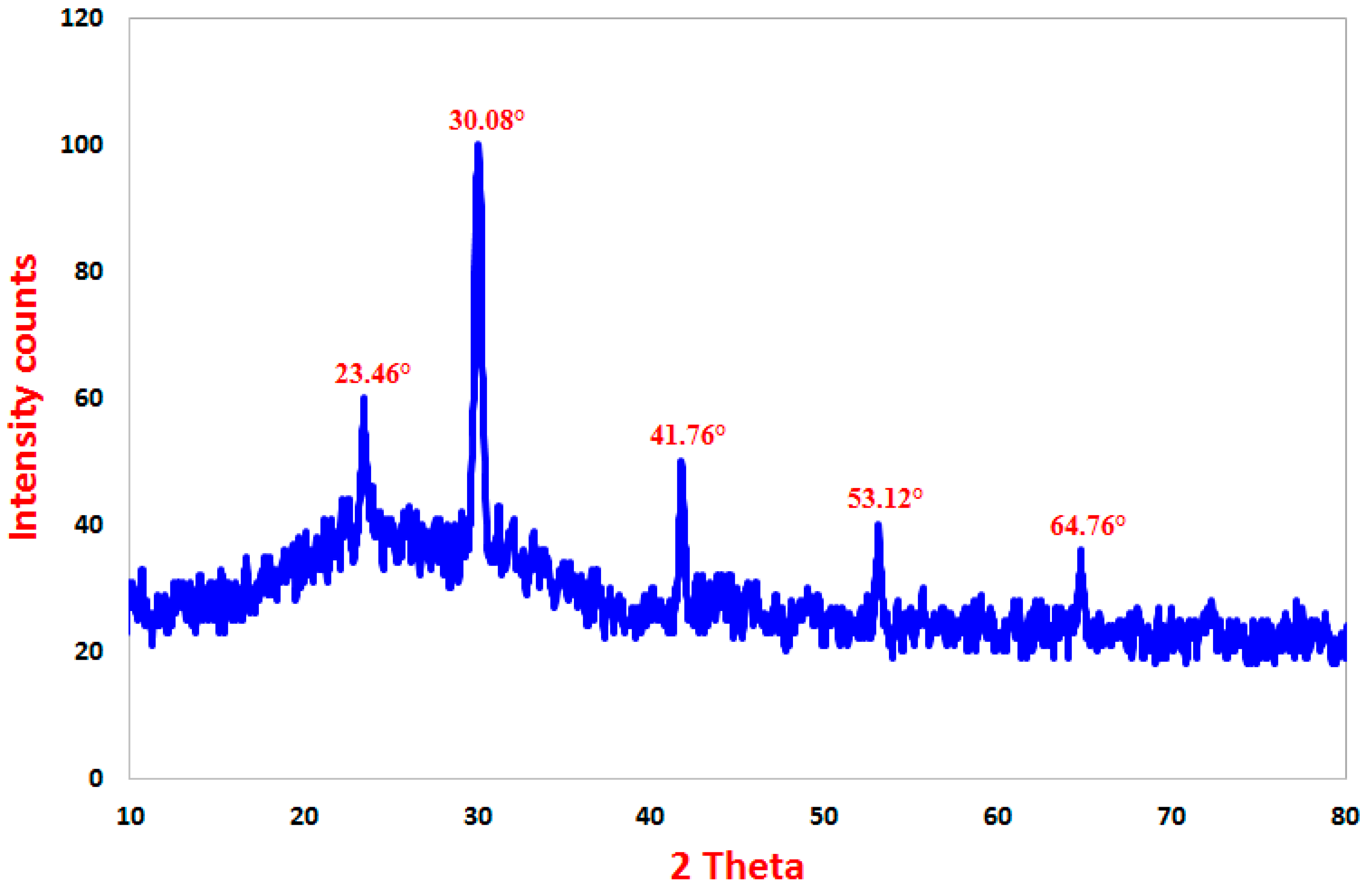
X-ray Diffraction (XRD) Spectroscopy
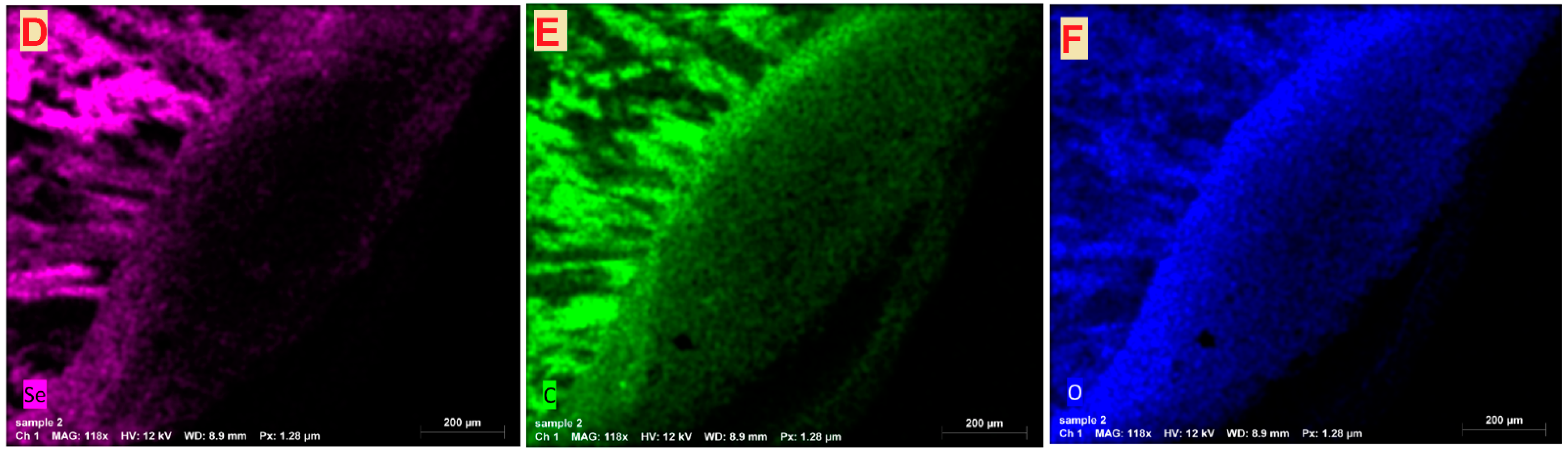
Transmission and Scanning Electron Microscopy
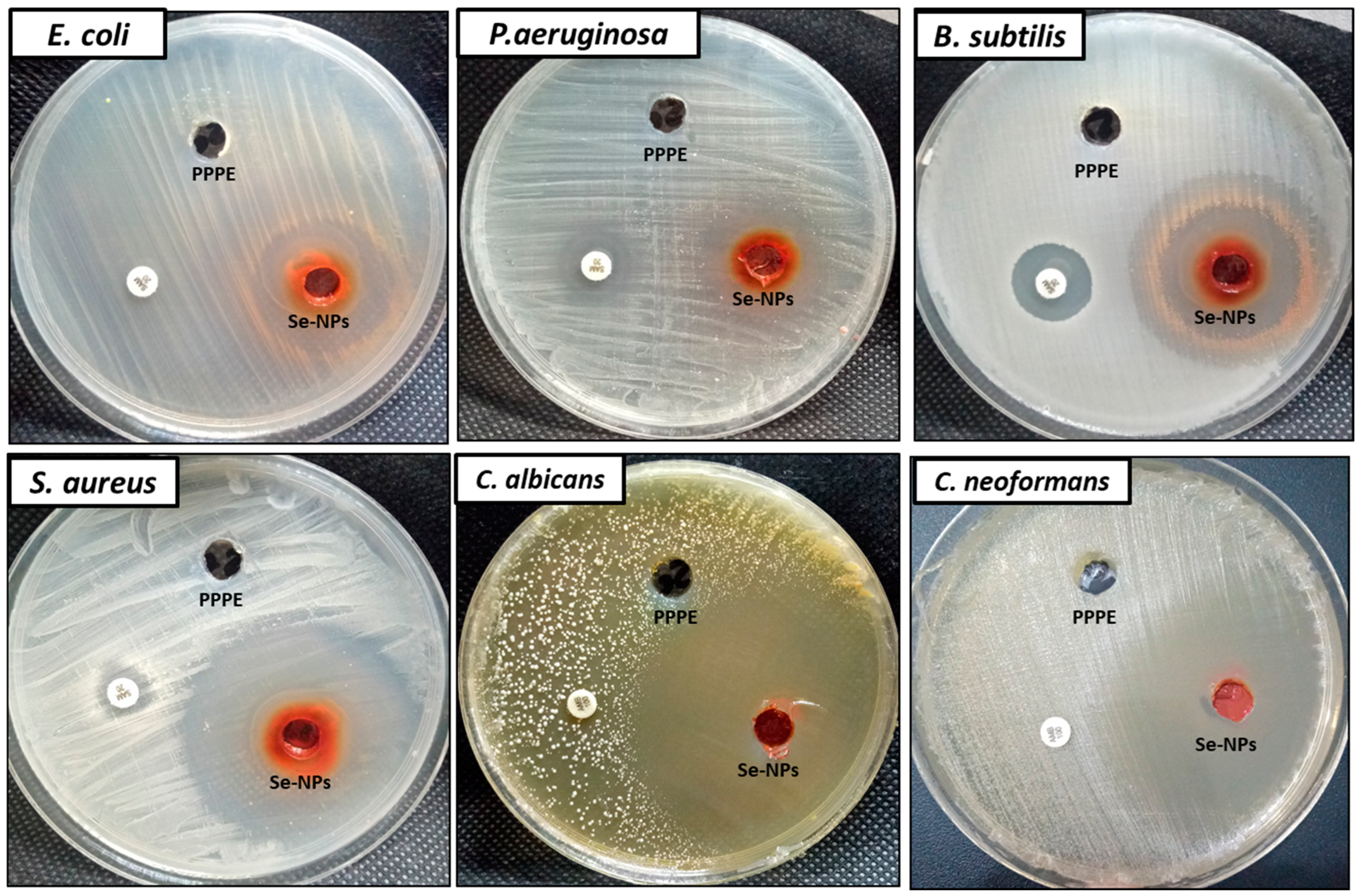
2.2.4. Antimicrobial Activity
2.2.5. Insecticidal Activity
Mosquito Colony
Larvicidal Activity
Fecundity and Hatchability
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesis and Characterization of SeNPs
3.1.1. UV–Vis Spectroscopy
3.1.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.1.3. X-ray Diffraction (XRD) Spectroscopy
3.1.4. Transmission and Scanning Electron Microscopy
3.2. Antimicrobial Activity
3.3. Insecticidal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- GAFFI. How 150 People Die Every Hour from Fungal Infection While the World Turns a Blind Eye; Global Action Fund for Fungal Infections (GAFFI): Geneva, Switzerland, 2013. [Google Scholar]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, E.A.; Schmidt, C.A.; Kwong, K.T.; Pigott, D.M.; Mupfasoni, D.; Biswas, G.; Shirude, S.; Hill, E.; Donkers, K.M.; Abdoli, A. The global distribution of lymphatic filariasis, 2000–18: A geospatial analysis. Lancet Glob. Health 2020, 8, e1186–e1194. [Google Scholar] [CrossRef]
- Abdallah, F.; Rady, M.; Merdan, B.; Shaarawi, F.; Mohammed, A.; Alshammery, K.; Al-Khalaf, A.; Selim, T.; Dahab, A. Effects of blood sources and artificial blood feeding membranes on the biological parameters and hepatitis C virus infectivity of Culex pipiens (Diptera: Culicidae). Afr. Entomol. 2021, 29, 262–273. [Google Scholar] [CrossRef]
- Gabarty, A.; Selim, T.A.; Hassaballah, A.I. Effect of gamma irradiation on protease and nuclease enzymes activity and egg oviposition of Culex pipiens mosquito engorged with Hepatitis C Virus (HCV). J. Radiat. Res. Appl. Sci. 2022, 15, 1–6. [Google Scholar] [CrossRef]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 135, 581. [Google Scholar] [PubMed]
- Hasaballah, A.; Selim, T.; Tanani, M.; Nasr, E. Lethality and vitality efficiency of different extracts of Salix safsaf leaves against the house fly, Musca domestica L.(Diptera: Muscidae). Afr. Entomol. 2021, 29, 479–490. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Elghait, M.; Hasanin, M.; Hashem, A.H.; Salem, S.S. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. Int. J. Biol. Macromol. 2021, 175, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Hashem, A.H. Eco-Friendly Synthesis of Superhydrophobic Antimicrobial Film Based on Cellulose Acetate/Polycaprolactone Loaded with the Green Biosynthesized Copper Nanoparticles for Food Packaging Application. J. Polym. Environ. 2021, 30, 1820–1832. [Google Scholar] [CrossRef]
