Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Summary Measures
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quantitative Assessment of Bone Healing
3.4. Risk of Bias Assessment
4. Discussion
4.1. Animal Models
4.2. Bone Defect
4.3. Bone infection Model
4.4. Scaffolds
4.5. Antibacterial Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Excluded | Reason for Exclusion |
| Long Bi, 2007 | Quantitative data not showed |
| Tamazawa et al. 2011 | Quantitative data not showed |
| Li et al. 2015 | In vitro |
| Cheng et al. 2015 | In vitro |
| Amin Yavari et al. 2016 | In vitro |
| Zhang et al. 2017 | In vitro |
| Azeena et al. 2017 | In vitro |
| Souza te al. 2017 | In vitro |
| García-González et al. 2018 | In ovo |
| Cao et al. 2018 | In vitro |
| Ando et al. 2018 | Absence of infection model |
| Cai et al. 2018 | Absence of infection model |
| Tang et al. 2018 | In vitro |
| Wang et al. 2019 | In vitro |
| Martin et al. 2019 | In vitro |
| Kuang et al. 2019 | In vitro |
| Balkaya et al. 2019 | In vitro |
| De Mori et al. 2019 | In vitro |
| Carvalho et al. 2019 | Absence of infection model |
| Bigham et al. 2019 | In vitro |
| Áragon et al. 2019 | In vitro |
| Wang et al. 2020 | In vitro |
| Peng et al. 2020 | Did not evaluate antimicrobial activity in vivo, just in vitro |
| Makvandi et al. 2020 | In vitro |
| Benedini et al. 2020 | In vitro |
| Avani et al. 2020 | In vitro |
| Kobata et al. 2020 | Did not evaluate bone regeneration |
| Ter boo et al. 2018 | Did not evaluate bone regeneration by microtomography |
| Xie et al. 2013 | Did not evaluate bone regeneration by microtomography |
| Pajares-Chamorro et al. 2021 | Did not evaluate antimicrobial activity in vivo, just in vitro |
References
- Rothe, R.; Hauser, S.; Neuber, C.; Laube, M.; Schulze, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Advances and challenges in drug delivery approaches. Pharmaceutics 2020, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Nöth, U.; Berner, A.; Hutmacher, D.W. Bone. In Regenerative Medicine—From Protocol to Patient: 5 Regenerative Therapies II, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 289–304. [Google Scholar]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Velard, F.; Gangloff, S.C. Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis. Front. Cell Infect. Microbiol. 2015, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; van der Wal, B.C.H.; Vogely, H.C. Impact of Bacterial Infections on Osteogenesis: Evidence From In Vivo Studies. J. Orthop. Res. 2019, 37, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Horswill, A.R. Staphylococcus aureus osteomyelitis: Bad to the bone. Cell Host Microbe. 2013, 13, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Gu, F.; Sui, Z.; Su, Z.; Yu, T. The Process of Osteoblastic Infection by Staphylococcus Aureus. Int. J. Med. Sci. 2020, 17, 1327–1332. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Wenke, J.C.; Guelcher, S.A. Dual delivery of an antibiotic and a growth factor addresses both the microbiological and biological challenges of contaminated bone fractures. Expert Opin. Drug Deliv. 2011, 8, 1555–1569. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Cano-Vicent, A.; i Serra, R.S.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio 2022, 16, 100412. [Google Scholar] [CrossRef]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: Applications to ex vivo and in vivo studies. Bonekey Rep. 2014, 3, 564. [Google Scholar] [CrossRef]
