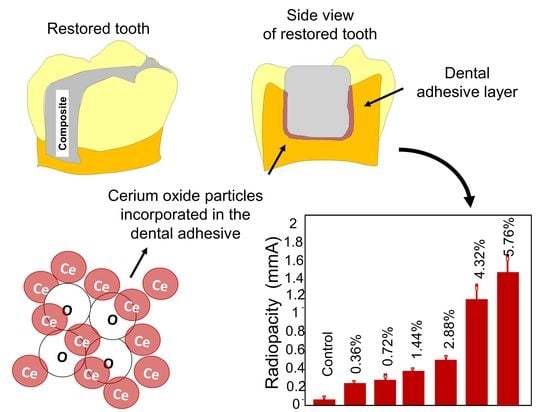
Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
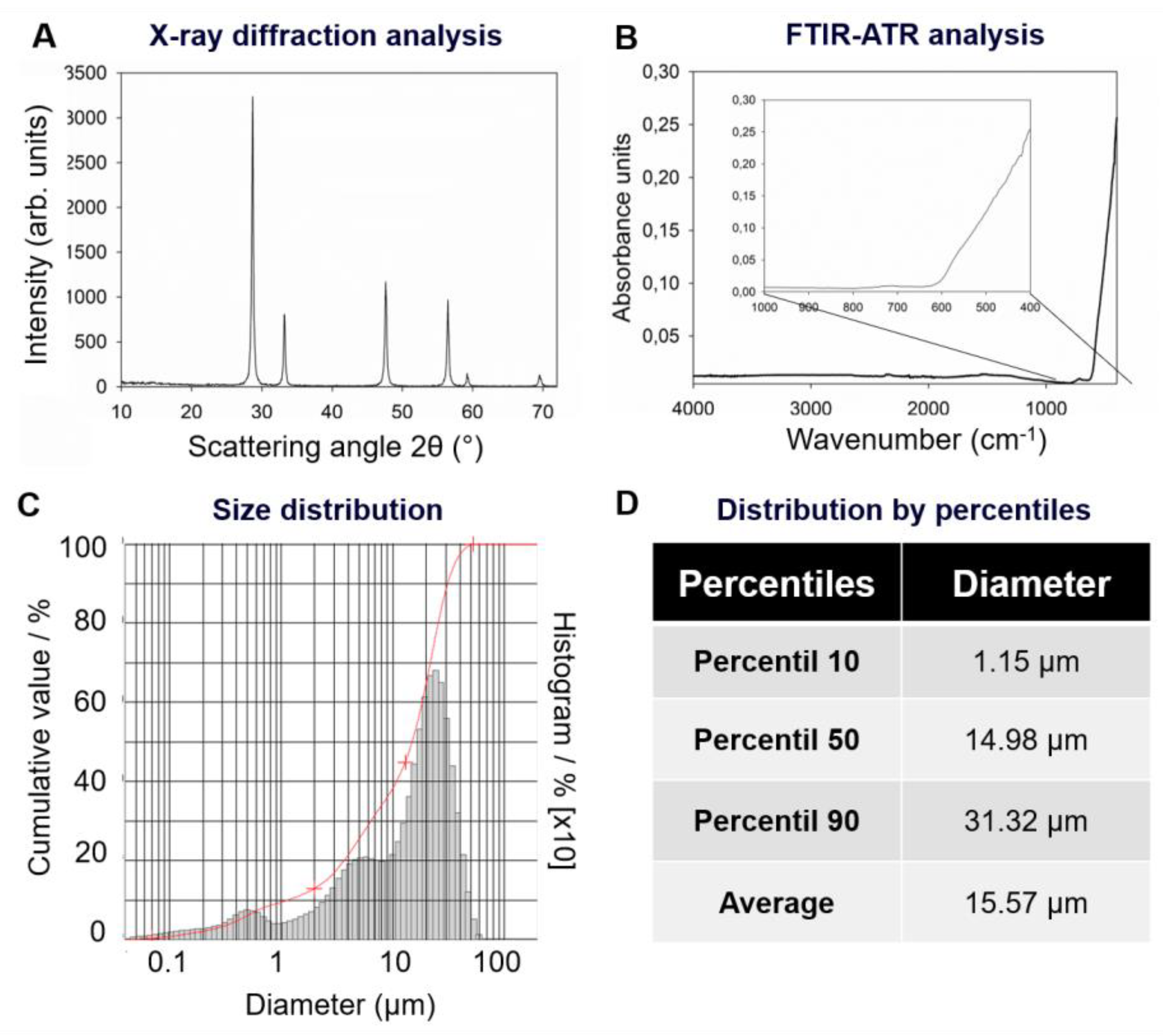
4.1. X-ray Diffraction Analysis of CeO2
4.2. FTIR Analysis of CeO2
4.3. Particle Size Distribution of CeO2
4.4. Preparation of Dental Adhesives
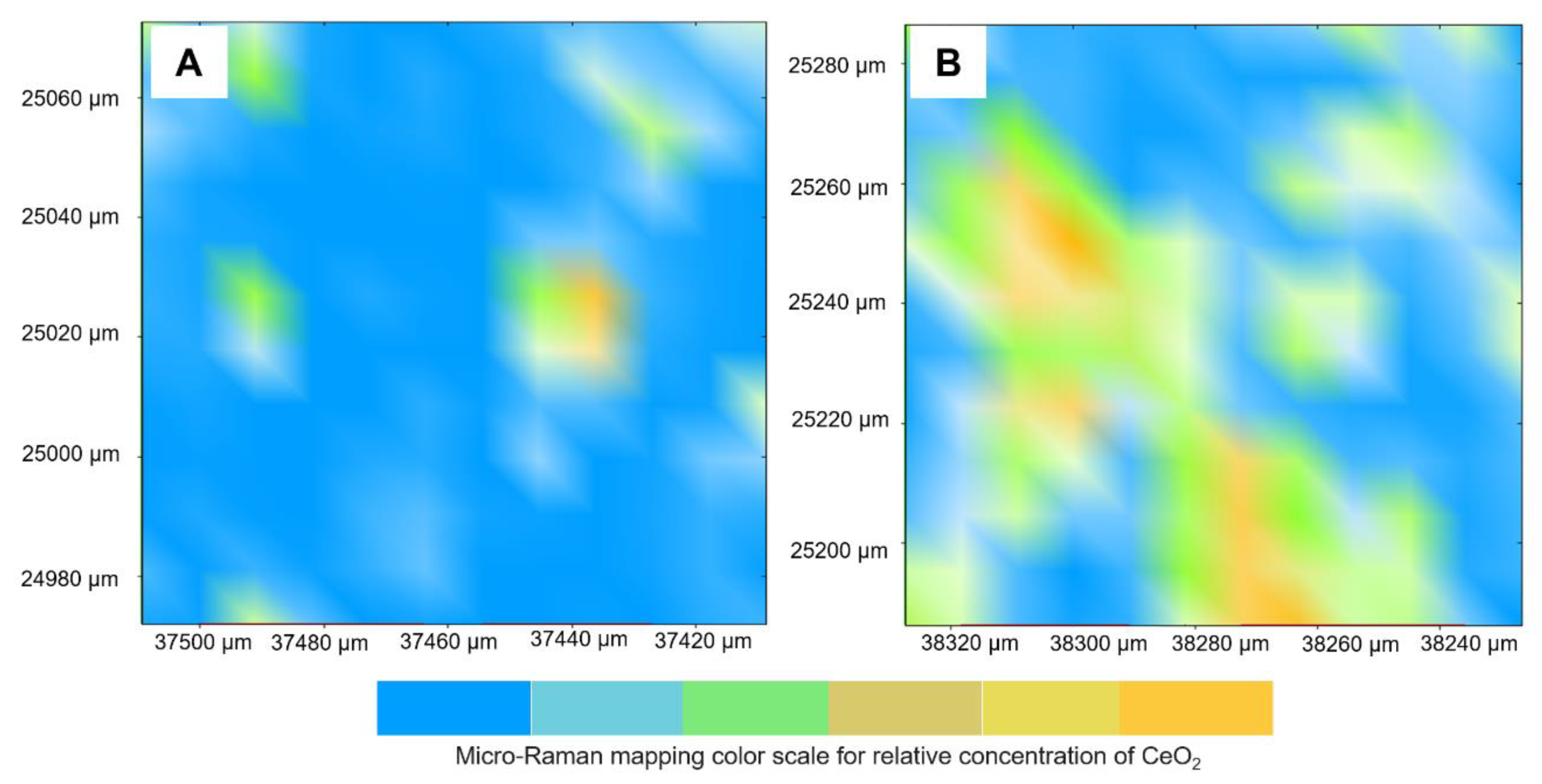
4.5. Qualitative Analysis of CeO2 into Dental Adhesives
4.6. Radiopacity Evaluation
4.7. Degree of Conversion
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper. Dent. 2018, 43, E173–E190. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.A.; Scherrer, S.S.; Ferracane, J.L.; Della Bona, A. Microstructural Characterization and Fracture Behavior of a Microhybrid and a Nanofill Composite. Dent. Mater. 2008, 24, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Oztas, B.; Kursun, S.; Dinc, G.; Kamburoglu, K. Radiopacity evaluation of composite restorative resins and bonding agents using digital and film x-ray systems. Eur. J. Dent. 2012, 6, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.R.; Keenan, A.V. Accuracy of dental radiographs for caries detection. Evid. Based Dent. 2016, 17, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, G.; Ozcan, M. Radiopacity of different shades of resin-based restorative materials compared to human and bovine teeth. Gen. Dent. 2012, 60, e237–e243. [Google Scholar] [PubMed]
- Raitz, R.; Moruzzi, P.D.; Vieira, G.; Fenyo-Pereira, M. Radiopacity of 28 Composite Resins for Teeth Restorations. J. Contemp. Dent. Pract. 2016, 17, 136–142. [Google Scholar] [CrossRef]
