Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities
Abstract
:1. Introduction
2. Results
2.1. Structural Verification of AMsils
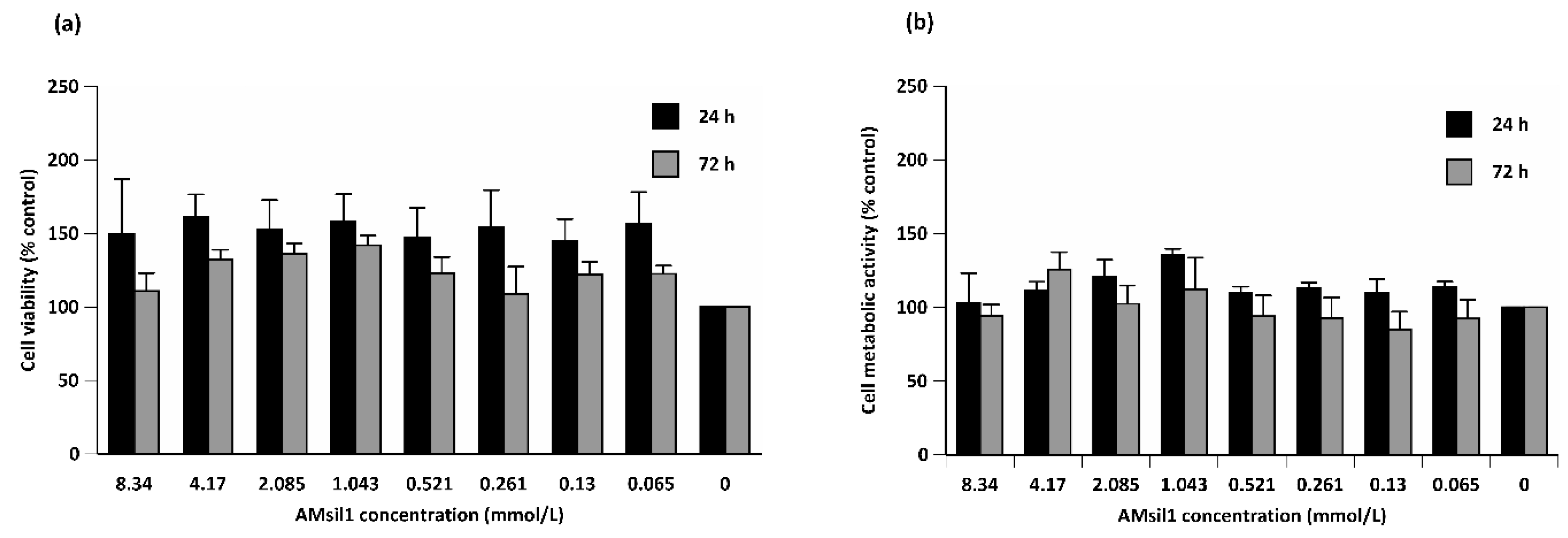
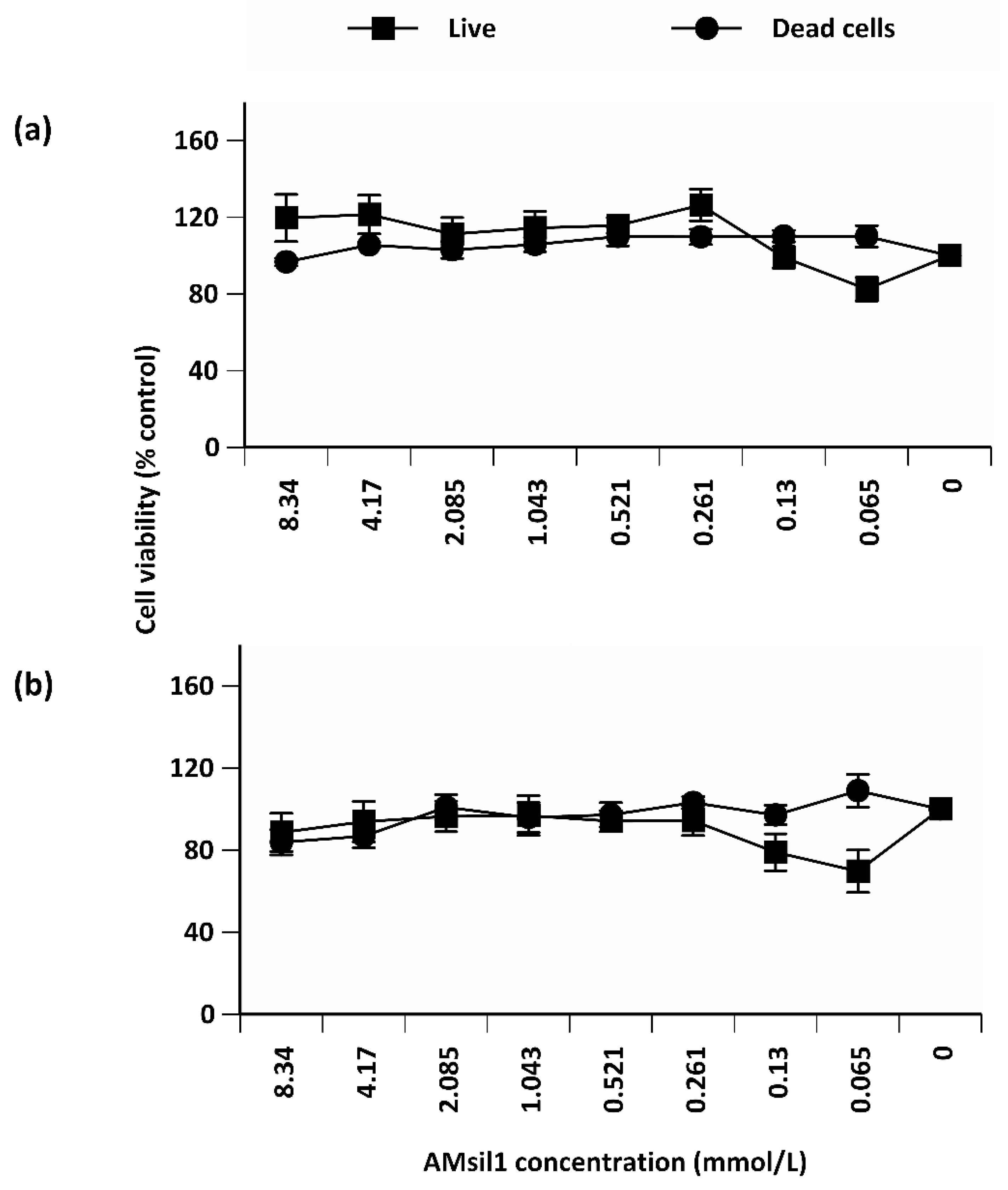
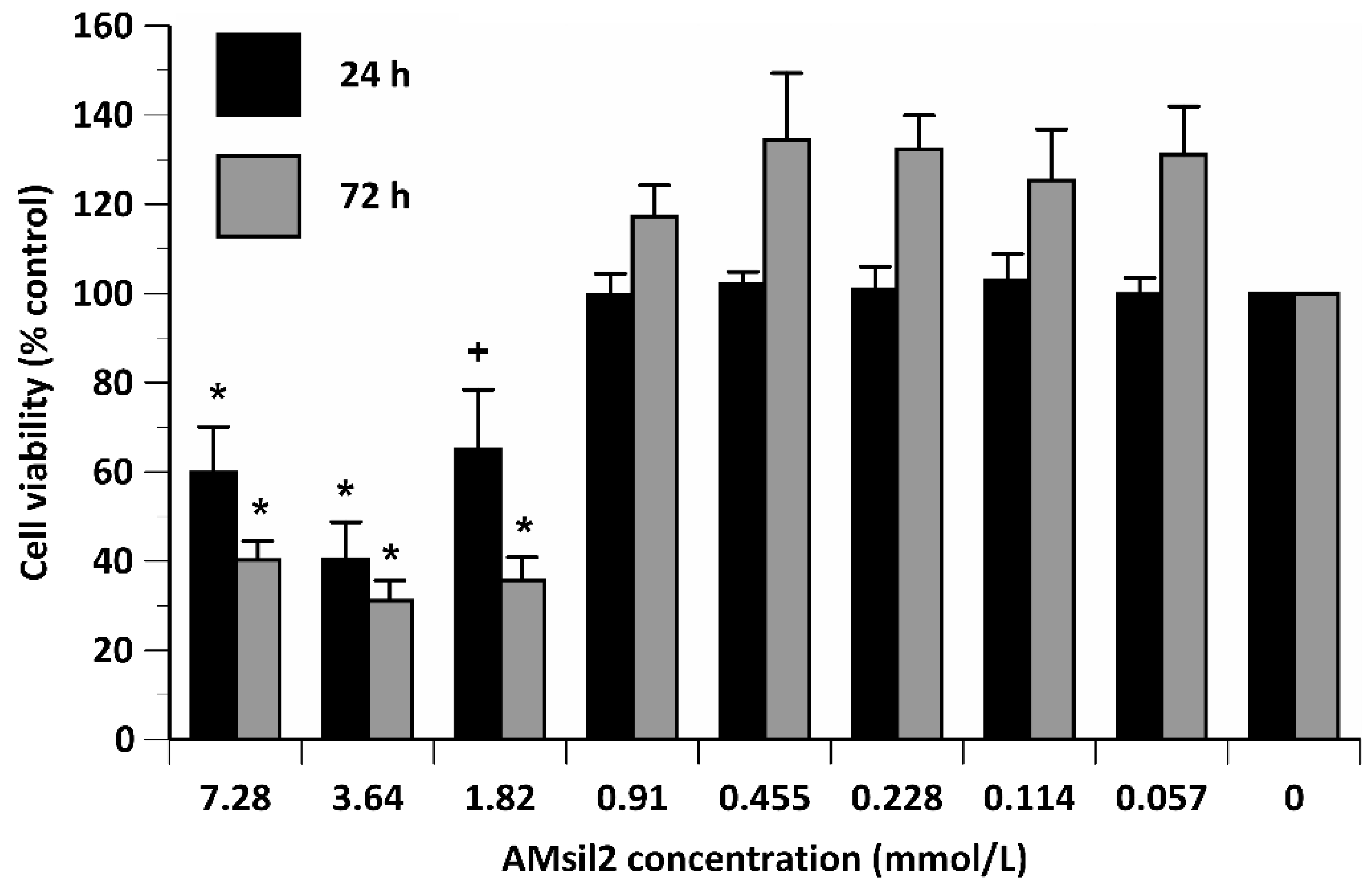
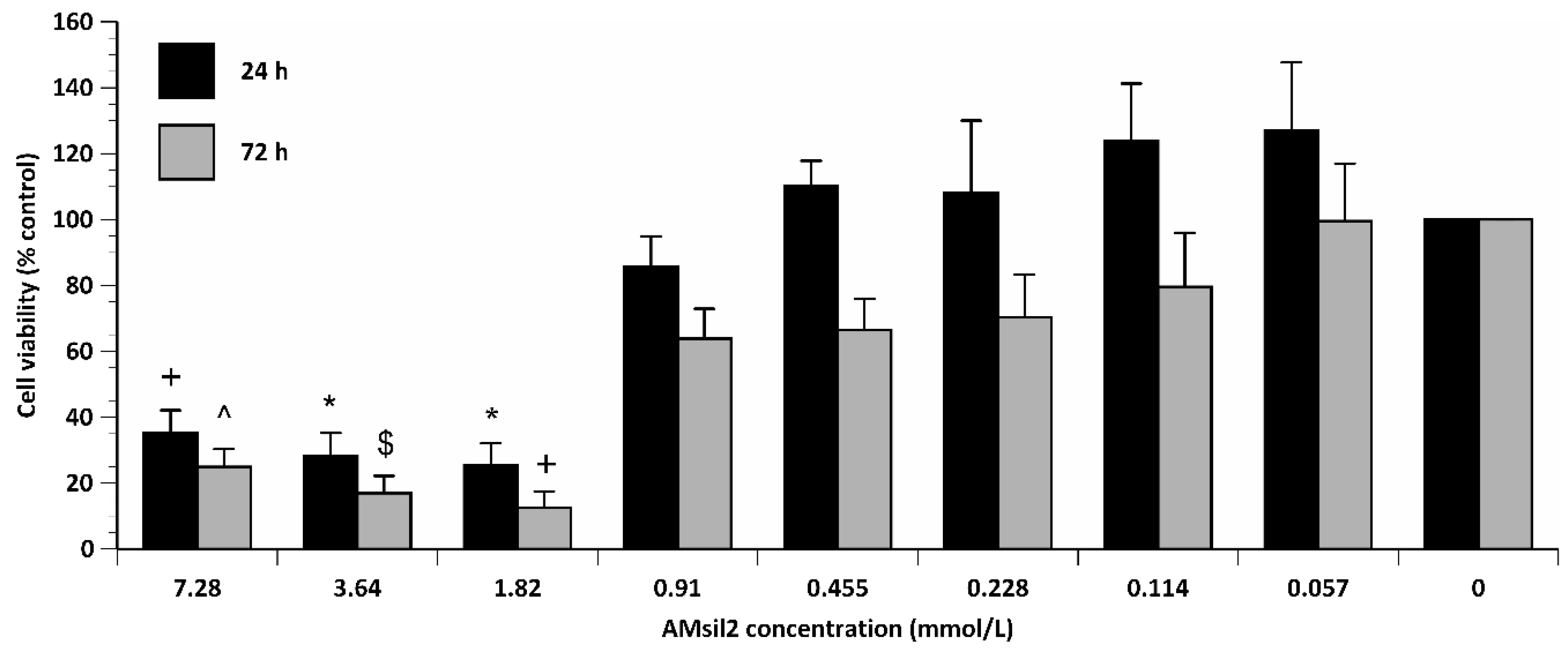
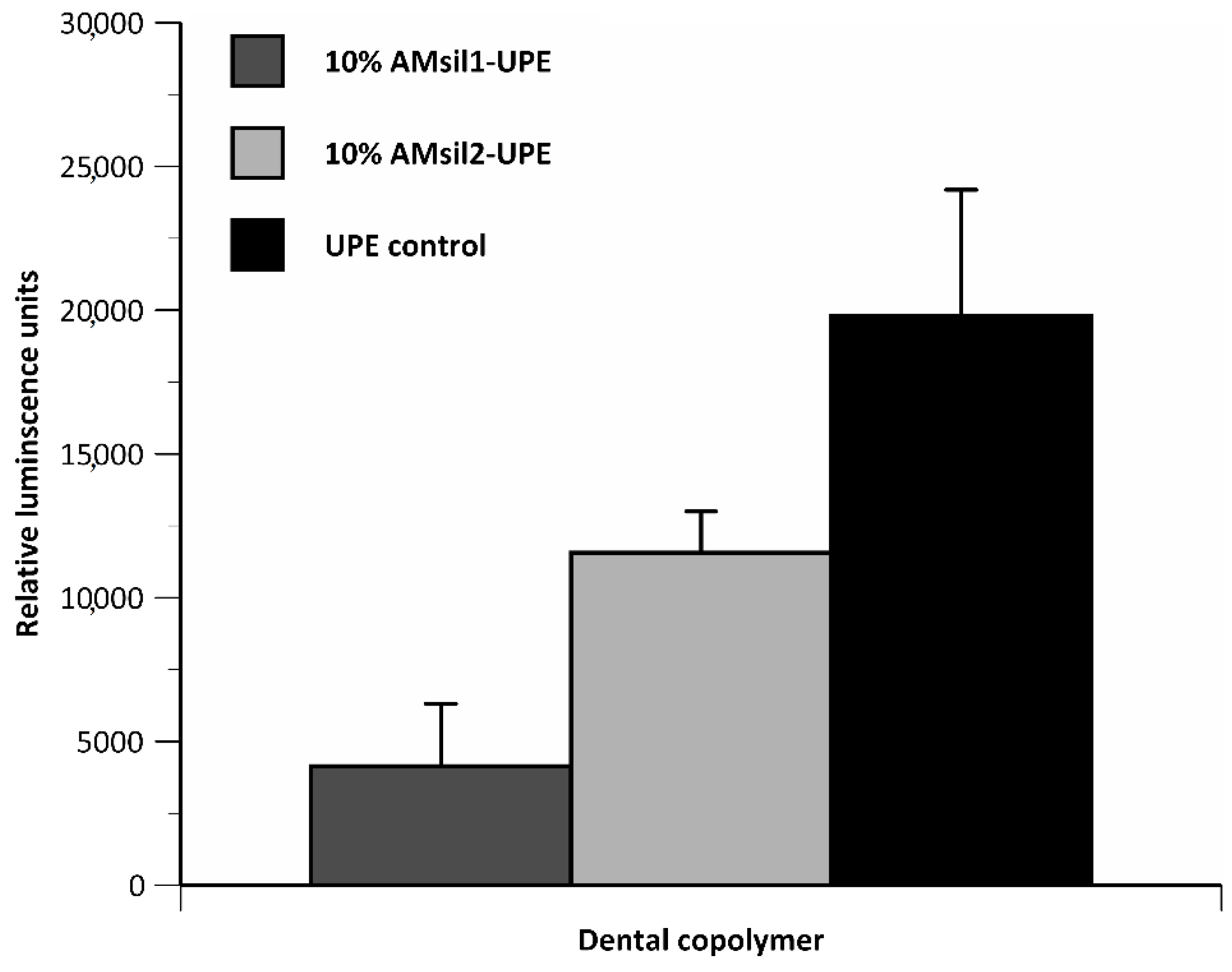
2.2. Biocompatibility Testing
2.2.1. AMsil1
2.2.2. AMsil2
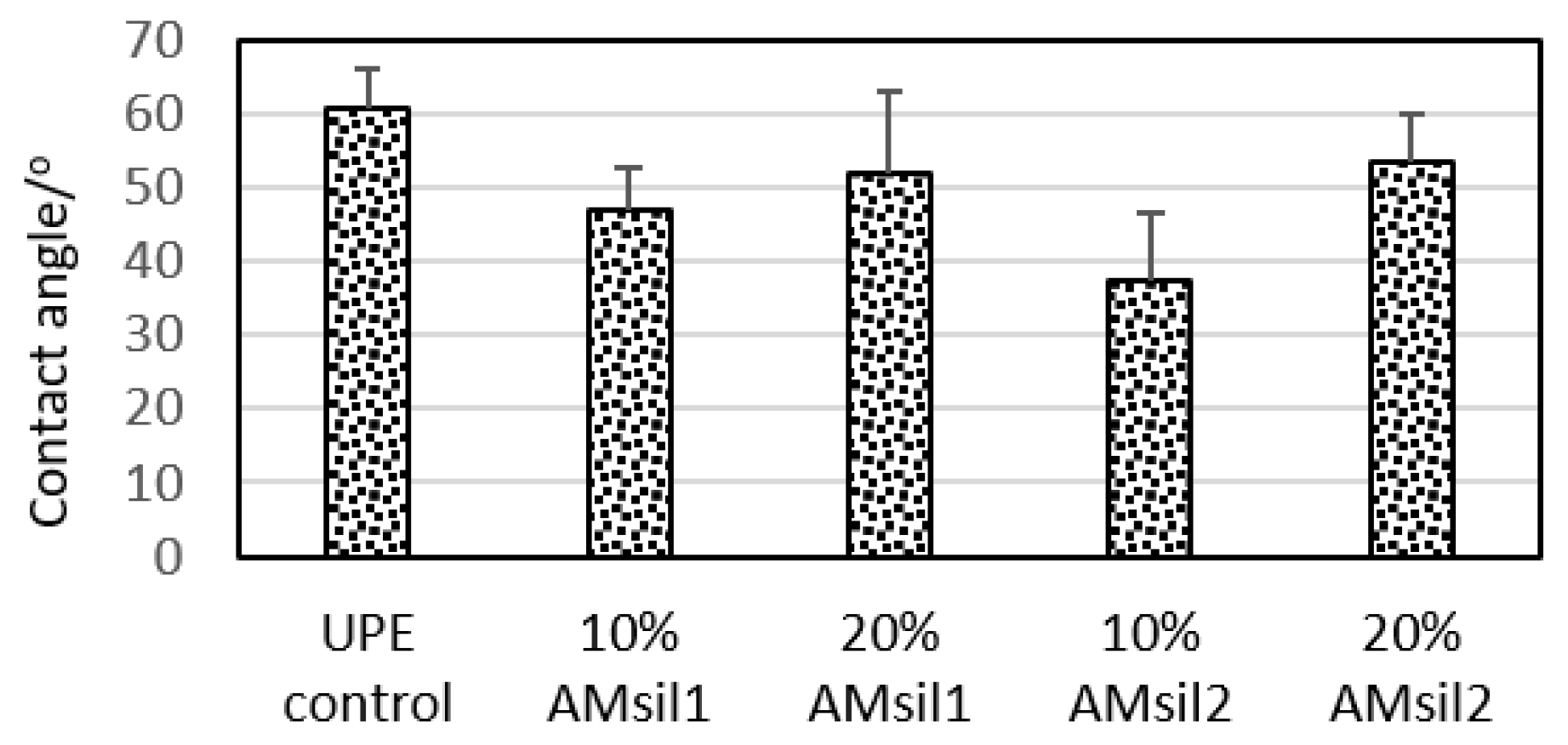
2.3. Hydrophobicity/Hydrophilicity of the Resins
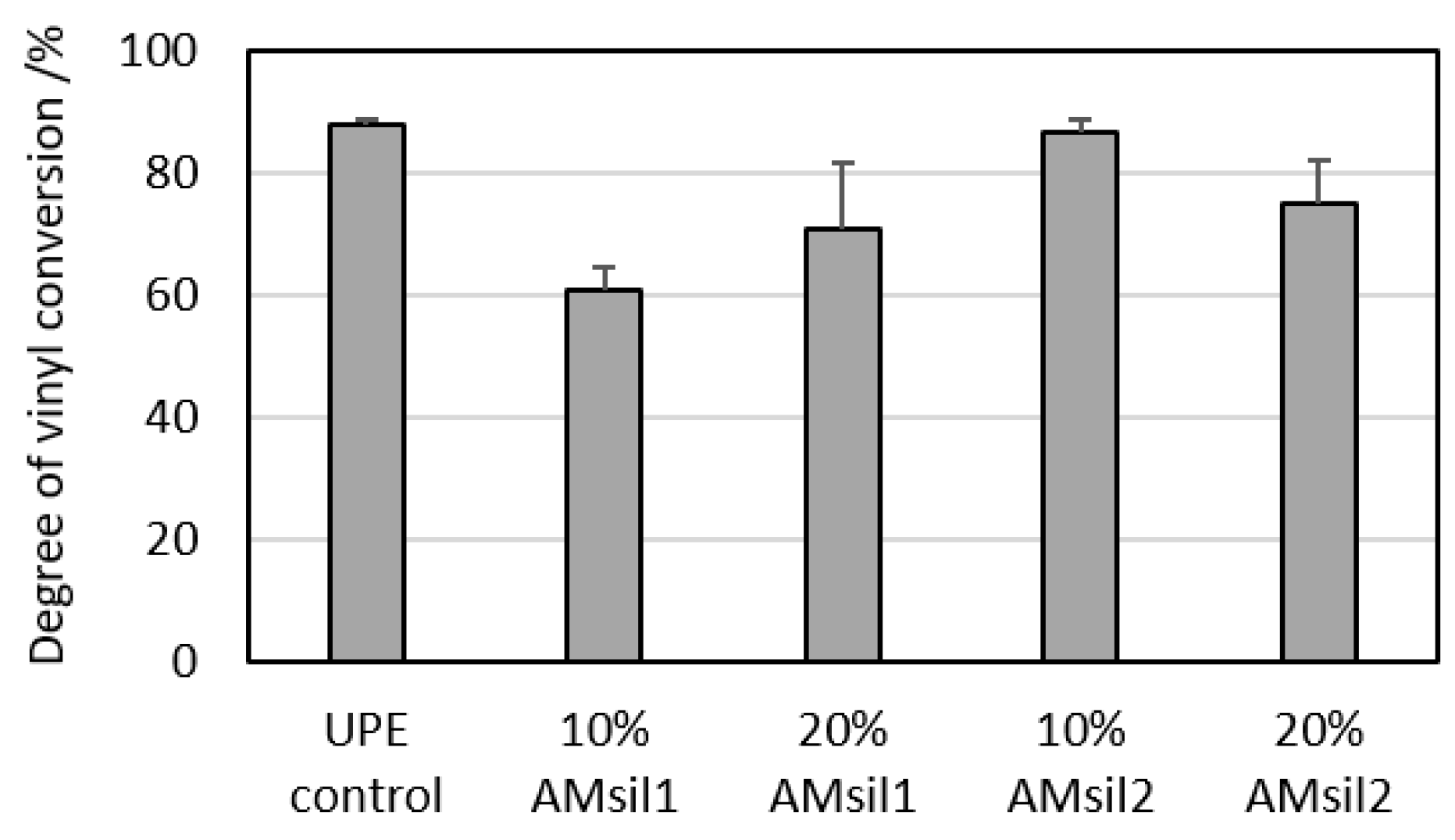
2.4. Effect of AMsils on Degree of Vinyl Conversion (DVC)
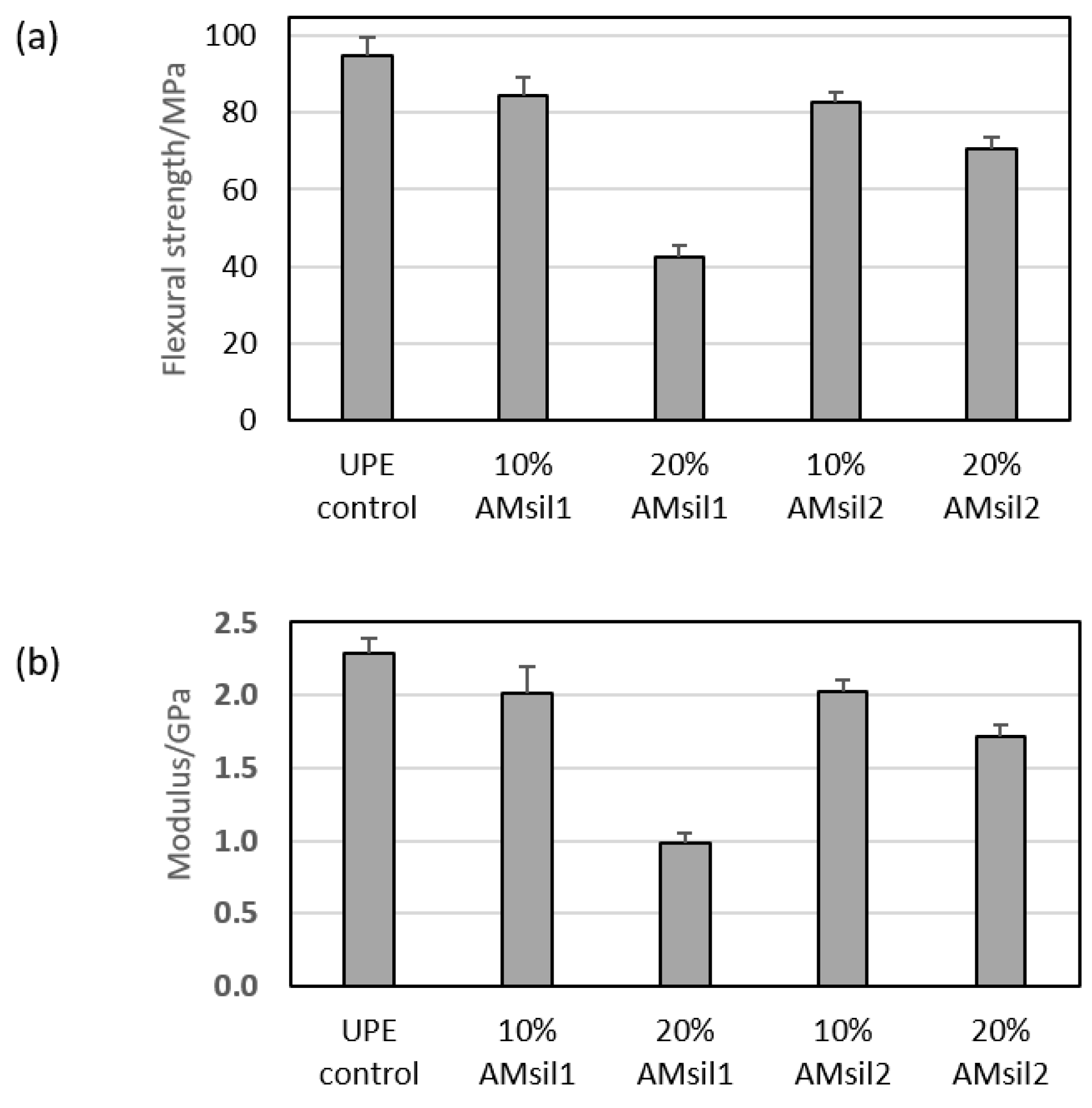
2.5. Mechanical Properties of AMsil–UPE Copolymers
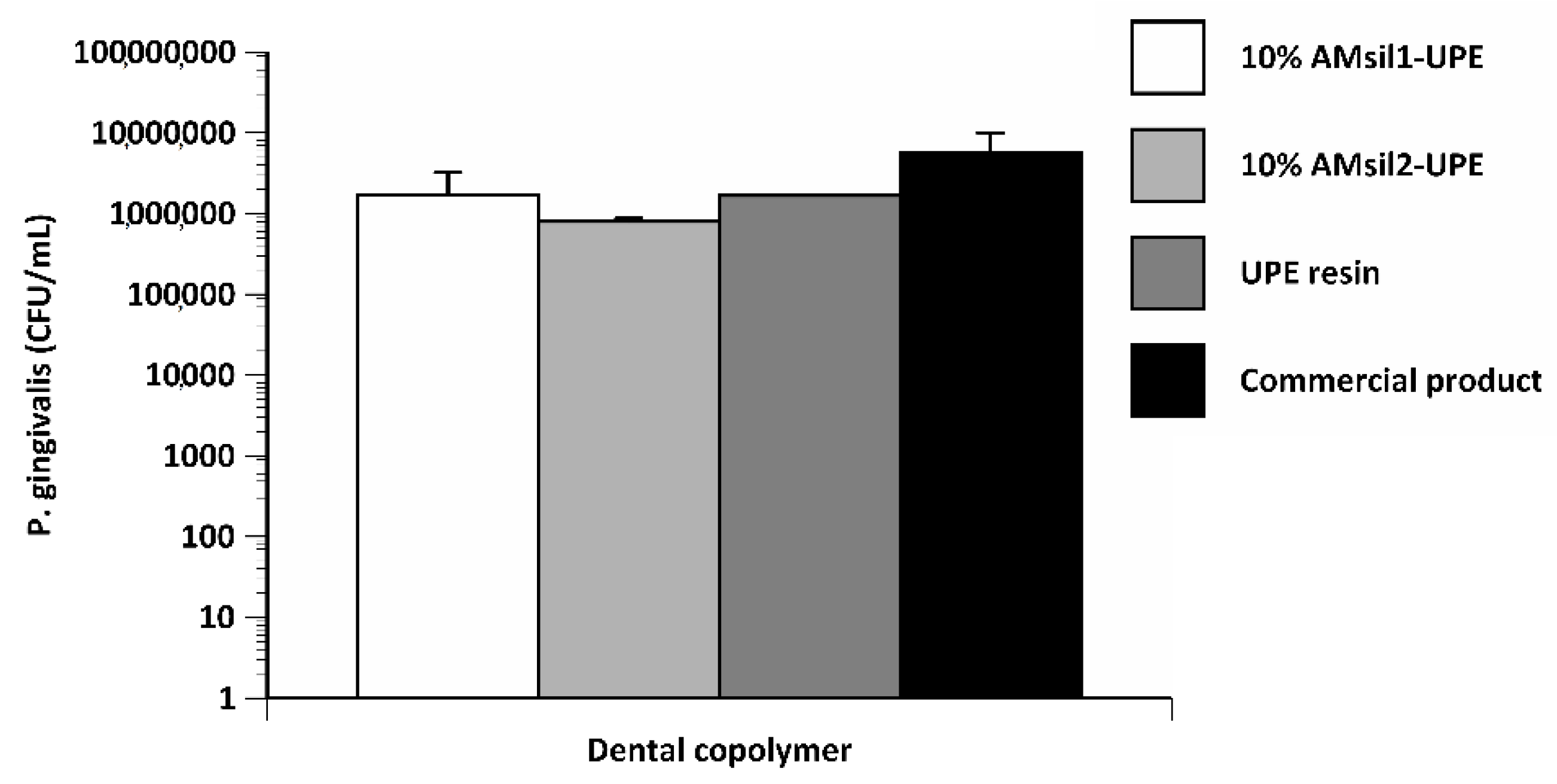
2.6. Bacterial Testing
3. Discussion
4. Materials and Methods
4.1. Monomer Synthesis
4.2. Structural Verification
4.3. Experimental Resin Formulation
4.4. Biocompatibility Tests
4.5. Contact Angle (CA)
4.6. Copolymer Specimen Preparation
4.7. Degree of Vinyl Conversion (DVC)
4.8. Mechanical Properties of Copolymers
4.9. Bacterial Testing
4.9.1. Planktonic
4.9.2. Biofilm
4.10. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| ACP | amorphous calcium phosphate |
| AM | antimicrobial |
| AMRE | antimicrobial and remineralizing |
| AMsil1 | N-(2-(methacryloyloxy)ethyl-N,N-dimethyl-3-(trimethoxysilyl)propan-1-aminium iodide |
| AMsil2 | N-(2-(methacryloyloxy)ethyl-N,N-dimethyl-3-(trimethoxysilyl)undecan-1-aminium bromide |
| Bis-GMA | 2,2-bis[p-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane |
| BrUDTMS | (11-bromoundecyl)trimethoxy silane |
| CA | contact angle |
| CCL1 | immortalized mouse subcutaneous connective tissue fibroblasts |
| CQ | camphorquinone |
| DMAEMA | 2-(dimethylamino)ethyl methacrylate |
| DVC | degree of vinyl conversion |
| 4EDMAB | ethyl-4-N,N-dimethylamino benzoate |
| EHMA | ethyl 2-(hydroxymethyl) acrylate |
| HGF | human gingival fibroblasts |
| IPTMS | (3-iodopropyl)trimethoxy silane |
| MDBP | methacryloyloxydodecyl pyrimidinium bromide |
| NMR | nuclear magnetic resonance |
| PEG-U | poly(ethylene glycol)-extended UDMA |
| QA | quaternary ammonium |
| TEGDMA | triethyleneglycol dimethacrylate |
| UDMA | urethane dimethacrylate |
| UPE | UDMA/PEG-U/EHMA resin |
References
- Gluzman, R.; Katz, R.V.; Frey, B.J.; McGowan, R. Prevention of root caries: A literature review of primary and secondary preventive agents. Spec. Care Dentist. 2013, 33, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L. Resin composite—state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Cenci, T.; Cenci, M.S.; Fedorowicz, Z.; Marchesan, M.A. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [Green Version]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Jedrychowski, J.R.; Caputo, A.A.; Kerper, S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J. Oral Rehabil. 1983, 10, 373–381. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Knetsch, M.L.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Osinaga, P.W.; Grande, R.H.; Ballester, R.Y.; Simionato, M.R.; Delgado Rodrigues, C.R.; Muench, A. Zinc sulfate addition to glass-ionomer-based cements: Influence on physical and antibacterial properties, zinc and fluoride release. Dent. Mater. 2003, 19, 212–217. [Google Scholar] [CrossRef]
