In Vitro Study on the Effect of a New Bioactive Desensitizer on Dentin Tubule Sealing and Bonding
Abstract
1. Introduction
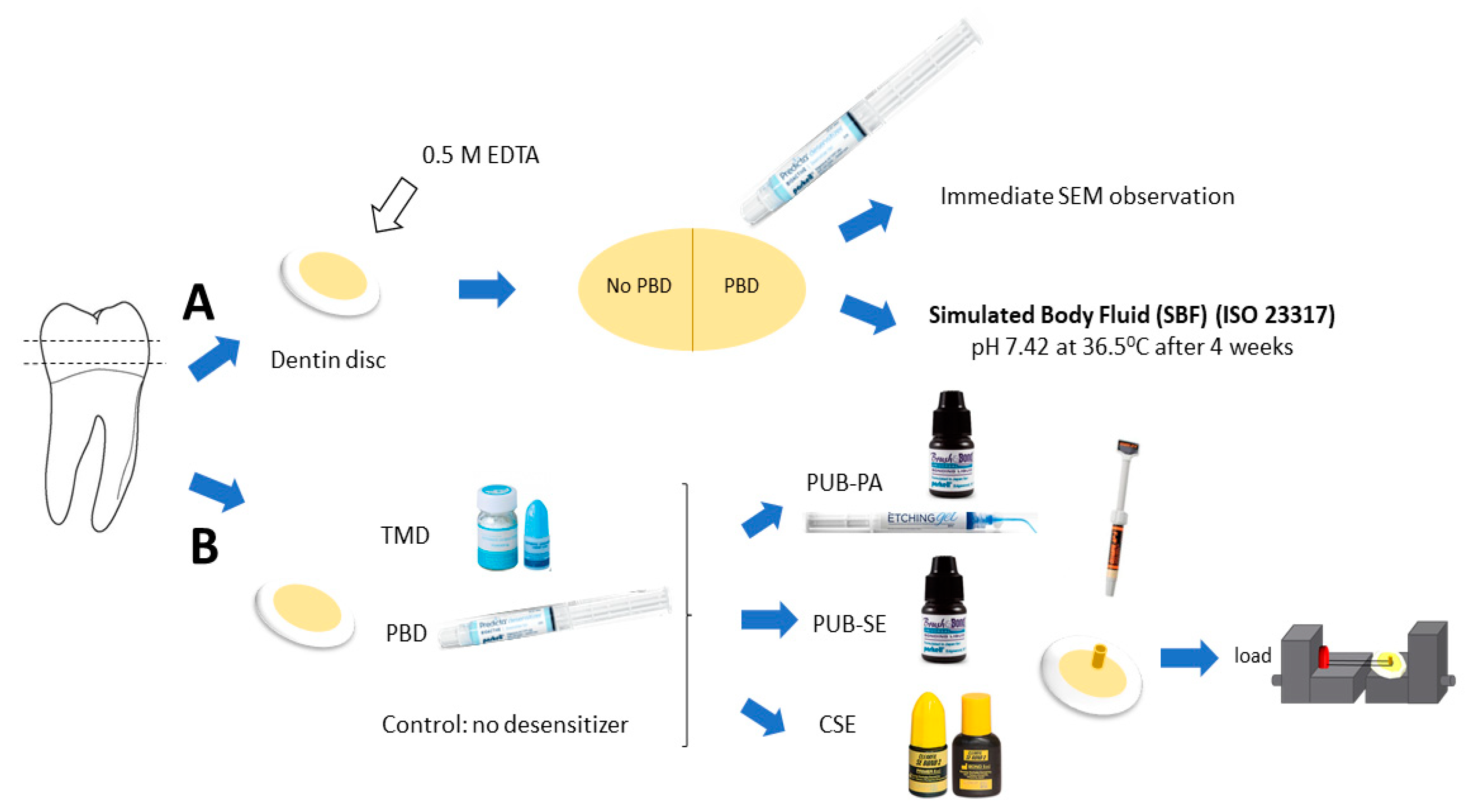
2. Materials and Methods
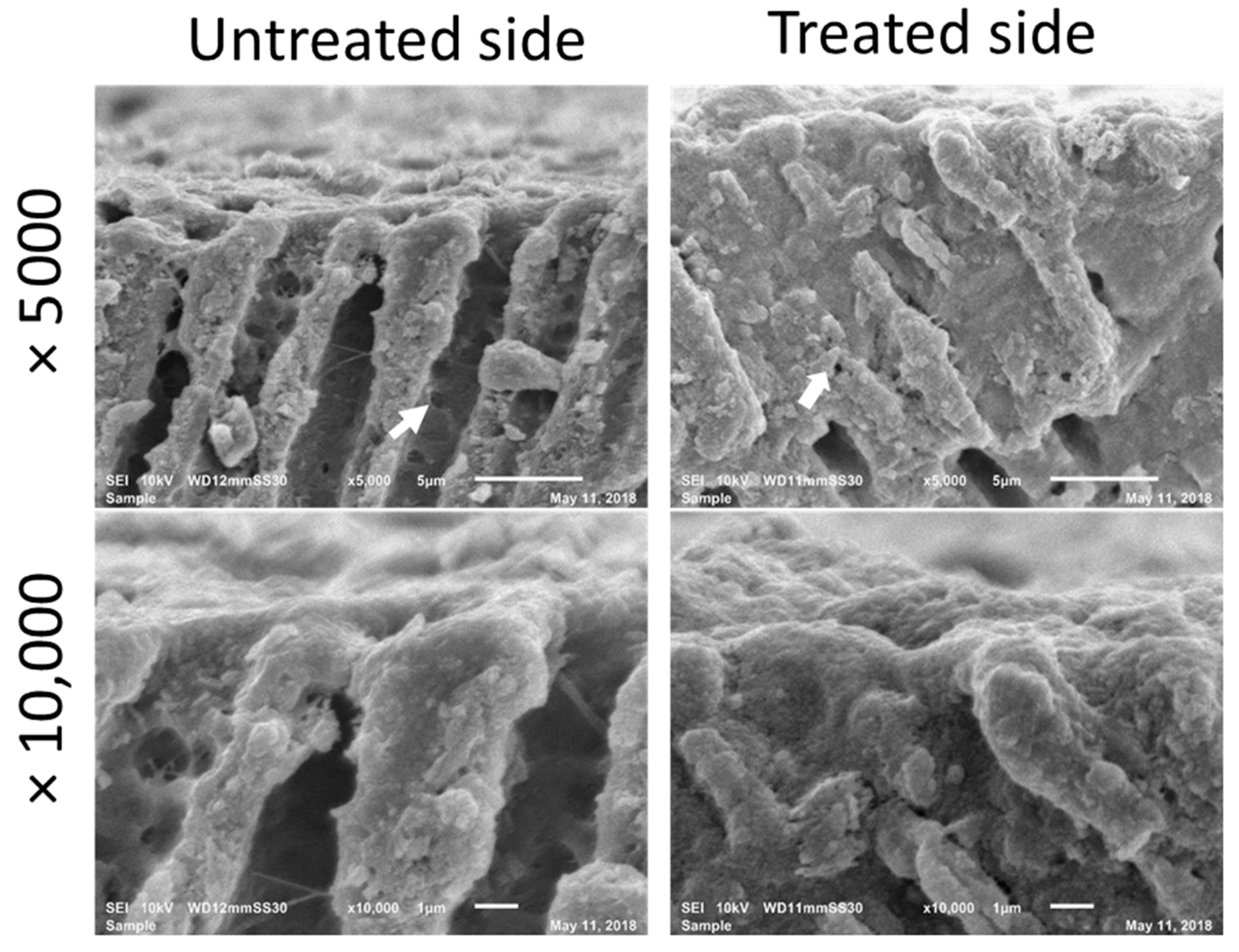
2.1. Morphological Observation
2.2. Observation after Application of PBD
2.3. Microshear Bond Strength Test
2.4. Evaluation of Failure Mode
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brännström, M.; Åström, A. A Study on the Mechanism of Pain Elicited from the Dentin. J. Dent. Res. 1964, 43, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Orchardson, R.; Gangarosa, L.P.; Holland, G.R.; Pashley, D.H. Towards a standard code of practice for evaluating the effectiveness of treatments for hypersensitive dentine. Arch. Oral Biol. 1994, 39, 121S–124S. [Google Scholar] [CrossRef]
- Miglani, S.; Aggarwal, V.; Ahuja, B. Dentin hypersensitivity: Recent trends in management. J. Conserv. Dent. 2010, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Andrade, A.K.M.; Montes, M.A.J.R. Diagnosis and treatment of dentinal hypersensitivity. J. Oral Sci. 2009, 51, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okiji, T. Dentin tubule occluding ability of dentin desensitizers. Am. J. Dent. 2015, 28, 90–94. [Google Scholar]
- Ishihata, H.; Kanehira, M.; Finger, W.J.; Takahashi, H.; Tomita, M.; Sasaki, K. Effect of two desensitizing agents on dentin permeability in vitro. J. Appl. Oral Sci. 2017, 25, 34–41. [Google Scholar] [CrossRef]
- Endo, H.; Kawamoto, R.; Takahashi, F.; Takenaka, H.; Yoshida, F.; Nojiri, K.; Takamizawa, T.; Miyazaki, M. Evaluation of a calcium phosphate desensitizer using an ultrasonic device. Dent. Mater. J. 2013, 32, 456–461. [Google Scholar] [CrossRef]
- Siso, S.H.; Dönmez, N.; Kahya, D.S.; Uslu, Y.S. The effect of calcium phosphate-containing desensitizing agent on the microtensile bond strength of multimode adhesive agent. Niger. J. Clin. Pract. 2017, 20, 964–970. [Google Scholar]
- Thanatvarakorn, O.; Nakashima, S.; Sadr, A.; Prasansuttiporn, T.; Ikeda, M.; Tagami, J. In vitro evaluation of dentinal hydraulic conductance and tubule sealing by a novel calcium-phosphate desensitizer. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 303–309. [Google Scholar] [CrossRef]
