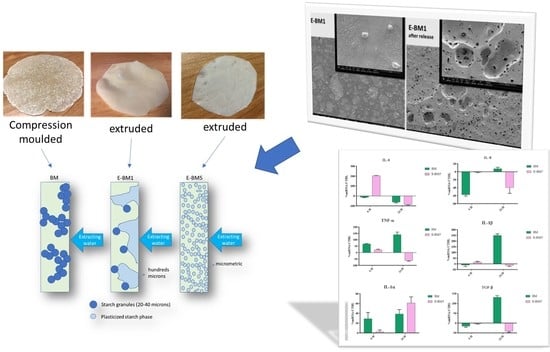
Skin-Compatible Biobased Beauty Masks Prepared by Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples and Films
2.3. Characterization
2.3.1. Material Characterization
2.3.2. Epidermal Cell Culture and Viability Assay
2.3.3. Evaluation of Immunomodulatory Properties
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN. World Population Aging 2017: Highlights; United Nations Report; UN: New York, NY, USA, 2017. [Google Scholar]
- Research Report. 15 Trends Changing the Face of the Beauty Industry in 2020. Available online: https://www.cbinsights.com/research/report/beauty-trends-2019/ (accessed on 6 January 2020).
- Global Cosmetics Products Market—Segmented by Product Type, Distribution Channel (Direct Selling, Supermarket, Specialty Stores), and Region—Growth, Trend and Forecasts (2018–2023). 360 Research Report. 20 February 2018. Available online: https://www.360researchreports.com/global-cosmetics-products-market-13100793 (accessed on 2 April 2020).
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact for pollution on skin and proper testing for anti-pollution claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef]
- Pacheco, G.; Vespoli de mehlo, C.; Galdorfini, B.; Borges Isaac, V.-L.; Lima Ribeiro, S.J.; Pecoraro, E.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosmet. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.L.; Swanson, C.L.; Thompson, A.R. Extrudates of Cornstarch with Urea and Glycols: Structure/Mechanical Property Relations. Starch Stärke 1992, 44, 335–338. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez-Rivera, M.A.; Sanfuentes, E.A.; Peña-Farfal, C. Antioxidant and antifungal effects of eugenol incorporated in bionanocomposites of poly(3-hydroxybutyrate)-thermoplastic starch. LWT Food Sci. Technol. 