- Syafiuddin, T.; Hisamitsu, H.; Toko, T.; Igarashi, T.; Goto, N.; Fujishima, A.; Miyazaki, T. In vitro inhibition of caries around a resin composite restoration containing antibacterial filler. Biomaterials 1997, 18, 1051–1057. [Google Scholar] [CrossRef]
- Takahashi, Y.; Imazato, S.; Kaneshiro, A.V.; Ebisu, S.; Frencken, J.E.; Tay, F.R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent. Mater. 2006, 22, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tanagawa, M.; Atsuta, M. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J. Biomed. Mater. Res. 1999, 47, 516–522. [Google Scholar] [CrossRef]
- Weng, Y.; Guo, X.; Chong, V.J.; Howard, L.; Gregory, R.L.; Xie, D. Synthesis and evaluation of a novel antibacterial dental resin composite with quaternary ammonium salts. J. Biomed. Sci. Eng. 2011, 4, 147. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.; Russell, A.J. Permanent, nonleaching antibacterial surfaces. 1. Synthesis by atom transfer radical polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Thome, T.; Mayer, M.P.; Imazato, S.; Geraldo-Martins, V.R.; Marques, M.M. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J. Dent. 2009, 37, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M. Polymerizable biomedical composition. U.S. Patent 8,217,081 B2, 10 July 2012. [Google Scholar]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef]