- Brouwer, F.; Askar, H.; Paris, S.; Schwendicke, F. Detecting Secondary Caries Lesions: A Systematic Review and Meta-analysis. J. Dent. Res. 2016, 95, 143–151. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Takahashi, H.; Iwasaki, N.; Honda, E.; Kurabayashi, T. Radiopacity of experimental composite resins containing radiopaque materials. Dent. Mater. J. 2005, 24, 315–320. [Google Scholar] [CrossRef][Green Version]
- Schwendicke, F.; Tzschoppe, M.; Paris, S. Radiographic caries detection: A systematic review and meta-analysis. J. Dent. 2015, 43, 924–933. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef]
- Espelid, I.; Tveit, A.B.; Erickson, R.L.; Keck, S.C.; Glasspoole, E.A. Radiopacity of restorations and detection of secondary caries. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1991, 7, 114–117. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent. Mater. 2013, 29, 231–240. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Cerium Oxide Nanostructures and their Applications. Funct. Nanomater. 2016, 32, 521–529. [Google Scholar]
- Scire, S.; Palmisano, L. Cerium Oxide (CeO2): Synthesis, Properties and Applications—1st Edition. Available online: https://www.elsevier.com/books/cerium-oxide-ceo2-synthesis-properties-and-applications/scire/978-0-12-815661-2 (accessed on 13 December 2019).
- Hosseini, A.; Sharifi, A.M.; Abdollahi, M.; Najafi, R.; Baeeri, M.; Rayegan, S.; Cheshmehnour, J.; Hassani, S.; Bayrami, Z.; Safa, M. Cerium and yttrium oxide nanoparticles are neuroprotective. Biol. Trace Elem. Res. 2015, 164, 80–89. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles—A Review. Biotechnol. Rep. 2017, 17, 1–5. [Google Scholar] [CrossRef]