- Hasanin, M.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hashem, A.H. Ecofriendly Synthesis of Biosynthesized Copper Nanoparticles with Starch-Based Nanocomposite: Antimicrobial, Antioxidant, and Anticancer Activities. Biol. Trace Elem. Res. 2021, 200, 2099–2112. [Google Scholar] [CrossRef]
- Hasanin, M.; Elbahnasawy, M.A.; Shehabeldine, A.M.; Hashem, A.H. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. BioMetals 2021, 34, 1313–1328. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneim, H.E.M.; Wassel, M.A.; Elfeky, A.S.; Bendary, S.H.; Awad, M.A.; Salem, S.S.; Mahmoud, S.A. Multiple Applications of CdS/TiO2 Nanocomposites Synthesized via Microwave-Assisted Sol-Gel. J. Clust. Sci. 2022, 33, 1119–1128. [Google Scholar] [CrossRef]
- Hashem, A.H.; Shehabeldine, A.M.; Ali, O.M.; Salem, S.S. Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities. Polymers 2022, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; El-Wakil, D.A.; Hashem, A.H.; Abdelaziz, A.M. Antagonistic Effect of Plant Growth-Promoting Fungi Against Fusarium Wilt Disease in Tomato: In vitro and In vivo Study. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Ali, O.M.; Hasanin, M.S.; Suleiman, W.B.; Helal, E.E.-H.; Hashem, A.H. Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: Antimicrobial, antioxidant, and anticancer activities. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Res. Appl. Chem. 2022, 13, 2023. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Sharaf, M.H.; Nagiub, A.M.; Salem, S.S.; Kalaba, M.H.; El Fakharany, E.M.; El-Wahab, H.A. A new strategy to integrate silver nanowires with waterborne coating to improve their antimicrobial and antiviral properties. Pigment Resin Technol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A.M. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Hashem, A.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hasanin, M. Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Stärke 2022, 74, e2100165. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Tantawy, H.; Hashem, A.H. Promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Adv. 2021, 11, 25961–25975. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Salem, S.S.; Alharbi, A.A.; Abdelghany, T.M. Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab. J. Chem. 2022, 15, 103927. [Google Scholar] [CrossRef]