- Christiansen, B.A. Effect of micro-computed tomography voxel size and segmentation method on trabecular bone microstructure measures in mice. Bone Rep. 2016, 5, 136–140. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- De Vries, R.B.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid. Based Preclin. Med. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- McLaren, J.S.; White, L.; Cox, H.C.; Ashraf, W.; Rahman, C.V.; Blunn, G.W.; Goodship, A.E.; Quirk, R.A.; Shakesheff, K.; Bayston, R.; et al. A biodegradable antibiotic-impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. Eur. Cells Mater. 2014, 27, 332–349. [Google Scholar] [CrossRef]
- Wei, P.-F.; Yuan, Z.-Y.; Jing, W.; Guan, B.-B.; Liu, Z.-H.; Zhang, X.; Mao, J.-P.; Chen, D.-F.; Cai, Q.; Yang, X.-P. Regenerating infected bone defects with osteocompatible microspheres possessing antibacterial activity. Biomater. Sci. 2018, 7, 272–286. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Zhou, W.; Fan, D.; Lin, X.; Jing, Z.; Cai, H.; Cheng, Y.; Liu, X.; et al. Sustainable release of vancomycin from micro-arc oxidised 3D-printed porous Ti6Al4V for treating methicillin-resistant Staphylococcus aureus bone infection and enhancing osteogenesis in a rabbit tibia osteomyelitis model. Biomater. Sci. 2020, 8, 3106–3115. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zhou, J.; Zhou, Z.; Wu, X.-L.; Wang, L.; Yao, Q. 3D printed dual-functional biomaterial with self-assembly micro-nano surface and enriched nano argentum for antibacterial and bone regeneration. Appl. Mater. Today 2019, 17, 206–215. [Google Scholar] [CrossRef]
- Egawa, S.; Hirai, K.; Matsumoto, R.; Yoshii, T.; Yuasa, M.; Okawa, A.; Sugo, K.; Sotome, S. Efficacy of Antibiotic-Loaded Hydroxyapatite/Collagen Composites Is Dependent on Adsorbability for Treating Staphylococcus aureus Osteomyelitis in Rats. J. Orthop. Res. 2020, 38, 843–851. [Google Scholar] [CrossRef]
- Boyle, K.K.; Sosa, B.; Osagie, L.; Turajane, K.; Bostrom, M.P.G.; Yang, X. Vancomycin-laden calcium phosphate-calcium sulfate composite allows bone formation in a rat infection model. PLoS ONE 2019, 14, e0222034. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Wu, X.-D.; He, X.; Lin, X.; Wang, H.; Li, J.; Jiang, J.; Huang, W. Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. J. Mater. Sci. Mater. Med. 2019, 30, 59. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Tian, X.; Lu, Z.; Ma, C.; Wu, M.; Zhang, C.; Yuan, Y.; Yuan, X.; Xie, D.; Liu, C.; Guo, J. Antimicrobial hydroxyapatite and its composites for the repair of infected femoral condyle. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111807. [Google Scholar] [CrossRef]
- Hasan, R.; Schaner, K.; Mulinti, P.; Brooks, A. A Bioglass-Based Antibiotic (Vancomycin) Releasing Bone Void Filling Putty to Treat Osteomyelitis and Aid Bone Healing. Int. J. Mol. Sci. 2021, 22, 7736. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, D.; Xu, M.; Yao, Q.; Zhu, H.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication 2017, 9, 025037. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Z.; Tian, B.; Zhang, X.; Wang, N. Study on antibacterial properties and cytocompatibility of EPL coated 3D printed PCL/HA composite scaffolds. RSC Adv. 2020, 10, 4805–4816. [Google Scholar] [CrossRef]