- Akça, B.; Erzeneoğlu, S.Z. The Mass Attenuation Coefficients, Electronic, Atomic, and Molecular Cross Sections, Effective Atomic Numbers, and Electron Densities for Compounds of Some Biomedically Important Elements at 59.5 keV. Available online: https://www.hindawi.com/journals/stni/2014/901465/ (accessed on 14 January 2020).
- Mullan, B.F.; Madsen, M.T.; Messerle, L.; Kolesnichenko, V.; Kruger, J. X-ray attenuation coefficients of high-atomic-number, hexanuclear transition metal cluster compounds: A new paradigm for radiographic contrast agents. Acad. Radiol. 2000, 7, 254–259. [Google Scholar] [CrossRef]
- Leitune, V.C.B.; Collares, F.M.; Takimi, A.; de Lima, G.B.; Petzhold, C.L.; Bergmann, C.P.; Samuel, S.M.W. Niobium pentoxide as a novel filler for dental adhesive resin. J. Dent. 2013, 41, 106–113. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Ferreira, C.J.; Collares, F.M. Tantalum oxide as filler for dental adhesive resin. Dent. Mater. J. 2018, 37, 897–903. [Google Scholar] [CrossRef]
- Gu, S.; Rasimick, B.J.; Deutsch, A.S.; Musikant, B.L. Radiopacity of dental materials using a digital X-ray system. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2006, 22, 765–770. [Google Scholar] [CrossRef]
- Demarco, F.F.; Collares, K.; Correa, M.B.; Cenci, M.S.; de Moraes, R.R.; Opdam, N.J. Should my composite restorations last forever? Why are they failing? Braz. Oral Res. 2017, 31, e56. [Google Scholar] [CrossRef]
- Kanzow, P.; Wiegand, A.; Schwendicke, F. Cost-effectiveness of repairing versus replacing composite or amalgam restorations. J. Dent. 2016, 54, 41–47. [Google Scholar] [CrossRef]
- 14:00–17:00 ISO 4049:2009. Available online: http://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/28/42898.html (accessed on 16 January 2020).