- Cunha-Cruz, J.; Wataha, J.C.; Zhou, L.; Manning, W.; Trantow, M.; Bettendorf, M.M.; Heaton, L.J.; Berg, J. Treating dentin hypersensitivity: Therapeutic choices made by dentists of the northwest PRECEDENT network. J. Am. Dent. Assoc. 2010, 141, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Shen, Y.; Wang, H.; Hannig, M. Sealing ability of dentin adhesives/desensitizer. Oper. Dent. 2007, 32, 496–503. [Google Scholar] [CrossRef]
- Grégoire, G.; Joniot, S.; Guignes, P.; Millas, A. Dentin permeability: Self-etching and one-bottle dentin bonding systems. J. Prosthet. Dent. 2003, 90, 42–49. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Have dentin adhesives become too hydrophilic? J. Can. Dent. Assoc. 2003, 69, 726–732. [Google Scholar] [PubMed]
- Yu, X.; Liang, B.; Jin, X.; Fu, B.; Hannig, M. Comparative in vivo study on the desensitizing efficacy of dentin desensitizers and one-bottle self-etching adhesives. Oper. Dent. 2010, 35, 279–286. [Google Scholar] [CrossRef]
- Sengun, A.; Koyuturk, A.E.; Sener, Y.; Ozer, F. Effect of desensitizers on the bond strength of a self-etching adhesive system to caries-affected dentin on the gingival wall. Oper. Dent. 2005, 29, 176–181. [Google Scholar]
- Pashley, D.H.; Carvalho, R.M. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef]
- Seara, S.F.; Erthal, B.S.; Ribeiro, M.; Kroll, L.; Pereira, G.D.S. The influence of a dentin desensitizer on the microtensile bond strength of two bonding systems. Oper. Dent. 2002, 27, 154–160. [Google Scholar]
- Plant, C.G.; Browne, R.M.; Knibbs, P.J.; Britton, A.S.; Sorahan, T. Pulpal effects of glass ionomer cements. Int. Endod. J. 1984, 17, 51–59. [Google Scholar] [CrossRef]
- Pashley, D.H. Smear layer: Physiological considerations. Oper. Dent. Suppl. 1984, 3, 13–29. [Google Scholar]
- Richardson, D.; Tao, L.; Pashley, D.H. Dentin permeability: Effects of crown preparation. Int. J. Prosthodont. 1991, 4, 219–225. [Google Scholar] [PubMed]
