The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Characteristics of Surfactants
2.3. The Extraction Procedure
2.4. Determination of Polyphenol Content in the Extracts
2.5. In Vitro Antioxidant Assay
2.6. Particle Size Measurement and Zeta Potential Determination
2.7. Computational Details
3. Results and Discussion
3.1. Extraction
3.2. Particle Size and ξ-Potential Determinations
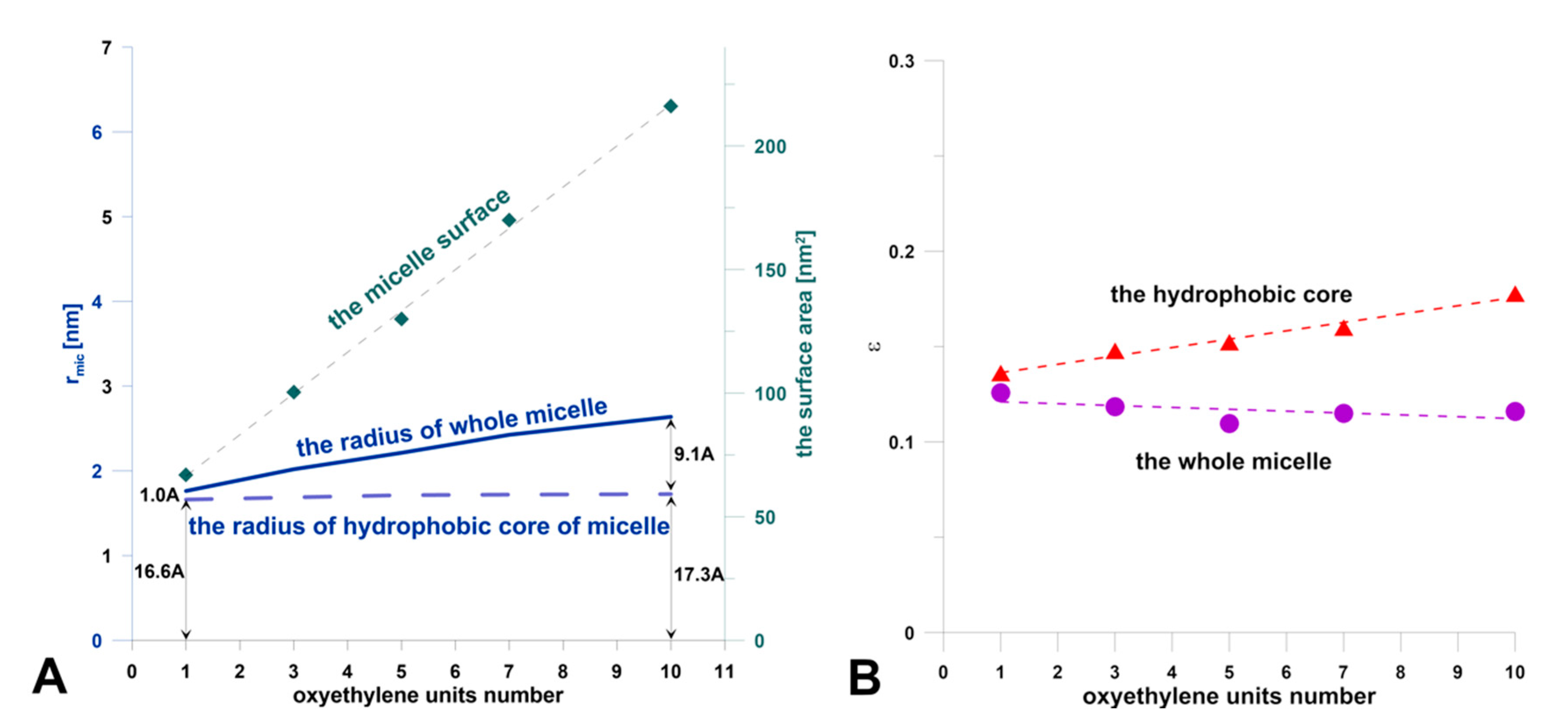
3.3. Molecular Dynamic Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oh, S.G.; Shah, D.O. The effect of micellar lifetime on the rate of solubilization and detergenxy in SDS.pdf. J. Am. Oil Chem. 1993, 70, 673–678. [Google Scholar] [CrossRef]
- Melnyk, A.; Namieśnik, J.; Wolska, L. Theory and recent applications of coacervate-based extraction techniques. TrAC Trends Anal. Chem. 2015, 71, 282–292. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Sicilia, D.; Rubio, S.; Pérez-Bendito, D. Evaluation of the factors affecting extraction of organic compounds based on the acid-induced phase cloud point approach. Anal. Chim. Acta 2002, 460, 13–22. [Google Scholar] [CrossRef]
- Śliwa, K.; Śliwa, P.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Application of Polyethylene/polypropylene Glycol Ethers of Fatty Alcohols for micelle-mediated extraction of calendula anthodium. J. Surfactants Deterg. 2019, 22, 655–661. [Google Scholar] [CrossRef]
- Śliwa, P.; Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Incorporation of bioflavonoids from Bidens tripartite into micelles of non-ionic surfactants—Experimental and theoretical studies. Colloids Surf. B Biointerfaces 2019, 184, 110553. [Google Scholar] [CrossRef]
- Morisue, T.; Moroi, Y.; Shibata, O. Solubilization of Benzene, Naphthalene, Anthracene, and Pyrene in Dodecylammonium Trifluoroacetate micelles. J. Phys. Chem. 1994, 98, 12995–13000. [Google Scholar] [CrossRef]
- Liu, J.C.; Chang, P.S. Solubility and adsorption behaviors of chlorophenols in the presence of surfactant. Water Sci. Technol. 1997, 35, 123–130. [Google Scholar] [CrossRef]
- Zarei, A.R. Spectrophotometric determination of trace amounts of furfural in water samples after mixed micelle-mediated extraction. Acta Chim. Slov. 2009, 56, 322–328. [Google Scholar] [CrossRef]
- Vinarov, Z.; Dobreva, P.; Tcholakova, S. Effect of surfactant molecular structure on Progesterone solubilization. J. Drug Deliv. Sci. Technol. 2018, 43, 44–49. [Google Scholar] [CrossRef]