- Hammad, E.N.; Salem, S.S.; Zohair, M.M.; Mohamed, A.A.; El-Dougdoug, W. Purpureocillium lilacinum Mediated Biosynthesis Copper Oxide Nanoparticles with Promising Removal of Dyes. Biointerface Res. Appl. Chem. 2022, 12, 1397–1404. [Google Scholar] [CrossRef]
- Salem, S.S.; Husen, A. Chapter 14-Effect of engineered nanomaterials on soil microbiomes and their association with crop growth and production. In Engineered Nanomaterials for Sustainable Agricultural Production, Soil Improvement and Stress Management; Husen, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 311–336. [Google Scholar] [CrossRef]
- Hammad, E.N.; Salem, S.S.; Mohamed, A.A.; El-Dougdoug, W. Environmental Impacts of Ecofriendly Iron Oxide Nanoparticles on Dyes Removal and Antibacterial Activity. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.A.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. BioMetals 2022, 35, 601–616. [Google Scholar] [CrossRef]
- Darroudi, M.; Yazdi, M.E.T.; Amiri, M.S. Plant-mediated biosynthesis of nanoparticles. In 21st Century Nanoscience—A Handbook; CRC Press: Boca Raton, FL, USA, 2020; pp. 1-1–1-18. [Google Scholar]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostructure Chem. 2021, 12, 467–480. [Google Scholar] [CrossRef]
- Elkodous, M.A.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Schomburg, L.; Arnér, E.S.J. Selenium Metabolism in Herbivores and Higher Trophic Levels Including Mammals. In Selenium in Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 123–139. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Divya, M.; Durán-Lara, E.F.; Prasannakumar, M.; Vaseeharan, B. A Review on Biogenic Synthesis of Selenium Nanoparticles and Its Biological Applications. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2355–2370. [Google Scholar] [CrossRef]
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Javed, B.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; Akram, A. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Johnson, J.; Shanmugam, R.; Lakshmi, T. A review on plant-mediated selenium nanoparticles and its applications. J. Popul. Ther. Clin. Pharmacol. 2022, 28, e29–e40. [Google Scholar]
- Valgas, C.; Souza, S.M.D.; Smânia, E.; Smânia, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Hassan, M.I.; Mohamed, A.; Hammad, K.M.; Selim, T.A. Evaluation of the Role of Irradiated, Culex pipiens, Mosquito (Diptera; Culicidae) in the Transmission of Hepatitis C Virus (HCV). Egypt. Acad. J. Biol. Sci. Entomol. 2018, 11, 139–148. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Sheikhlou, K.; Allahyari, S.; Sabouri, S.; Najian, Y.; Jafarizadeh-Malmiri, H. Walnut leaf extract-based green synthesis of selenium nanoparticles via microwave irradiation and their characteristics assessment. Open Agric. 2020, 5, 227–235. [Google Scholar] [CrossRef]