2018, 98, 260–267. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Applications of PHAs in Medicine and Pharmacy. In Biopolymers Online; Wiley: Hoboken, NJ, USA, 2005; Chapter 20. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.-H.; Chou, I.-N.; Tseng, S.-H.; Wu, H.-S. Application of Biodegradable Polyhydroxyalkanoates as Surgical Films for Ventral Hernia Repair in Mice. Int. J. Polym. Sci. 2014, 2014, 789681. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.X.L. The Greenhouse Gas Emissions and Fossil Energy Requirement of Bioplastics from Cradle to Gate of a Biomass Refinery. Environ. Sci. Technol. 2008, 42, 6961–6966. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A. Novel Sustainable Composites Based on Poly(hydroxybutyrate-co-hydroxyvalerate and Seagrass Beach-CAST Fibers: Performance and Degradability in Marine Environments. Materials 2018, 11, 772. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-hydroxybutyrateco-3-hydroxyhexanoate)(PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 15; pp. 321–342. [Google Scholar]
- Coltelli, M.-B.; Danti, S.; Trombi, L.; Morganti, P.; Donnarumma, G.; Baroni, A.; Fusco, A.; Lazzeri, A. Preparation of Innovative Skin Compatible Films to Release Polysaccharides for Biobased Beauty Masks. Cosmetics 2018, 5, 70. [Google Scholar] [CrossRef]
- Gigante, V.; Coltelli, M.-B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat Die Extruded Biocompatible Poly(Lactic Acid) (PLA)/Poly(ButyleneSuccinate) (PBS) BasedFilms. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Gigante, V.; Cinelli, P.; Lazzeri, A. Flexible Food Packaging Using Polymers from Biomass. In Bionanotechnology to Save the Environment. Plant and Fishery’s Biomass as Alternative to Petrol; Morganti, P., Ed.; MDPI: Basel, Switzerland, 2018; pp. 272–296. [Google Scholar]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Marcondes Agnelli, J.A. The Influence of the Industrial Processing on the Degradation of Poly(hydroxybutyrate)–PHB. Mater. Res. 2013, 16, 327–332. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable biocomposites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kreuzberger, S.; Hinrichsen, G. Influence of thermal degradation on tensile strength and Young’s modulus of poly(hydroxybutyrate). Polym. Bull. 1994, 33, 355–359. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Parulekar, Y.; Mohanty, A.K. Extruded Biodegradable Cast Films from Polyhydroxyalkanoate and Thermoplastic Starch Blends: Fabrication and Characterization. Macromol. Mater. Eng. 2007, 292, 1218–1228. [Google Scholar] [CrossRef]
- Godbole, S.; Gote, S.; Latkar, M.; Chakrabarti, T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate–starch blend films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Willett, J.L.; Kotnis, M.A.; O’Brien, G.S.; Fanta, G.F.; Gordon, S.H. Properties of starch-graft-poly(glycidyl methacrylate)–PHBV composites. J. Appl. Polym. Sci. 1998, 70, 1121–1127. [Google Scholar] [CrossRef]
- Lai, S.-M.; Sun, W.-W.; Don, T.-M. Preparation and characterization of biodegradable polymer blends from poly(3-hydroxybutyrate)/poly(vinyl acetate)-modified corn starch. Polym. Eng. Sci. 2015, 55, 1321–1329. [Google Scholar] [CrossRef]
- De Paula, F.C.; De Paula, C.B.C.; Contiero, J. Prospective Biodegradable Plastics from Biomass Conversion Processes. In BioFuels-State of Development; Biernat, K., Ed.; IntechOpen: London, UK, 2018; Chapter 12. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS blends via In Situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng. 2011, 297, 402–410. [Google Scholar] [CrossRef]