- Weng, W.; Li, X.; Nie, W.; Liu, H.; Liu, S.; Huang, J.; Wang, D. One-Step Preparation of an AgNP-nHA@RGO Three-Dimensional Porous Scaffold and Its Application in Infected Bone Defect Treatment. Int. J. Nanomed. 2020, 15, 5027–5042. [Google Scholar] [CrossRef]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur. Cell Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- Calabro, L.; Richards, R.G.; Moriarty, T.F. Preclinical Models of Infection in Bone and Joint Surgery. In Bone and Joint Infections: From Microbiology to Diagnostics and Treatment; John Wiley & Sons: Oxford, UK, 2015. [Google Scholar]
- Cvetkovic, V.; Najman, S.; Rajkovic, J.; Zabar, A.; Vasiljevic, P.; Djordjevic, L.; Trajanovic, M.D. A comparison of the microarchitecture of lower limb long bones between some animal models and humans: A review. Vet. Med. 2013, 58, 339–351. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect. Tissue Res. 2015, 56, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Feitosa, C.C.; Kawamoto, F.Y.K.; Marinho, P.V.T.; Dal-Bó, Í.d.S.; Monteiro, B.F.; Ferrigno, C.R.A. Animal modeling in bone research-Should we follow the White Rabbit? Anim. Model Exp. Med. 2019, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. S5), S20–S22. [Google Scholar] [CrossRef]
- Blokhuis, T.J. Management of traumatic bone defects: Metaphyseal versus diaphyseal defects. Injury 2017, 48 (Suppl. S1), S91–S93. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Ignatius, A. Animal models for studying metaphyseal bone fracture healing. Eur. Cell Mater. 2020, 40, 172–188. [Google Scholar] [CrossRef]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin. Microbiol. Rev. 2018, 31, e00084-17. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- Schepers, E.J.; Ducheyne, P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1-24 month experiment with several materials and particle sizes and size ranges. J. Oral Rehabil. 1997, 24, 171–181. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef]
- Alvarez, R.; Cortes, L.E.L.; Molina, J.; Cisneros, J.M.; Pachon, J. Optimizing the Clinical Use of Vancomycin. Antimicrob. Agents Chemother. 2016, 60, 2601–2609. [Google Scholar] [CrossRef]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Wang, C.; Canden, A.; Burr, M.; Agarwal, J. Local Intramedullary Delivery of Vancomycin Can Prevent the Development of Long Bone Staphylococcus aureus Infection. PLoS ONE 2016, 11, e0160187. [Google Scholar] [CrossRef]
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96. [Google Scholar] [CrossRef]
- Bee, S.L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Hamid, Z.A.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Fiore, M.; Bruschi, A.; Giannini, C.; Morante, L.; Rondinella, C.; Filippini, M.; Sambri, A.; De Paolis, M. Is Silver the New Gold? A Systematic Review of the Preclinical Evidence of Its Use in Bone Substitutes as Antiseptic. Antibiotics 2022, 11, 995. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic Acid Induced Self-Assembly of Three-Dimensional Graphene with Good Adsorption and Antibacterial Properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]

| Author and Year | Specie | Total Number of Animals | Bone Defect Type and Location | Biomaterial/Scaffold | Antibacterial Agent | Bacteria | Strain | Inoculum Size | Time to Insert the Scaffold |
|---|---|---|---|---|---|---|---|---|---|