- Belli, R.; Kreppel, S.; Petschelt, A.; Hornberger, H.; Boccaccini, A.R.; Lohbauer, U. Strengthening of dental adhesives via particle reinforcement. J. Mech. Behav. Biomed. Mater. 2014, 37, 100–108. [Google Scholar] [CrossRef]
- Abdalla, A.I.; Feilzer, A.J. Four-year water degradation of a total-etch and two self-etching adhesives bonded to dentin. J. Dent. 2008, 36, 611–617. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; de Oliveira Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef]
- Marins, N.H.; Meereis, C.T.W.; Silva, R.M.; Ruas, C.P.; Takimi, A.S.; Carreño, N.L.V.; Ogliari, F.A. Radiopaque dental adhesive with addition of niobium pentoxide nanoparticles. Polym. Bull. 2018, 75, 2301–2314. [Google Scholar] [CrossRef]
- Debnath, S.; Islam, M.R.; Khan, M.S.R. Optical properties of CeO2 thin films. Bull. Mater. Sci. 2007, 30, 315–319. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Watts, D.C.; Lassila, L.V.J. Effect of nanofiller fractions and temperature on polymerization shrinkage on glass fiber reinforced filling material. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 606–610. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Bramante, C.M.; García-Godoy, F.; Moldauer, B.I.; Gagliardi Minotti, P.; Tercília Grizzo, L.; Duarte, M.A.H. The effect of radiopacifiers agents on pH, calcium release, radiopacity, and antimicrobial properties of different calcium hydroxide dressings. Microsc. Res. Tech. 2015, 78, 620–625. [Google Scholar] [CrossRef]
- Martins, G.C.; Reis, A.; Loguercio, A.D.; Zander-Grande, C.; Meier, M.; Mazur, R.F.; Gomes, O.M.M. Does Making An Adhesive System Radiopaque by Filler Addition Affect Its Bonding Properties? J. Adhes. Dent. 2015, 17, 513–519. [Google Scholar]
- Collares, F.M.; Ogliari, F.A.; Lima, G.S.; Fontanella, V.R.C.; Piva, E.; Samuel, S.M.W. Ytterbium trifluoride as a radiopaque agent for dental cements. Int. Endod. J. 2010, 43, 792–797. [Google Scholar] [CrossRef]
- Farias, I.A.P.; Dos Santos, C.C.L.; Sampaio, F.C. Antimicrobial Activity of Cerium Oxide Nanoparticles on Opportunistic Microorganisms: A Systematic Review. BioMed Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef]
- Varini, E.; Sánchez-Salcedo, S.; Malavasi, G.; Lusvardi, G.; Vallet-Regí, M.; Salinas, A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: Bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 109971. [Google Scholar] [CrossRef]
- Walkey, C.; Das, S.; Seal, S.; Erlichman, J.; Heckman, K.; Ghibelli, L.; Traversa, E.; McGinnis, J.F.; Self, W.T. Catalytic Properties and Biomedical Applications of Cerium Oxide Nanoparticles. Environ. Sci. Nano 2015, 2, 33–53. [Google Scholar] [CrossRef]
- Gaglianone, L.A.; Lima, A.F.; Gonçalves, L.S.; Cavalcanti, A.N.; Aguiar, F.H.B.; Marchi, G.M. Mechanical properties and degree of conversion of etch-and-rinse and self-etch adhesive systems cured by a quartz tungsten halogen lamp and a light-emitting diode. J. Mech. Behav. Biomed. Mater. 2012, 12, 139–143. [Google Scholar] [CrossRef]
- Maktabi, H.; Ibrahim, M.; Alkhubaizi, Q.; Weir, M.; Xu, H.; Strassler, H.; Fugolin, A.P.P.; Pfeifer, C.S.; Melo, M.A.S. Underperforming light curing procedures trigger detrimental irradiance-dependent biofilm response on incrementally placed dental composites. J. Dent. 2019, 88, 103110. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, I.M.; Leitune, V.C.B.; Takimi, A.S.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Collares, F.M. Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation. J. Funct. Biomater. 2020, 11, 7. https://doi.org/10.3390/jfb11010007
Garcia IM, Leitune VCB, Takimi AS, Bergmann CP, Samuel SMW, Melo MA, Collares FM. Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation. Journal of Functional Biomaterials. 2020; 11(1):7. https://doi.org/10.3390/jfb11010007
Chicago/Turabian StyleGarcia, Isadora Martini, Vicente Castelo Branco Leitune, Antonio Shigueaki Takimi, Carlos Pérez Bergmann, Susana Maria Werner Samuel, Mary Anne Melo, and Fabrício Mezzomo Collares. 2020. "Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation" Journal of Functional Biomaterials 11, no. 1: 7. https://doi.org/10.3390/jfb11010007
APA StyleGarcia, I. M., Leitune, V. C. B., Takimi, A. S., Bergmann, C. P., Samuel, S. M. W., Melo, M. A., & Collares, F. M. (2020). Cerium Dioxide Particles to Tune Radiopacity of Dental Adhesives: Microstructural and Physico-Chemical Evaluation. Journal of Functional Biomaterials, 11(1), 7. https://doi.org/10.3390/jfb11010007