- Rajendra, K.; Krishnaswamy, R.K.; Sun, X. PHA Compositions Comprising PBS and PBSA and Methods for Their Production. U.S. Patent US9056947B2, 16 June 2015. [Google Scholar]
- Zhang, G.; Xie, W.; Wu, D. Selective localization of starch nanocrystals in the biodegradable nanocomposites probed by crystallization temperatures. Carbohydr. Polym. 2020, 227, 115341. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of Modified Tapioca Starch on Mechanical, Thermal, and Morphological Properties of PBS Blends for Food Packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Zhou, Y. Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch Stärke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Herrera Brandelero, R.P.; Eiras Grossmann, M.V.; Yamashita, F. Effect of the method of production of the blends on mechanical and structural properties of biodegradable starch films produced by blown extrusion. Carbohydr. Polym. 2011, 86, 1344–1350. [Google Scholar] [CrossRef]
- Olivato, J.B.; Müller, C.M.O.; Carvalho, G.M.; Yamashita, F.; Grossmann, M.V.E. Physical and structural characterisation of starch/polyester blends with tartaric acid. Mater. Sci. Eng. C 2014, 39, 35–39. [Google Scholar] [CrossRef]
- Larsson, M.; Markbo, O.; Jannasch, P. Melt processability and thermomechanical properties of blends based on polyhydroxyalkanoates and poly(butylene adipateco-terephthalate). RSC Adv. 2016, 6, 44354–44363. [Google Scholar] [CrossRef]
- De Matos Costa, A.R.; Marques Santos, R.; Noriyuki Ito, E.; Hecker de Carvalho, L.; Luís Canedo, E. Melt and cold crystallization in a poly(3-hydroxybutyrate) poly(butylene adipate-co-terephthalate. J. Therm. Anal. Calorim. 2019, 137, 1341–1346. [Google Scholar] [CrossRef]