- Taechangam, P.; Scamehorn, J.F.; Osuwan, S.; Rirksomboon, T. Effect of nonionic surfactant molecular structure on cloud point extraction of phenol from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 200–209. [Google Scholar] [CrossRef]
- Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Kolniak, J. A micelle mediated extraction as a new method of obtaining the infusion of Bidens tripartita. Acta Biochim. Pol. 2016, 63, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Mucsi, Z.; Szabó, A.; Hermecz, I.; Kucsman, Á.; Csizmadia, I.G. Modeling rate-controlling solvent effects. The pericyclic Meisenheimer rearrangement of N-propargylmorpholine N-oxide. J. Am. Chem. Soc. 2005, 127, 7615–7631. [Google Scholar] [CrossRef] [PubMed]
- Miastkowska, M.; Śliwa, P. Influence of terpene type on the release from an O/W nanoemulsion: Experimental and theoretical studies. Molecules 2020, 25, 2747. [Google Scholar] [CrossRef]
- ChemAxon. Instant J. Chem 15.2.16.0 2015. Available online: http://www.chemaxon.com (accessed on 15 January 2020).
- Śliwa, K.; Tomaszkiewicz-Potępa, A.; Sikora, E.; Ogonowski, J. Micelle-mediated extraction of elderberry blossom by whey protein and naturally derived surfactants. Acta Biochim. Pol. 2013, 60, 803–806. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Pietro, W.J.; Francl, M.M.; Hehre, W.J.; Defrees, D.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods. 24. Supplemented small split-valence basis-sets for 2nd-row elements. J. Am. Chem. Soc. 1982, 104, 5039–5048. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-Consistent Molecular Orbital Methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Dobbs, K.D.; Hehre, W.J. Molecular-orbital theory of the properties of inorganic and organometallic compounds. 5. Extended basis-sets for 1st-row transition-metals. J. Comp. Chem. 1987, 8, 861–879. [Google Scholar] [CrossRef]
- Dobbs, K.D.; Hehre, W.J. Molecular-orbital theory of the properties of inorganic and organometallic compounds. 6. Extended basis-sets for 2nd-row transition-metals. J. Comp. Chem. 1987, 8, 880–893. [Google Scholar] [CrossRef]
- Dobbs, K.D.; Hehre, W.J. Molecular-orbital theory of the properties of inorganic and organometallic compounds. 4. Extended basis-sets for 3rd row and 4th row, main-group elements. J. Comp. Chem. 1986, 7, 359–378. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. Packmol: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Martínez, L. Packing optimization for automated generation of complex system’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Markidis, S.; Laure, E. Solving software challenges for exascale: International Conference on Exascale Applications and Software, EASC 2014 Stockholm, Sweden, April 2–3, 2014 revised selected papers. Lect. Notes Comput. Sci. Incl. Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinform. 2015, 8759, 3–27. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef]