| McLaren et al., 2014 [17] | Sheep | 30 | Ø: 8 mm and height: 4 mm Medial femoral condyle | PLGA/PEG | Gentamicin sulfate and Clindamycin hydrochloride | S. aureus | F2789 | 20 μL of a 2 × 106 cfu/mL (40,000 CFU) | At the same time as inoculation |
| Wei PF et al., 2019 [18] | Rats | 45 | Ø: 8 mm in the cranium | PLLA/PEG | Silver nanoparticles (AgNPs) | S. aureus | Not reported | 1 × 107 CFU in 100 μL of sterile normal saline | 1 week |
| Li et al., 2019 [20] | Rabbits | 16 | Ø: 4 mm and height: 4 mm External tibial epicondyle of left limb. | PCL | Silver nanoparticles (AgNPs) | S. aureus | ATCC 25923 | 0.1 mL of 1 × 105 CFU/mL | At the same time as inoculation |
| Boyle et al., 2019 [22] | Rats | 64 | Ø: 3 mm and height: 3 mm Right proximal tibia | Cap/Cas | Vancomycin (10%) | S. aureus | ATCC 29213 | 10 μL of 1.5 × 106 CFU/ml | At the same time of inoculation and 3 weeks after inoculation |
| Zhang et al., 2019 [23] | Rabbits | 54 | A cortical bone window of 10 × 6 mm the proximal tibia. | Ag/n-HA/PU | Silver phosphate | S. aureus | ATCC 25923 | 0.1 mL of 3 × 107 CFU/mL | 4 weeks |
| Zhang et al., 2020 [19] | Rabbits | 18 | Ø: 5 mm tibial plateau | TS-M/P/V | Vancomycin | MRSA | Not reported | 1 × 108 CFU | At the same time as inoculation |
| Egawa et al., 2020 [21] | Rats | 54 | Ø: 1 mm hole made in the first surgery was dilated to Ø: 3 mm Lateral epicondyle of the bilateral femur. | HAp/Col | Cefotiam and Vancomycin | MSSA | Not reported | 1 × 107 CFU | 1 week |
| Jin Tao et al., 2020 [24] | Rabbits | Ø: 2 mm Tibia | VCM-NPs/Gel | Vancomycin | S. aureus | ATCC 96 | 1 × 108 CFU/mL | 4 weeks | |
| Tian et al., 2021 [25] | Rats | 60 | Ø: 3.5 mm × 5 mm the lateral condyle of the femur | PU/Ag-THA HA/PU | silver nitrate (AgNO3) and Tannin (T) | S. aureus | Not reported | 1 × 106 CFUs/mL | 10 days |
| Hasan et al., 2021 [26] | Rats | 6 | A 4.2 mm hole the tibial metaphysis. | ABVF-BG | Vancomycin | S. aureus | ATC49230 | 1 × 108 CFUs | At the same time as inoculation |
| BV/TV | ||
|---|---|---|
| Study | Time point | Outcome |
| Wei PF et al., 2018 [18] | 24 weeks | Antimicrobial strategies: 47.5 ± 1.39% mg cm−3 and Control group: 52 ± 0.99% mg cm−3 (p < 0.01) |
| Li et al., 2019 [20] | 8 weeks | Antimicrobial strategies showed significantly higher values of BV/TV in PCL/AgNPs and PCL/PDA/AgNPs groups, compared with other groups, with the highest levels on the PCL/PDA/AgNPs (p < 0.05) |
| Boyle et al., 2019 [22] | 6 weeks | Inoculation + CaS/CaP (at the same time) group: 20.55% Inoculation + after 3 weeks: CERAMENT + Vancomycin group: 31.27% (p < 0.05) |
| Zhang et al., 2019 [23] | 4 days | Almost no new bone formation was seen in any group |
| 3 weeks | The new bone formation was observed in each group, the n-HA/PU3 group (0.113 ± 0.047) showed the most obvious change, but there were no significant differences | |
| 6 weeks | Levels were increased in each group, and there was a significant difference between the n-HA/PU3 group (0.488 ± 0.100) and n-HA/PU group (0.131 ± 0.064; p = 0.01) | |
| 12 weeks | Levels were increased in the n-HA/PU3 group (0.607 ± 0.043), as well as in the n-HA/PU10 group (0.636 ± 0.088), and both the n-HA/PU3 group and n-HA/PU10 group showed significantly higher bone formation than the n-HA/PU group (0.057 ± 0.057). There were no significant differences in the rate of bone formation between the n-HA/PU3 group and the n-HA/PU10 group | |