- Boskey, A.L. Amorphous calcium phosphate: The contention of bone. J. Dent. Res. 1997, 76, 1433–1436. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3–56. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef]
- Tsuji, T.; Onuma, K.; Yamamoto, A.; Iijima, M.; Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 16866–16870. [Google Scholar] [CrossRef] [Green Version]
- Weiner, S. Transient precursor strategy in mineral formation of bone. Bone 2006, 39, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Sagi, I.; Addadi, L. Structural biology. Choosing the crystallization path less traveled. Science 2005, 309, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Peters, F.; Epple, M. Continuous synthesis of amorphous carbonated apatites. Biomaterials 2002, 23, 2553–2559. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Skrtic, D. Fine-tuning of polymeric resins and their interfaces with amorphous calcium phosphate. A strategy for designing effective remineralizing dental composites. Polymers 2010, 2, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Skrtic, D.; Antonucci, J.M. Dental composites based on amorphous calcium phosphate - resin composition/physicochemical properties study. J. Biomater. Appl. 2007, 21, 375–393. [Google Scholar] [CrossRef] [Green Version]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stan. 2003, 108, 167–182. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D.; Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141–1150. [Google Scholar] [CrossRef]
- Zhang, F.; Allen, A.J.; Levine, L.E.; Vaudin, M.D.; Skrtic, D.; Antonucci, J.M.; Hoffman, K.M.; Giuseppetti, A.A.; Ilavsky, J. Structural and dynamical studies of acid-mediated conversion in amorphous-calcium-phosphate based dental composites. Dent. Mater. 2014, 30, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Okeke, U.C.; Synder, C.R.; Frukhtbeyn, S.A. Synthesis, purification and characterization of polymerizable multifunctional quaternary ammonium compounds. Molecules 2019, 24, 1464. [Google Scholar] [CrossRef] [Green Version]
- Esteban Florez, F.L.; Hiers, R.D.; Smart, K.; Kreth, J.; Qi, F.; Merritt, J.; Khajotia, S.S. Real-time assessment of Streptococcus mutans biofilm metabolism on resin composite. Dent. Mater. 2016, 32, 1263–1269. [Google Scholar] [CrossRef] [Green Version]
- Forssten, S.D.; Bjorklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenesy, K.E. Periodontal disease: An overview for physicians. Mt. Sinai J. Med. 1998, 65, 362–369. [Google Scholar] [PubMed]
- Rafiei, M.; Kiani, F.; Sayehmiri, K.; Sayehmiri, F.; Tavirani, M.; Dousti, M.; Sheikhi, A. Prevalence of Anaerobic Bacteria (P.gingivalis) as Major Microbial Agent in the Incidence Periodontal Diseases by Meta-analysis. J. Dent. 2018, 19, 232–242. [Google Scholar]
- Antonucci, J.M.; Davis, C.H.; Sun, J.; O’Donnell, J.N.; Skrtic, D. Leachability and Cytotoxicity of an Experimental Polymeric ACP Composite. PMSE Prepr. Am. Chem. Soc. Div. Polym. Mater. Sci. Eng. Meet. 2011, 104, 250–252. [Google Scholar]