- Lebecque, S.; Crowet, J.M.; Nasir, M.N.; Deleu, M.; Lins, L. Molecular dynamics study of micelles properties according to their size. J. Mol. Graph. Model. 2017, 72, 6–15. [Google Scholar] [CrossRef]

| Surfactant INCI | Acronym | Type of Alcohol | OE | Cloud Point [°C] | Surface Tension [mN/m] | HLB 2 | CMC [mg/dm3] |
|---|---|---|---|---|---|---|---|
| Oleth-5 | O5 | C18:1 | 5 | 16–22 1 | 30 | 9.7 | 9.3 |
| Oleth-10 | O10 | C18:1 | 10 | 47–55 1 | 31 | 12.9 | 19.0 1 |
| Oleth-20 | O20 | C18:1 | 20 | >100 1 | 32 | 15.6 | 25.0 1 |
| Steareth-20 | S20 | C18 | 20 | 73–77 1 | 45 | 15.6 | 25.0 1 |
| Ceteareth-20 | CS20 | C16/C18 | 20 | >100 1 | 37 | 16.0 | 55.6 |
| Ceteareth-12 | CS12 | C16/C18 | 12 | 89 | 35 | 13.4 | 18.3 |
| C12-C15 Pareth-12 | T12 | C12/C15 | 12 | 96 | 31 | 14.4 | 22.3 |
| C12-C13 Pareth-12 | LT12 | C12/C13 | 12 | 86 | 29 | 15.0 | 35.0 |
| Extraction Medium | Number of CH2 and OE Units | Total Polyphenols Content per Rutin [mg/dm3] | Total Flavonoids Content per Luteolin [mg/dm3] | Chlorogenic Acid [mg/dm3] | Luteolin 7-O-Glucoside [mg/dm3] |
|---|---|---|---|---|---|
| O5 | 18:1; 5 | 424.9 ± 1.1 | 83.2 ± 2.1 | 43.9 | 23.1 |
| O10 | 18:1; 10 | 469.3 ± 10.0 | 72.2 ± 0.3 | 25.3 | 11.3 |
| O20 | 18:1; 20 | 339.2 ± 0.8 | 53.1 ± 0.7 | 25.4 | 10.2 |
| S20 | 18; 20 | 334.1 ± 0.8 | 62.2 ± 8.5 | 39.8 | 18.2 |
| CS20 | 16/18; 20 | 334.1 ± 0.5 | 56.3 ± 1.6 | 40.3 | 20.2 |
| CS12 | 16/18; 12 | 253.5 ± 2.5 | 75.5 ± 1.2 | 66.4 | 28.5 |
| T12 | 12/15; 12 | 301.5 ± 15.5 | 68.4 ± 0.3 | 59.6 | 20.3 |
| LT12 | 12/13; 12 | 371.6 ± 8.9 | 62.9 ± 0.5 | 55.0 | 13.8 |
| Water | - | 34.0 1 | 25.6 1 | 9.0 | 0.44 |
| Ethanol | - | 40 | 16 | 0.2 | 3.5 |
| Surfactant | Hydrodynamic Diameter 1 [nm] | PDI [%] | Size Distribution by Intensity [nm] 2 | ξ [mV] | Conductivity [mS/cm] | ||
|---|---|---|---|---|---|---|---|
| 1st Peak/%Intensity | 2nd Peak/%Intensity | 3rd Peak/%Intensity | |||||
| O5 | 110.3 ± 5.0 | 25.9 ± 1.6 | 141.6/100 | - | - | −9.4 ± 0.4 | 0.049 |
| O10 | 10.9 ± 0.4 | 20.2 ± 1.9 | 9.5/84 | 429.0/16 | - | −5.3 ± 1.8 | 0.061 |
| O20 | 11.9 ± 0.3 | 23.3 ± 1.4 | 9.1/70 | 4517/24 | 270/6 | −19.2 ± 1.5 | 0.066 |
| S20 | 12.4 ± 0.7 | 22.9 ± 1.9 | 8.6/55 | 6471/35 | 623/10 | −14.9 ± 5.2 | 0.031 |
| CS20 | 9.5 ± 0.3 | 8.5 ± 5.1 | 9.7/100 | - | - | −19.2 ± 5.4 | 0.030 |