- Salem, S.S. Bio-fabrication of Selenium Nanoparticles Using Baker’s Yeast Extract and Its Antimicrobial Efficacy on Food Borne Pathogens. Appl. Biochem. Biotechnol. 2022, 194, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Mollania, N.; Tayebee, R.; Narenji-Sani, F. An environmentally benign method for the biosynthesis of stable selenium nanoparticles. Res. Chem. Intermed. 2016, 42, 4253–4271. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, Cytotoxicity and Larvicidal Activity of Green Synthesized Selenium Nanoparticles Using Penicillium corylophilum. J. Clust. Sci. 2021, 32, 351–361. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of Selenium Nanoparticles from Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B Biol. 2019, 191, 123–127. [Google Scholar] [CrossRef]
- Sarkar, R.D.; Kalita, M.C. Se nanoparticles stabilized with Allamanda cathartica L. flower extract inhibited phytopathogens and promoted mustard growth under salt stress. Heliyon 2022, 8, e09076. [Google Scholar] [CrossRef] [PubMed]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V.; Easwaran, M.; Liu, W.C.; Balasubramanian, B.; Arumugam, M. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021, 11, 1032. [Google Scholar] [CrossRef]
- Ramamurthy, C.; Sampath, K.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green syn-thesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Shakibaie, M.; Mohazab, N.S.; Mousavi, S.A.A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus species Msh-1 Against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8, e26381. [Google Scholar] [CrossRef] [Green Version]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Omar, A.A.; Mohamed, A.H.; Satti, S.H.; Zohra, E. Phytogenic Selenium Nanoparticles Elicited the Physiological, Biochemical, and Antioxidant Defense System Amelioration of Huanglongbing-Infected ‘Kinnow’ Mandarin Plants. Nanomaterials 2022, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C 2018, 90, 104–112. [Google Scholar] [CrossRef]
- Hashem, A.H.; Salem, S.S. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2022, 17, 2100432. [Google Scholar] [CrossRef]