- Da Silva, E.M.; Almeida, G.S.; Poskus, L.T.; Guimaraes, J.G. Relationship between the degree of conversion, solubility and salivary sorption of a hybrid and a nanofilled resin composite. J. Appl. Oral Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienek, D.R.; Giuseppetti, A.A.; Skrtic, D. Amorphous calcium phosphates as bioactive filler in polymeric dental composites. In Calcium Phosphates-From Fundamentals to Applications; Dutour-Sikiric, M., Furedi-Milhofer, H., Eds.; IntechOpen Limited: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure−activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic biocides with pendant active groups: Molecular weight dependence of antibacterial activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. Molecular weight dependence of antibacterial activity in cationic disinfectants. J. Bioact. Compat. Polym. 1990, 5, 31–41. [Google Scholar] [CrossRef]
- Kapralos, V.; Koutroulis, A.; Ørstavik, D.; Sunde, P.T.; Rukke, H.V. Antibacterial Activity of Endodontic Sealers against Planktonic Bacteria and Bacteria in Biofilms. J. Endod. 2018, 44, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.J.; Ceri, H.; Stremick, C.; Turner, R.J. Differences in biofilm and planktonic cell mediated reduction of metalloid oxyanions. FEMS Microbiol. Lett. 2004, 235, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Frukhtbeyn, S.A.; Giuseppetti, A.A.; Okeke, U.C.; Pires, R.M.; Antonucci, J.M.; Skrtic, D. Ionic dimethacrylates for antimicrobial and remineralizing dental composites. Ann. Dent. Oral Disord. 2018, 1, 108. [Google Scholar]