- Siriphannon, P.; Kameshima, Y.; Yasumori, A.; Okada, K.; Hayashi, S. Formation of hydroxyapatite on CaSiO3 powders in simulated body fluid. J. Eur. Ceram. Soc. 2002, 22, 511–520. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Larsen, M.J.; Pearce, E.I.F. Saturation of human saliva with respect to calcium salts. Arch. Oral Biol. 2003, 48, 317–322. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takagi, S.; Chow, L.C.; Suzuki, K. Reaction of calcium phosphate cements with different amounts of tetracalcium phosphate and dicalcium phosphate anhydrous. J. Biomed. Mater. Res. 1999, 46, 504–510. [Google Scholar] [CrossRef]
- Thanatvarakorn, O.; Nakashima, S.; Sadr, A.; Prasansuttiporn, T.; Thitthaweerat, S.; Tagami, J. Effect of a calcium-phosphate based desensitizer on dentin surface characteristics. Dent. Mater. J. 2013, 32, 615–621. [Google Scholar] [CrossRef]
- Garcia, R.N.; Giannini, M.; Takagaki, T.; Sato, T.; Matsui, N.; Nikaido, T.; Tagami, J. Effect of dentin desensitizers on resin cement bond strengths. RSBO 2016, 12, 14–22. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Sano, H.; Tay, F.R.; Kaga, M.; Kudou, Y.; Oguchi, H.; Araki, Y.; Kubota, M. Micromorphological changes in resin-dentin bonds after 1 year of water storage. J. Biomed. Mater. Res. 2002, 63, 306–311. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.; Itthagarun, A. Single-step adhesives are permeable membranes. J. Dent. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Yang, H.; Pei, D.; Chen, Z.; Lei, J.; Zhou, L.; Huang, C. Effects of the application sequence of calcium-containing desensitising pastes during etch-and-rinse adhesive restoration. J. Dent. 2014, 42, 1115–1123. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Tay, F.R. Self-etch vs. etch-and-rinse adhesives: Effect of thermo-mechanical fatigue loading on marginal quality of bonded resin composite restorations. Dent. Mater. 2005, 21, 397–412. [Google Scholar] [CrossRef] [PubMed]


| Materials | Composition | Application |
|---|---|---|
| Teethmate Desensitizer (TMD) (Kuraray Noritake Dental Inc, Tokyo, Japan) | Powder: tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) Liquid: water | Mix powder and water within 30 s, apply slurry with microapplicator 15 s, rub 60 s rinse with water spray. |
| Predicta Bioactive Desensitizer gel (PBD, Parkell, Edgewood, NY, USA) | Calcium, phosphate, nanohydroxyapatite | Rinse surface with warm water, dry with absorbent cotton. Paint a coat and rub gently the liquid in 10–20 s using an applicator. Wipe the product off using a cotton pledget before air blowing. |