- Lashin, I.; Hasanin, M.; Hassan, S.A.M.; Hashem, A.H. Green biosynthesis of zinc and selenium oxide nanoparticles using callus extract of Ziziphus spina-christi: Characterization, antimicrobial, and antioxidant activity. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnology 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef]
- Pavela, R. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol. Res. 2008, 102, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.-S.; Wang, L.; Dinesh, D.; Suresh, U.; Roni, M.; Higuchi, A. Eco-friendly drugs from the marine environment: Spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ. Sci. Pollut. Res. 2016, 23, 16671–16685. [Google Scholar] [CrossRef]
- Murugan, K.; Anitha, J.; Suresh, U.; Rajaganesh, R.; Panneerselvam, C.; Aziz, A.T.; Tseng, L.-C.; Kalimuthu, K.; Alsalhi, M.S.; Devanesan, S. Chitosan-fabricated Ag nanoparticles and larvivorous fishes: A novel route to control the coastal malaria vector Anopheles sundaicus? Hydrobiologia 2017, 797, 335–350. [Google Scholar] [CrossRef]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, J.; Dey, P.; Saha, S.; Acharya, K. Mycosynthesis of selenium nanoparticles. Micro Nano Lett. 2011, 6, 599–602. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Raza, M.; Ghosh, P.C.; Sardar, M. Synthesis and characterization of nano selenium using plant biomolecules and their potential applications. BioNanoScience 2019, 9, 96–104. [Google Scholar] [CrossRef]
- Krishnan, M.; Ranganathan, K.; Maadhu, P.; Thangavelu, P.; Kundan, S.; Arjunan, N. Leaf extract of Dillenia indica as a source of selenium nanoparticles with larvicidal and antimicrobial potential toward vector mosquitoes and pathogenic microbes. Coatings 2020, 10, 626. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004, 75, 237–244. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Green nanoparticles for mosquito control. Sci. World J. 2014, 2014, 496362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpana, V.; Alarjani, K.M.; Rajeswari, V.D. Enhancing malaria control using Lagenaria siceraria and its mediated zinc oxide nanoparticles against the vector Anopheles stephensi and its parasite Plasmodium falciparum. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Hasaballah, A.I.; El-Naggar, H.A.; Abdelbary, S.; Bashar, M.A.; Selim, T.A. Eco-friendly Synthesis of Zinc Oxide Nanoparticles by Marine Sponge, Spongia officinalis: Antimicrobial and Insecticidal Activities Against the Mosquito Vectors, Culex pipiens and Anopheles pharoensis. BioNanoScience 2022, 12, 89–104. [Google Scholar] [CrossRef]