| CS12 | 852.9 ± 33.2 | 22.8 ± 2.9 | 890.4/97 | 41.6/3 | - | −5.2 ± 0.3 | 0.068 |
| T12 | 32.4 ± 38.7 | 23.2 ± 1.7 | 7.4/64 | 208.0/16 | 3206/20 | −13.8 ± 1.2 | 0.051 |
| LT12 | 218.7 ± 124 | 37.8 ± 14.0 | 7.0/40 | 440.2/50 | 3859/10 | −19.0 ± 4.3 | 0.037 |
| extracts | |||||||
| O5 | 79.2 ± 2.3 | 24.9 ± 1.1 | 97.0/100 | - | - | −1.6 ± 0.1 | 1.961 |
| O10 | 358.2 ± 44.1 | 24.3 ± 1.2 | 447.7/60 | 16.4/40 | - | −2.5 ± 0.2 | 2.468 |
| O20 | 414.4 ± 70.7 | 32.1 ± 2.5 | 329.5/61 | 5713/22 | 9.2/17 | −3.3 ± 0.1 | 2.213 |
| S20 | 4658 ± 3659 | 35.3 ± 9.5 | 370.7/50 | 6966/35 | 9.7/15 | −3.3 ± 0.0 | 1.993 |
| CS20 | 18.4 ± 0.2 | 21.0 ± 1.9 | 340.7/54 | 9.8/46 | - | −2.8 ± 0.5 | 1.556 |
| CS12 | 4838 ± 1205 | 28.5 ± 2.2 | 3067/94 | 1612/4 | 55.3/2 | −2.8 ± 0.1 | 2.108 |
| T12 | 1188 ± 683.1 | 27.3 ± 9.1 | 525.7/60 | 4910/34 | 8.4/6 | −5.7 ± 0.4 | 2.034 |
| LT12 | 1102 ± 87.8 | 58.5 ± 2.3 | 2508/63 | 391.0/30 | 11.2/7 | −4.4 ± 0.1 | 2.159 |
| Surfactant | Hydrodynamic Diameter [nm]/Temperature [°C] | ||||
|---|---|---|---|---|---|
| 10 | 25 | 30 | 40 | 50 | |
| O5 | 107.3 | 112.3 | 111.0 | 110.7 | 111.3 |
| O10 | 9.4 | 12.3 | 16.9 | 28.3 | 47.9 |
| O20 | 12.6 | 102.3 | 359.5 | 953.4 | 13.4 |
| S20 | 12.2 | 237.1 | 179.0 | 399.4 | 590.7 |
| CS20 | 9.9 | 11.1 | 10.7 | 10.7 | 10.5 |
| CS12 | 927.2 | 1458 | 1337 | 858.5 | 12.2 |
| T12 | 155.2 | 55.7 | 118.6 | 129.5 | 104.9 |
| LT12 | 9.1 | 10.9 | 10.3 | 142.0 | 148.1 |
| Surfactant Model | Denotation | HLBChemAxon | HLBGriffin |
|---|---|---|---|
| C10H21OC2H4OH | C10E1 | 5.97 | 6.04 |
| C10H21(OC2H4)3OH | C10E3 | 9.22 | 10.27 |
| C10H21(OC2H4)5OH | C10E5 | 11.69 | 12.54 |
| C10H21(OC2H4)7OH | C10E7 | 13.81 | 13.95 |
| C10H21(OC2H4)10OH | C10E10 | 16.69 | 15.28 |
| C10H21(OC2H4)15OH | C10E15 | 21.10 | 16.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwa, K.; Śliwa, P. The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. J. Funct. Biomater. 2020, 11, 57. https://doi.org/10.3390/jfb11030057
Śliwa K, Śliwa P. The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. Journal of Functional Biomaterials. 2020; 11(3):57. https://doi.org/10.3390/jfb11030057
Chicago/Turabian StyleŚliwa, Karolina, and Paweł Śliwa. 2020. "The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids" Journal of Functional Biomaterials 11, no. 3: 57. https://doi.org/10.3390/jfb11030057
APA StyleŚliwa, K., & Śliwa, P. (2020). The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. Journal of Functional Biomaterials, 11(3), 57. https://doi.org/10.3390/jfb11030057