| Parkell Etching (Parkell, Edgewood, NY, USA) | 32% PA Gel (H3PO4) | 10–15 s etch then rinse. |
| Parkell Universal Adhesive PBOND (Parkell, Edgewood, NY, USA) | Acetone, Ethyl Alcohol, 10-MDP, 2-Hydroxylethyl methacrylate, 2-Propenoic acid | Mix well, dispense 1–3 drops. Rub the liquid using an applicator, keep moist for 20 s. Blow gently for 10 s and light cure for 10 s. |
| Clearfil SE 2 (Kuraray Noritake Dental, Japan) | Primer: MDP, HEMA, camphorquinone, hydrophilic dimethacrylate, N,N-diethanol p-toludine, water. Bond: MDP, Bis-GMA, HEMA, camphorquinone, hydrophobic dimethacrylate, N,N-diethanol p-toludine, silanated colloidal silica. | With the applicator, prime for 20 s. Apply bond and light cure for 10 s |
| Z100 Restorative (3M ESPE, St. Paul, MN, USA) | Bis-GMA, TEDGMA, Zirconium/Silica filler | Build the resin cylinder then light cure for 40 s. |
| Mean ± SD. | TMD | PBD | Control |
|---|---|---|---|
| PUB-SE | 25.5 ± 4.7 | 19.5 ± 3.7 | 24.9 ± 4.0 |
| PUB-PA | 31.8 ± 5.8 | 30.2 ± 5.9 | 37.9 ± 5.0 |
| CSE | 29.2 ± 5.7 | 34.2 ± 3.7 | 40.3 ± 4.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luong, M.N.; Huang, L.; Chan, D.C.N.; Sadr, A. In Vitro Study on the Effect of a New Bioactive Desensitizer on Dentin Tubule Sealing and Bonding. J. Funct. Biomater. 2020, 11, 38. https://doi.org/10.3390/jfb11020038
Luong MN, Huang L, Chan DCN, Sadr A. In Vitro Study on the Effect of a New Bioactive Desensitizer on Dentin Tubule Sealing and Bonding. Journal of Functional Biomaterials. 2020; 11(2):38. https://doi.org/10.3390/jfb11020038
Chicago/Turabian StyleLuong, Minh N., Laurie Huang, Daniel C. N. Chan, and Alireza Sadr. 2020. "In Vitro Study on the Effect of a New Bioactive Desensitizer on Dentin Tubule Sealing and Bonding" Journal of Functional Biomaterials 11, no. 2: 38. https://doi.org/10.3390/jfb11020038
APA StyleLuong, M. N., Huang, L., Chan, D. C. N., & Sadr, A. (2020). In Vitro Study on the Effect of a New Bioactive Desensitizer on Dentin Tubule Sealing and Bonding. Journal of Functional Biomaterials, 11(2), 38. https://doi.org/10.3390/jfb11020038