- Benelli, G. Plant-mediated synthesis of nanoparticles: A newer and safer tool against mosquito-borne diseases? Asian Pac. J. Trop. Biomed. 2016, 6, 353–354. [Google Scholar] [CrossRef]
- Vinotha, V.; Yazhiniprabha, M.; Raj, D.S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Govindarajan, M.; Vaseeharan, B. Biogenic synthesis of aromatic cardamom-wrapped zinc oxide nanoparticles and their potential antibacterial and mosquito larvicidal activity: An effective eco-friendly approach. J. Environ. Chem. Eng. 2020, 8, 104466. [Google Scholar] [CrossRef]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Nicoletti, M.; Madhiyazhagan, P.; Dinesh, D.; Suresh, U.; Khater, H.F.; Wei, H. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 2015, 121, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Subramaniam, J.; Kumar, P.M.; Suresh, U.; Roni, M. S argassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef]


| Test Material | E. coli | P. aeruginosa | B. subtilis | S. aureus | C. albicans | C. neoformans | |
|---|---|---|---|---|---|---|---|
| SeNPs (µg/mL) | 2000 | 26.5 ± 0.70 a | 24.4 ± 0.85 a | 30.7 ± 0.53 a | 48.7 ± 1.06 a | 59.5 ± 0.70 a | 50.2 ± 1.13 a |
| 1000 | 21.7 ± 1.06 b | 21.6 ± 0.56 b | 27.5 ± 0.35 b | 41.5 ± 0.70 b | 54.7 ± 0.99 b | 46.4 ± 0.84 b | |
| 500 | 15.4 ± 0.56 c | 17.6 ± 0.85 c | 23.6 ± 0.46 c | 34.8 ± 1.20 c | 51.0 ± 1.34 c | 40.85 ± 1.20 c | |
| 250 | 11.7 ± 0.35 d | 11.3 ± 0.49 d | 19.2 ± 0.17 d | 29.6 ± 0.56 d | 45 ± 1.41 d | 36.8 ± 1.13 d | |
| 125 | 8.2 ± 0.35 e | 8.7 ± 0.42 e | 13.5 ± 0.32 e | 24.6 ± 0.84 e | 37.3 ± 0.91 e | 30.4 ± 0.64 e | |
| 62.5 | 0 ± 00 f | 0 ± 00 f | 8.7 ± 0.21 f | 20.7 ± 0.84 f | 34.25 ± 0.35 f | 26.4 ± 0.84 f | |
| 31.25 | 0 ± 00 f | 0 ± 00 f | 0 ± 00 g | 16.5 ± 0.70 g | 27.5 ± 0.70 g | 20.1 ± 1.20 g | |
| 15.62 | 0 ± 00 f | 0 ± 00 f | 0 ± 00 g | 10.6 ± 0.92 h | 24 ± 1.41 h | 14 ± 1.41 h | |
| 7.81 | 0 ± 00 f | 0 ± 00 f | 0 ± 00 g | 0 ± 00 i | 16.5 ± 0.70 i | 10.7 ± 0.35 i | |
| 3.9 | 0 ± 00 f | 0 ± 00 f | 0 ± 00 g | 0 ± 00 i | 12.3 ± 0.91 j | 0 ± 00 j | |
| 1.95 | 0 ± 00 f | 0 ± 00 f | 0 ± 00 g | 0 ± 00 i | 0 ± 00 k | 0 ± 00 j | |
| PPPW * | 0±00 | 0 ± 00 | 0 ± 00 | 0 ± 00 | 0 ± 00 | 0 ± 00 | |
| SAM/AMB ** | 0±00 | 14.5 ± 0.5 | 15.6 ± 0.4 | 10.1 ± 0.9 | 0 ± 00 | 0 ± 00 | |
| Treatments | Concentrations (mg/L) | n | Larval Mortality % ± SD | LC50 (LCL–UCL) (mg/L) | LC90 (LCL–UCL) (mg/L) | Statistic Summary |
|---|---|---|---|---|---|---|