| Jin Tao et al., 2020 [24] | 8 weeks | The BV/TV decreased further and was significantly lower in the control group than in the VCM/Gel, VCM-NPs/Gel, and VCS groups In addition, the BV/TV was markedly higher in the VCMNPs/Gel group than in the VCM/Gel group |
| BMD | ||
|---|---|---|
| Study | Time Points | Outcome |
| Wei PF et al., 2018 [18] | 24 weeks | Antimicrobial strategies: 359.05 ± 30.99 mg cm−3 and Control 65.41 ± 11.21 mg cm−3 showing significant differences (p < 0.01) |
| Jin Tao et al., 2020 [24] | 8 weeks | The BMD decreased further and was significantly lower in the control group than in the VCM/Gel, VCM-NPs/Gel, and VCS groups. In addition, the BMD was markedly higher in the VCMNPs/Gel group than in the VCM/Gel group |
| Tian et al., 2021 [25] | 4 weeks 8 weeks 12 weeks | The PU/Ag-THA group, at all three-time points, presented significantly higher results than that of PU/HA and PU/THA groups |
| TV | ||
|---|---|---|
| Study | Time Points | Outcome |
| Egawa et al., 2020 [21] | 1 week | The VCM group (0.45 ± 0.04 mm) showed a significant decrease in cortical destruction compared with the NS group (0.37 ± 0.04 mm) and the same trend was seen in the CEZ group (0.44 ± 0.06 mm), though this difference was not statistically significant |
| 2 weeks | Both the VCM (0.51 ± 0.07 mm) and CEZ (0.50 ± 0.04 mm) groups showed a marked decrease in cortical bone destruction. NS group showed 0.18 ± 0.07 mm of cortical destruction | |
| 4 weeks | A significant decrease in the treatment group with vancomycin was attained, compared with the control group. The same trend was noted in the cefotiam group, but the difference was not significant | |
| Bone Area | ||
|---|---|---|
| Study | Time Points | Outcome |
| Boyle et al., 2019 [22] | 6 weeks | An identical value of the bone area was attained for the antibacterial biomaterial and control group (1.46 mm2) |
| Tb.N | ||
|---|---|---|
| Study | Time Points | Outcome |
| Li et al., 2019 [20] | 8 weeks | The ratio of Tb.N was significantly higher in PCL/AgNPs and PCL/PDA/AgNPs groups, as compared with other groups, with the PCL/PDA/AgNPs group achieving the highest score |
| Zhang et al., 2019 [23] | 4 days | There were a few new bone trabeculas |
| 3 weeks | The n-HA/PU10 group (0.264 ± 0.139/mm) showed the highest number of new bone trabeculas, but without significant differences | |
| 6 weeks | The number of new bone trabeculas increased in each group, and a significant difference was detected between the n-HA/PU3 group (1.486 ± 0.129/mm) and n-HA/PU group (0.621 ± 0.256/mm; p = 0.013) | |
| 12 weeks | The number of new bone trabeculas continued to increase in all groups, but there was no difference between the n-HA/PU3 group (1.595 ± 0.319/mm) and n-HA/PU10 group (1.711 ± 0.379/mm) | |
| Tb.Th | ||
|---|---|---|
| Study | Time Points | Outcome |
| McLaren et al., 2014 [17] | 13 weeks | Antimicrobial strategies group showed a bone defect fill of 53.8 ± 17.2%, but no significant differences were attained, compared with the control |
| Zhang et al., 2019 [23] | 4 days | There were no significant differences in the thickness of the new bone trabecular thickness among all groups |
| 3 weeks | n-HA/PU3 group = 0.142 ± 0.031 μm and Control group: 0.029 ± 0.001 μm with statistical significance (p = 0.027) | |