- Bienek, D.R.; Frukhtbeyn, S.A.; Giuseppetti, A.A.; Okeke, U.C.; Skrtic, D. Antimicrobial monomers for polymeric dental restoratives: Cytotoxicity and physicochemical properties. J. Funct. Biomat. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrtic, D.; Antonucci, J.M. Bioactive polymeric composites for tooth mineral regeneration: Physicochemical and cellular aspects. J. Funct. Biomat. 2011, 2, 271–307. [Google Scholar] [CrossRef] [Green Version]
- Bienek, D.R.; Giuseppetti, A.A.; Okeke, U.C.; Frukhtbeyn, S.A.; Dupree, P.J.; Khajotia, S.S.; Esteban Florez, F.L.; Hiers, R.D.; Skrtic, D. Antimicrobial, biocompatibility, and physicochemical properties of novel adhesive methacrylate dental monomers. J. Bioact. Compat. Polym. 2019, in press. [Google Scholar]
- Merritt, J.; Kreth, J.; Qi, F.; Sullivan, R.; Shi, W. Non-disruptive, real-time analyses of the metabolic status and viability of Streptococcus mutans cells in response to antimicrobial treatments. J. Microbiol. Methods 2005, 61, 161–170. [Google Scholar] [CrossRef]
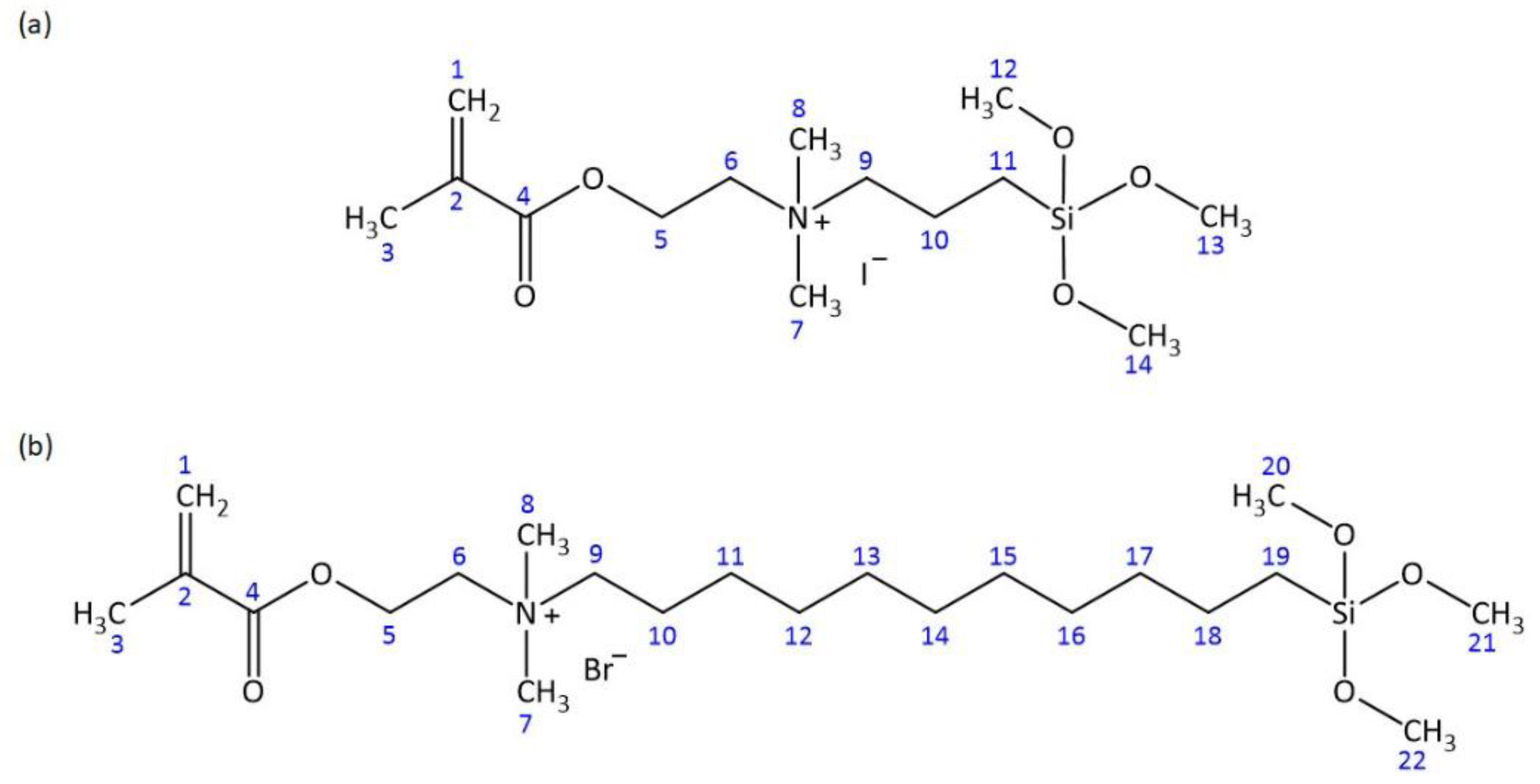
| Atom # | 13C Chemical Shift, ppm | 1H Chemical Shift, ppm | # of H’s | Signal Splitting |
|---|---|---|---|---|
| 1 | 126.6 (CH2) | 5.77, 6.09 | 1, 1 | singlets |
| 2 | 135.3 (C) | 0 | ||
| 3 | 17.8 (CH3) | 1.92 | 3 | singlet |
| 4 | 165.8 (C) | 0 | ||
| 5 | 58.0 (CH2) | 4.52 | 2 | multiplet |
| 6 | 61.7 (CH2) | 3.70 | 2 | multiplet |
| 7, 8 | 50.6 (CH3) | 3.09 | 6 | singlet |
| 9 | 65.9 (CH2) | 3.34 | 2 | multiplet |
| 10 | 15.6 (CH2) | 1.72 | 2 | multiplet |
| 11 | 5.2 (CH2) | 0.54 | 2 | multiplet |