- Lin, X.; Fan, X.; Li, R.; Li, Z.; Ren, T.; Ren, X.; Huang, T.-S. Preparation and characterization of PHB/PBAT–based biodegradable antibacterial hydrophobic nanofibrous membranes. Polym. Adv. Technol. 2018, 29, 481–489. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M. Improving the interfacial adhesion in a new renewable resourcebasedbiocomposites from biofuel coproduct and biodegradable plastic. J. Mater. Sci. 2013, 48, 6025–6038. [Google Scholar] [CrossRef]
- Belyamani, I.; Kim, K.; Rahimi, S.K.; Sahukhal, G.S.; Elasri, M.O.; Otaigbe, J.U. Creep, recovery, and stress relaxation behavior of nanostructured bioactive calcium phosphate glass–POSS/polymer composites for bone implants studied under simulated physiological conditions. J. Biomed. Mater. Res. Part B 2019, 107B, 2419–2432. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory Cytokines in Vascular Dysfunction and Vascular Disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, M.R. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef]
- Breulmann, M.; Künkel, A.; Philipp, S.; Reimer, V.; Siegenthaler, K.O.; Skupin, G.; Yamamoto, M. Polymers, Biodegradable. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim/Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Suhartini, M.; Mitomo, H.; Yoshii, F.; Nagasawa, N.; Kume, T. Radiation Crosslinking of Poly(Butylene Succinate) in the Presence of Inorganic Material and Its Biodegradability. J. Polym. Environ. 2001, 9, 163–171. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Ren, H.; Fu, T.; Ren, W.Y. Preparation, characterization and properties of binary and ternary blends with thermoplastic starch poly(lactic acid) and poly(butylene adipate-co-terephthalate). Carbohydr. Polym. 2009, 77, 576–582. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B. Infrared spectroscopy as a tool to characterise starch ordered structure-a joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2015, 139, 35–42. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Fabrication, characterization, and application of polyester/wood flour composites. J. Polym. Eng. 2017. [Google Scholar] [CrossRef]