| 6 weeks | n-HA/PU3 group = 0.327 ± 0.040 μm and Control group: 0.067 ± 0.026 μm with statistical significance (p = 0.035) | |
| 12 weeks | n-HA/PU3 group = 0.140 ± 0.095 μm and Control group: 0.157 ± 0.065 μm with statistical significance (p = 0.002) | |
| Hasan et al., 2021 [26] | 8 weeks | A healing bone without signs of infection was seen in the antimicrobial strategies group. Precisely, the drilled hole at the bone site was being filled by immature cancellous and cortical bone. However, the numerical data were not reported, neither the statistical significance of the result |
| Tb.Sp | ||
|---|---|---|
| Study | Time Points | Outcome |
| Zhang et al., 2019 [23] | 3 weeks | There were no significant differences between any of the groups. |
| 6 weeks | Ag 3%: 0.351 ± 0.099 μm and control group 1.245 ± 0.276 μm with statistical significance (p = 0.013). | |
| 12 weeks | Ag 3%: 0.250 ± 0.022 μm and control group 0.507 ± 0.122 μm with statistical significance (p = 0.042). Ag 10%: 0.218 ± 0.055 μm and control group 0.507 ± 0.122 μm with statistical significance (p = 0.038). | |
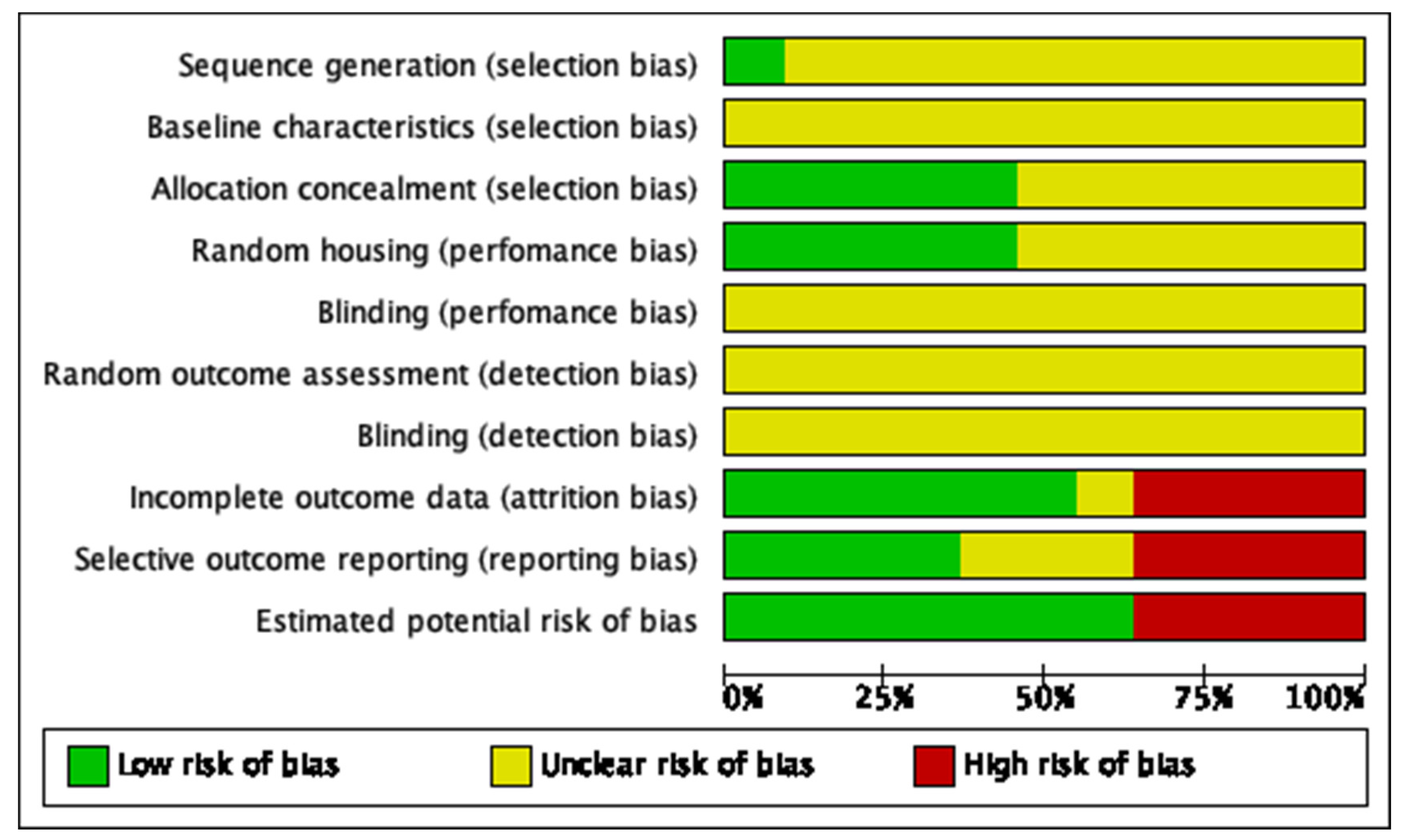
| Criteria [16] | McLaren et al., 2014 [17] | Wei et al., 2018 [18] | Li et al., 2019 [20] | Boyle et al., 2019 [22] | Zhang et al., 2019 [23] | Zhang et al., 2020 [19] | Egawa et al., 2020 [21] | Jin Tao et al., 2020 [24] | Tian et al., 2021 [25] | Hasan et al., 2021 [26] |
|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation (selection bias) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Baseline characteristics (selection bias) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Allocation concealment (selection bias) | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Unclear | Unclear | Unclear | Unclear |
| Random housing (performance bias) | Low risk | Unclear | Unclear | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | Unclear |
| Blinding (performance bias) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Random outcome assessment (detection bias) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Blinding (detection bias) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | High risk | Low risk | Low risk | High risk | Low risk | High risk | High risk | High risk | Unclear |
| Selective outcome reporting (reporting bias) | Low risk | High risk | High risk | Unclear | Low risk | High risk | Low risk | High risk | Low risk | Unclear |
| Estimated potential risk of bias | Low risk | High risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | High risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, L.C.; Fernandes, M.H.R.; Gomes, P.S. Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models. J. Funct. Biomater. 2022, 13, 193. https://doi.org/10.3390/jfb13040193
Mariano LC, Fernandes MHR, Gomes PS. Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models. Journal of Functional Biomaterials. 2022; 13(4):193. https://doi.org/10.3390/jfb13040193
Chicago/Turabian StyleMariano, Lorena Castro, Maria Helena Raposo Fernandes, and Pedro Sousa Gomes. 2022. "Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models" Journal of Functional Biomaterials 13, no. 4: 193. https://doi.org/10.3390/jfb13040193
APA StyleMariano, L. C., Fernandes, M. H. R., & Gomes, P. S. (2022). Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models. Journal of Functional Biomaterials, 13(4), 193. https://doi.org/10.3390/jfb13040193