| 12, 13, 14 | 50.1 (CH3) | 3.51 | 9 | singlet |
| Atom # | 13C Chemical Shift, ppm | 1H Chemical Shift, ppm | # of H’s | Signal Splitting |
|---|---|---|---|---|
| 1 | 126.5 (CH2) | 5.76, 6.08 | 1, 1 | singlets |
| 2 | 135.3 (C) | 0 | ||
| 3 | 17.8 (CH3) | 1.91 | 3 | singlet |
| 4 | 165.8 (C) | 0 | ||
| 5 | 58.1 (CH2) | 4.52 | 2 | multiplet |
| 6 | 61.6 (CH2) | 3.70 | 2 | multiplet |
| 7, 8 | 50.4 (CH3) | 3.09 | 6 | singlet |
| 9 | 63.8 (CH2) | 3.36 | 2 | multiplet |
| 10 | 21.7 (CH2) | 1.67 | 2 | multiplet |
| 11–18 | 22.1, 25.7, 28.4, 28.6, 28.7, 28.8, 28.9, 32.3 (CH2) | 1.25 | 16 | multiplet |
| 19 | 8.6 (CH2) | 0.57 | 2 | multiplet |
| 20, 21, 22 | 49.9 (CH3) | 3.46 | 9 | singlet |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienek, D.R.; Giuseppetti, A.A.; Frukhtbeyn, S.A.; Hiers, R.D.; Esteban Florez, F.L.; Khajotia, S.S.; Skrtic, D. Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities. J. Funct. Biomater. 2020, 11, 1. https://doi.org/10.3390/jfb11010001
Bienek DR, Giuseppetti AA, Frukhtbeyn SA, Hiers RD, Esteban Florez FL, Khajotia SS, Skrtic D. Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities. Journal of Functional Biomaterials. 2020; 11(1):1. https://doi.org/10.3390/jfb11010001
Chicago/Turabian StyleBienek, Diane R., Anthony A. Giuseppetti, Stanislav A. Frukhtbeyn, Rochelle D. Hiers, Fernando L. Esteban Florez, Sharukh S. Khajotia, and Drago Skrtic. 2020. "Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities" Journal of Functional Biomaterials 11, no. 1: 1. https://doi.org/10.3390/jfb11010001