- Brauchle, M.; Angermeyer, K.; Hubner, G.; Werner, S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 1994, 9, 3199–3204. [Google Scholar]
- Kristensen, M.; Chu, C.Q.; Eedy, D.J.; Feldmann, M.; Brennan, F.M.; Breathnach, S.M. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: Epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin. Exp. Immunol. 1993, 94, 354–362. [Google Scholar] [CrossRef]
- Raja Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound reepithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- White, L.A.; Mitchell, T.I.; Brinckerhoff, C.E. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim. Biophys. Acta 2000, 1490, 259–268. [Google Scholar] [CrossRef]
- Greenwel, P.; Inagaki, Y.; Hu, W.; Walsh, M.; Ramirez, F. Sp1 is required for the early response of alpha2(I) collagen to transforming growth factor-beta1. J. Biol. Chem. 1997, 272, 19738–19745. [Google Scholar] [CrossRef]
- Mauviel, A.; Chung, K.Y.; Agarwal, A.; Tamai, K.; Uitto, J. Cellspecific induction of distinct oncogenes of the Jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J. Biol. Chem. 1996, 271, 10917–10923. [Google Scholar] [CrossRef]
- Riedel, K.; Riedel, F.; Goessler, U.R.; Germann, G.; Sauerbier, M. Tgf-beta antisense therapy increases angiogenic potential in human keratinocytes In Vitro. Arch. Med. Res. 2007, 38, 45–51. [Google Scholar] [CrossRef]
- Zeng, G.; McCue, H.M.; Mastrangelo, L.; Millis, A.J. Endogenous TGF-beta activity is modified during cellular aging: Effects on metalloproteinase and TIMP-1 expression. Exp. Cell Res. 1996, 228, 271–276. [Google Scholar] [CrossRef]