| Crude extract of PPPW | Control | 75 | 0.0 ± 0.0 a | 219.841 (186.330–260.524) | 950.087 (715.547–1407.422) | d. f. = 5, F = 800.96, p < 0.001, χ2 = 2.396 |
| 50 | 75 | 10.67 ± 1.33 b | ||||
| 100 | 75 | 26.67 ± 1.33 c | ||||
| 200 | 75 | 41.33 ± 1.33 d | ||||
| 400 | 75 | 69.33 ± 1.33 e | ||||
| 800 | 75 | 89.33 ±1.33 f | ||||
| SeNPs | Control | 75 | 0.0 ± 0.0 a | 75.411 (66.163–85.797) | 208.289 (173.006–265.562) | d. f. = 5, F = 254.757, p < 0.001, χ2 = 13.331 |
| 25 | 75 | 13.3 ± 1.33 b | ||||
| 50 | 75 | 28.0 ± 4.0 c | ||||
| 100 | 75 | 52.0 ± 4.61d | ||||
| 200 | 75 | 93.3 ± 2.58 e | ||||
| 400 | 75 | 100.0 ± 0.0 e | ||||
| Se ions | Nil | Nil | Nil | Nil | Nil | Nil |
| Treatment | Concentration (mg/L) | Fecundity Mean ± SE | Hatchability Mean ± SE | Hatchability (%) |
|---|---|---|---|---|
| Crude extract of PPPW | Control | 142.0 ± 6.11 a | 138.3 ± 5.24 a | 97.46 |
| 50 | 133.3 ± 4.41 a | 124.0 ± 4.16 b | 93.02 | |
| 100 | 110.0 ± 1.54 b | 93.3 ± 2.40 c | 84.9 | |
| 200 | 93.3 ± 1.67 c | 76.0 ± 1.0 d | 81.52 | |
| 400 | 59.3 ± 2.96 d | 44.67 ± 2.60 e | 75.23 | |
| 800 | 48.3 ± 1.67 e | 29.3 ± 2.40 f | 60.74 | |
| Statistic Summary | p < 0.001, d. f. = 5 | p < 0.001, d. f. = 5 F = 172.918 | ||
| SeNPs | Control | 143.67 ± 1.85 a | 131.67 ± 6.01 a | 91.577 |
| 25 | 126.67 ± 1.67 b | 106.67 ± 1.67 b | 84.205 | |
| 50 | 98.0 ± 2.0 c | 72.33 ± 1.45 c | 73.929 | |
| 100 | 63.3 ± 1.67 d | 26.67 ± 1.76 d | 42.137 | |
| 200 | 30.67 ± 2.96 e | 10.0 ± 0.58 e | 32.893 | |
| 400 | 13.67 ± 1.85 f | 2.67 ± 0.88 f | 18.472 | |
| Statistic summary | p < 0.001, d. f. =5 | p < 0.001, d. f. =5 F = 380.721 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. J. Funct. Biomater. 2022, 13, 112. https://doi.org/10.3390/jfb13030112
Hashem AH, Selim TA, Alruhaili MH, Selim S, Alkhalifah DHM, Al Jaouni SK, Salem SS. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. Journal of Functional Biomaterials. 2022; 13(3):112. https://doi.org/10.3390/jfb13030112
Chicago/Turabian StyleHashem, Amr H., Tharwat A. Selim, Mohammed H. Alruhaili, Samy Selim, Dalal Hussien M. Alkhalifah, Soad K. Al Jaouni, and Salem S. Salem. 2022. "Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste" Journal of Functional Biomaterials 13, no. 3: 112. https://doi.org/10.3390/jfb13030112
APA StyleHashem, A. H., Selim, T. A., Alruhaili, M. H., Selim, S., Alkhalifah, D. H. M., Al Jaouni, S. K., & Salem, S. S. (2022). Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. Journal of Functional Biomaterials, 13(3), 112. https://doi.org/10.3390/jfb13030112