- Mitra, R.; Khar, A. Suppression of macrophage function in AK-5 tumor transplanted animals: Role of TGF-beta1. Immunol. Lett. 2004, 91, 189–195. [Google Scholar] [CrossRef]
- Tsunawaki, S.; Sporn, M.; Ding, A.; Nathan, C. Deactivation of macrophages by transforming growth factor-beta. Nature 1988, 334, 260–262. [Google Scholar] [CrossRef]
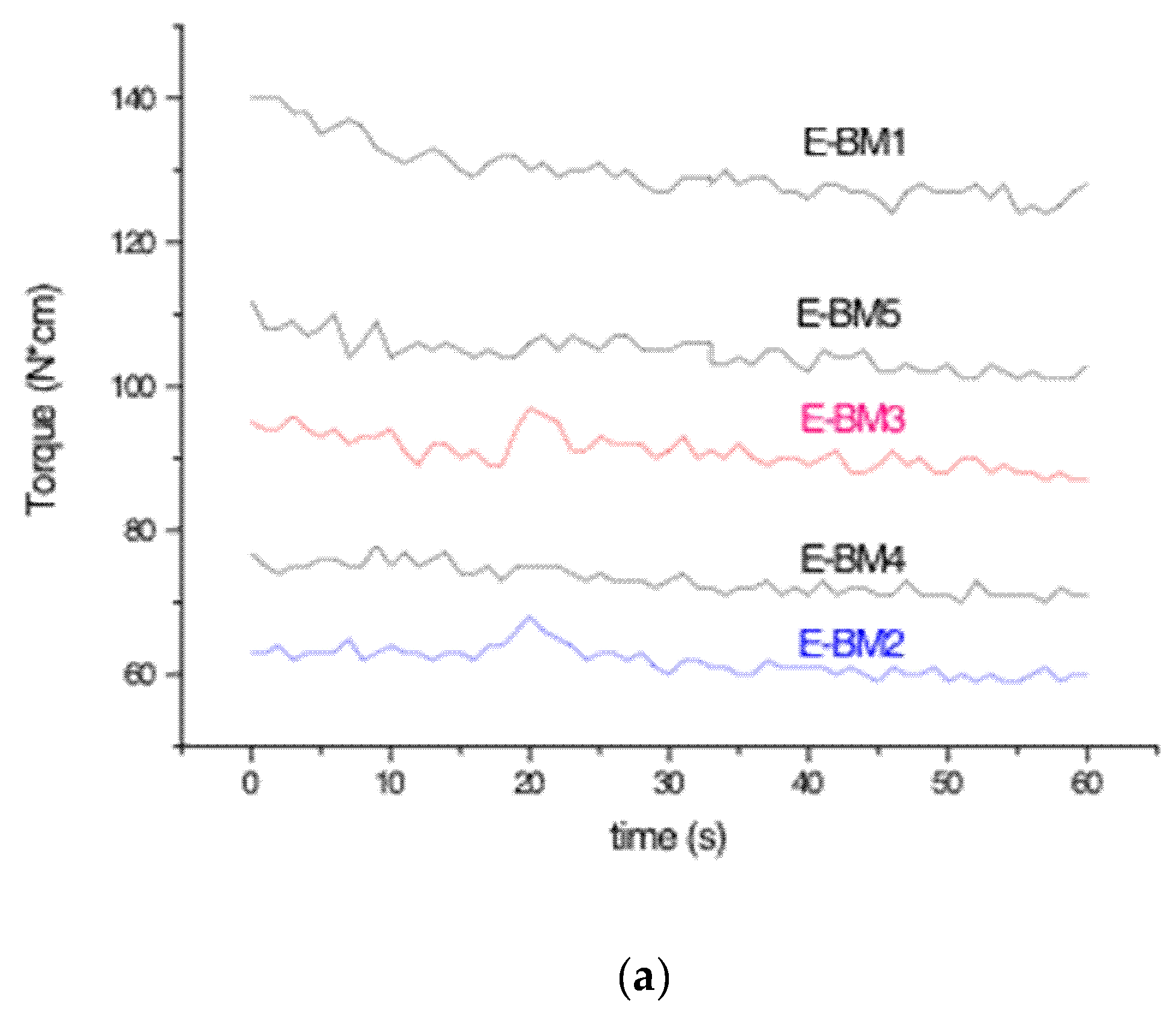
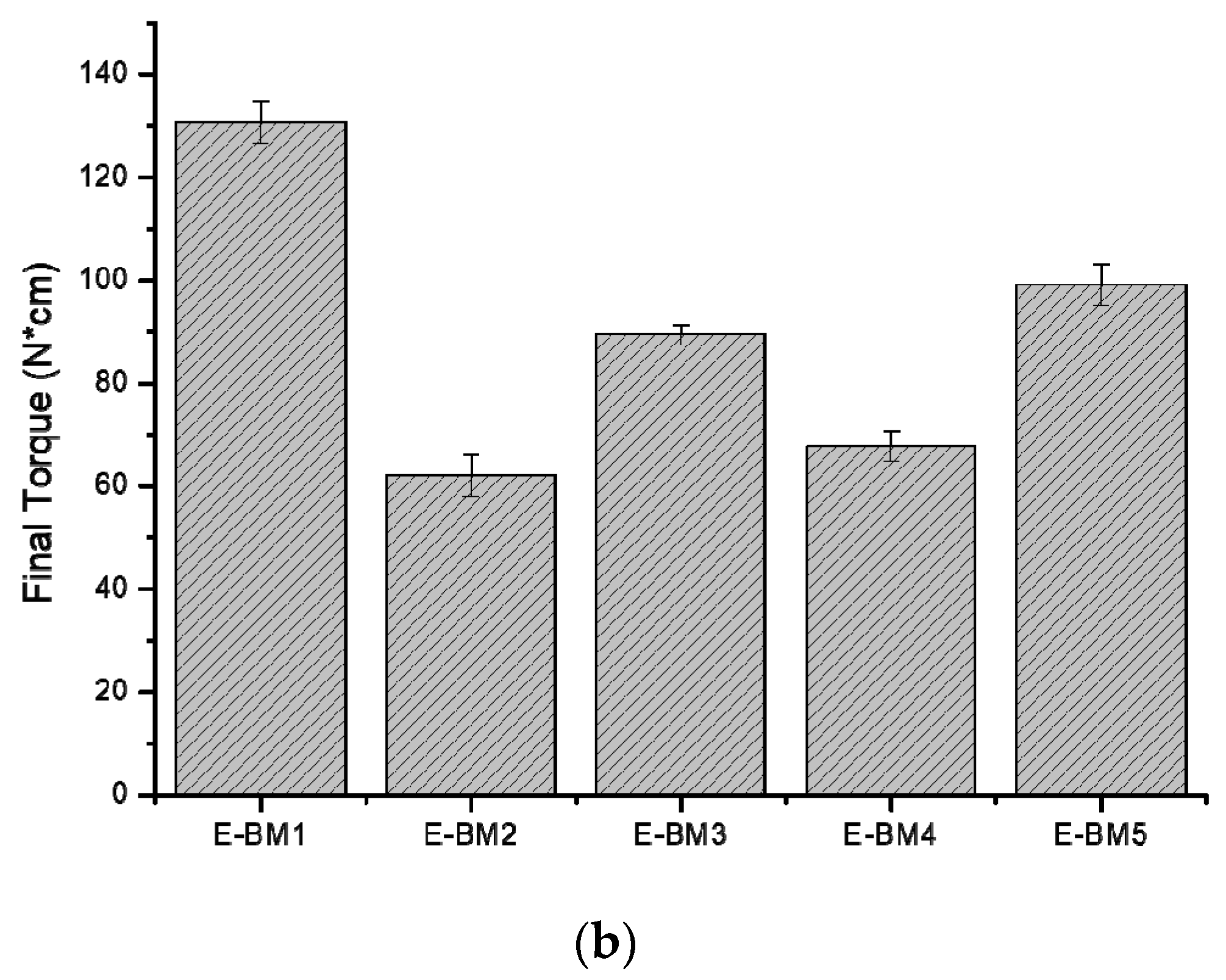
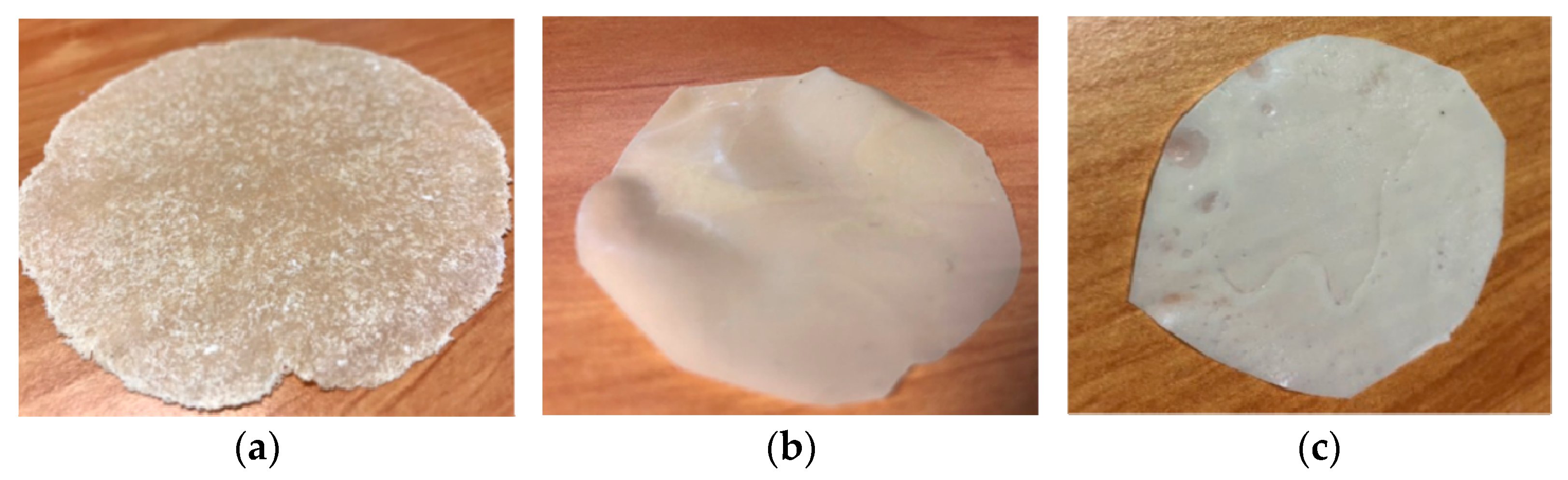
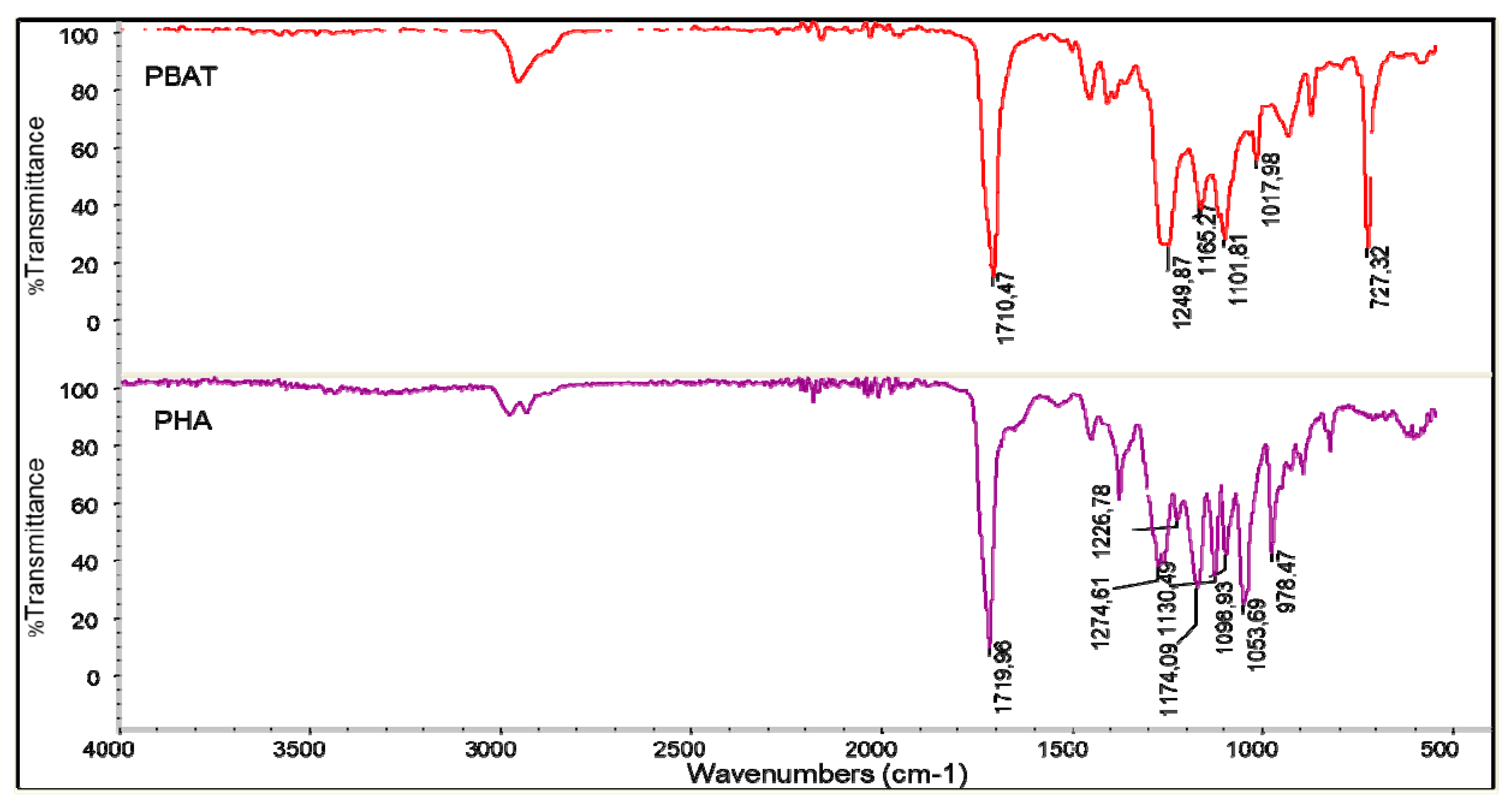


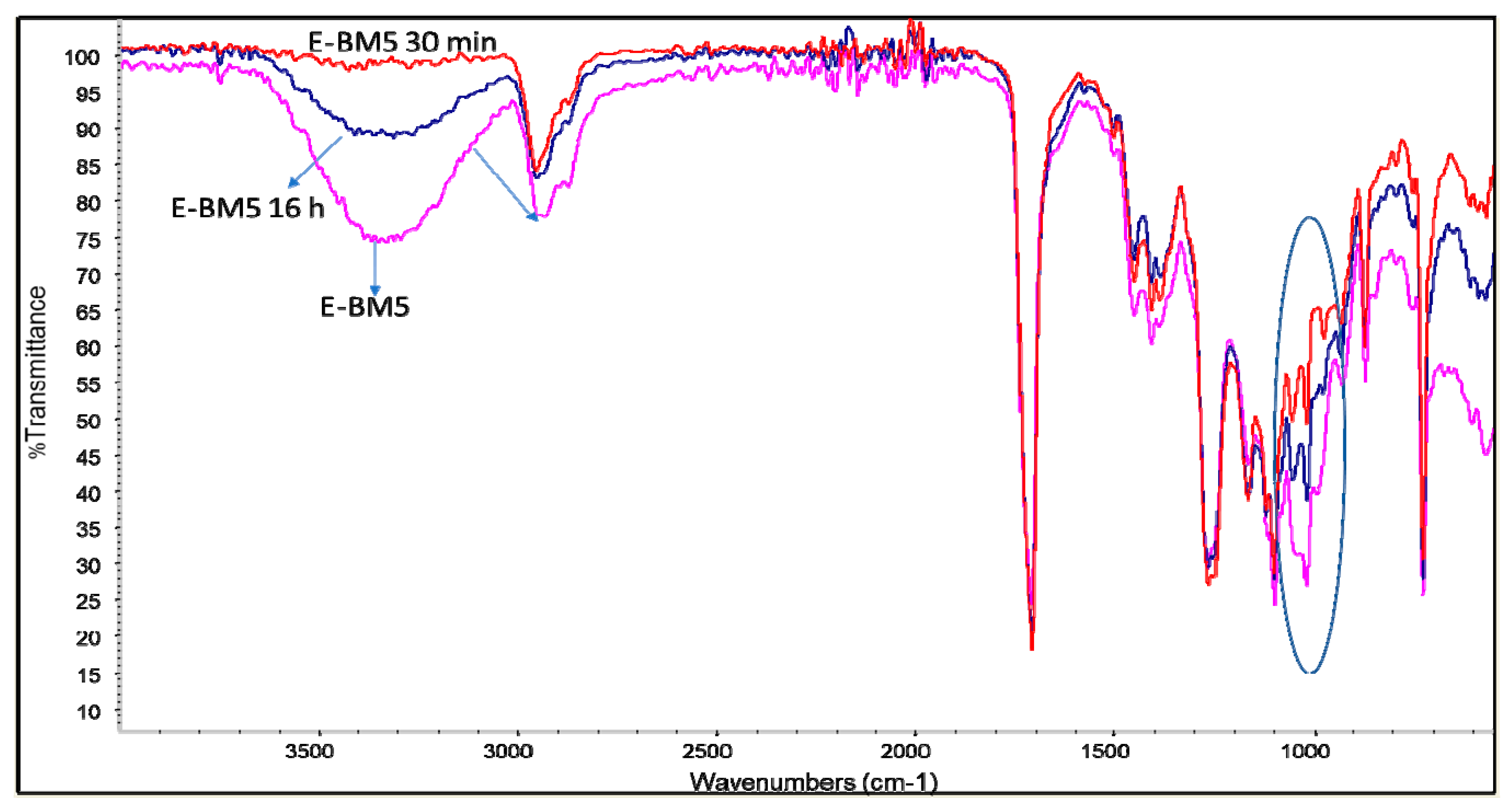






| Blends | P-PLST (wt%) | PHA (wt%) | PBSA (wt%) | PBAT (wt%) | CC (wt%) |
|---|---|---|---|---|---|
| E-BM1 | 46.5 | 46.5 | - | - | 7 |
| E-BM2 | 46.5 | - | 46.5 | - | 7 |
| E-BM3 | 46.5 | 23.25 | 23.25 | - | 7 |
| E-BM4 | 46.5 | - | - | 46.5 | 7 |
| E-BM5 | 46.5 | 23.25 | 23.25 | 7 |
| Gene | Primer Sequence | Conditions | Size (bp) |
|---|---|---|---|
| IL-1 α | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ | 5 s at 95 °C, 8 s at 55 °C, | 421 |
| 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | 17 s at 72 °C for 45 cycles | ||
| IL-1 β | 5′-GCATCCAGCTACGAATCTCC-3′ | 5 s at 95 °C, 14 s at 58 °C, | 708 |
| 5′-CCACATTCAGCACAGGACTC-3′ | 28 s at 72 °C for 40 cycles | ||
| TNF-α | 5′-CAGAGGGAAGAGTTCCCCAG-3′ | 5 s at 95 °C, 6 s at 57 °C, | 324 |
| 5′-CCTTGGTCTGGTAGGAGACG-3′ | 13 s at 72 °C for 40 cycles | ||
| IL-6 | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ | 5 s at 95 °C, 13 s at 56 °C, | 628 |
| 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | 25 s at 72 °C for 40 cycles | ||
| IL-8 | 5-ATGACTTCCAAGCTGGCCGTG-3′ | 5 s at 94 °C, 6 s at 55 °C, | 297 |
| 5-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′ | 12 s at 72 °C for 40 cycles | ||
| TGF-β | 5′-CCGACTACTACGCCAAGGAGGTCAC-3′ | 5 s at 94 °C, 9 s at 60 °C, | 439 |
| 5′-AGGCCGGTTCATGCCATGAATGGTG-3′ | 18 s at 72 °C for 40 cycles |
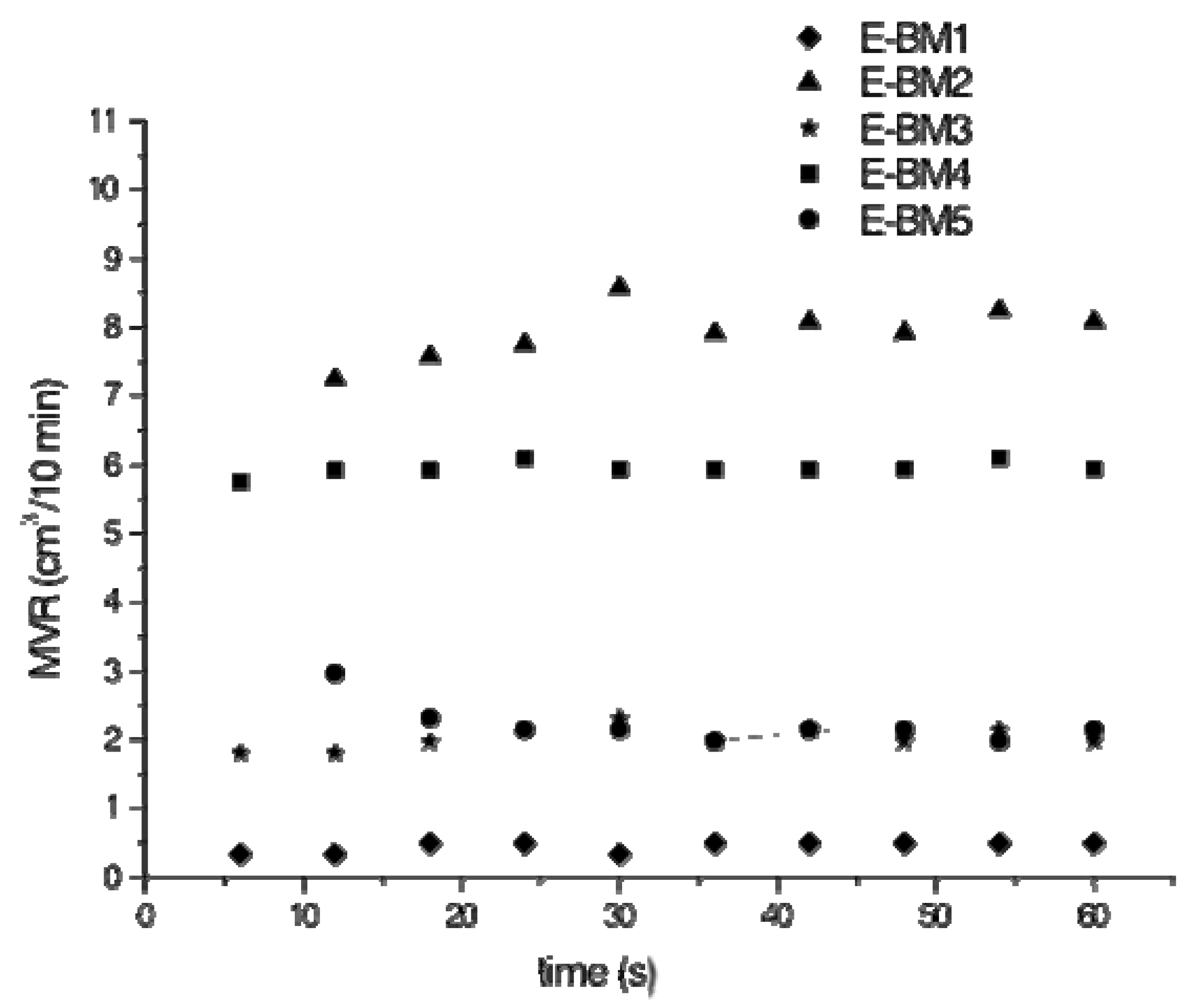
| Blends | MFR (g/10 min) | MVR (cm3/10 min) |
|---|---|---|
| PHA | 0 | 0 |
| PBSA | 2.7 ± 0.1 | 2.45 ± 0.09 |
| PBAT | 5.1 ± 0.1 | 4.7 ± 0.1 |
| E-BM1 | 0.53 ± 0.09 | 0.44 ± 0.08 |
| E-BM2 | 8.9 ± 0.4 | 7.9 ± 0.4 |
| E-BM3 | 2.3 ± 0.2 | 2.0 ± 0.2 |
| E-BM4 | 7.2 ± 0.1 | 5.94 ± 0.09 |
| E-BM5 | 2.3 ± 0.3 | 2.2 ± 0,3 |
| Blends | After 30 min (wt%) | After 16 h (wt%) | After 30 min with Respect to P-PLST (wt%) |
|---|---|---|---|
| BM | 24.6 | 25.0 | 49.0 |
| E-BM1 | 19.3 | 20.8 | 41.0 |
| E-BM5 | 17.6 | 22.2 | 38.0 |
| Sample | %ABRED |
|---|---|
| BM | 105 |
| EBM-5 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltelli, M.-B.; Panariello, L.; Morganti, P.; Danti, S.; Baroni, A.; Lazzeri, A.; Fusco, A.; Donnarumma, G. Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. J. Funct. Biomater. 2020, 11, 23. https://doi.org/10.3390/jfb11020023
Coltelli M-B, Panariello L, Morganti P, Danti S, Baroni A, Lazzeri A, Fusco A, Donnarumma G. Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. Journal of Functional Biomaterials. 2020; 11(2):23. https://doi.org/10.3390/jfb11020023
Chicago/Turabian StyleColtelli, Maria-Beatrice, Luca Panariello, Pierfrancesco Morganti, Serena Danti, Adone Baroni, Andrea Lazzeri, Alessandra Fusco, and Giovanna Donnarumma. 2020. "Skin-Compatible Biobased Beauty Masks Prepared by Extrusion" Journal of Functional Biomaterials 11, no. 2: 23. https://doi.org/10.3390/jfb11020023
APA StyleColtelli, M.-B., Panariello, L., Morganti, P., Danti, S., Baroni, A., Lazzeri, A., Fusco, A., & Donnarumma, G. (2020). Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. Journal of Functional Biomaterials, 11(2), 23. https://doi.org/10.3390/jfb11020023