Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro
Abstract
1. Introduction
2. Results
2.1. Wet Compressive Strength of PMC Formulations
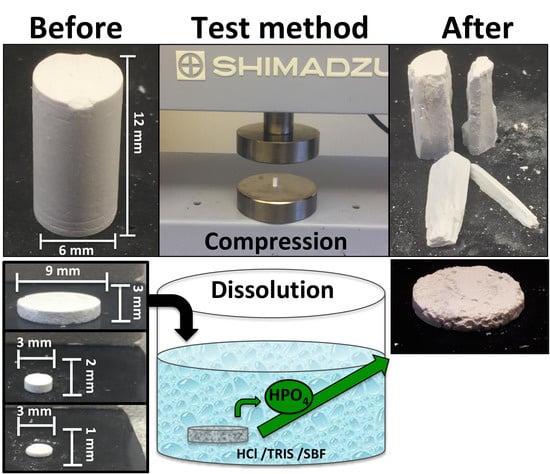
2.1.1. Sample Formulations for Compression Testing
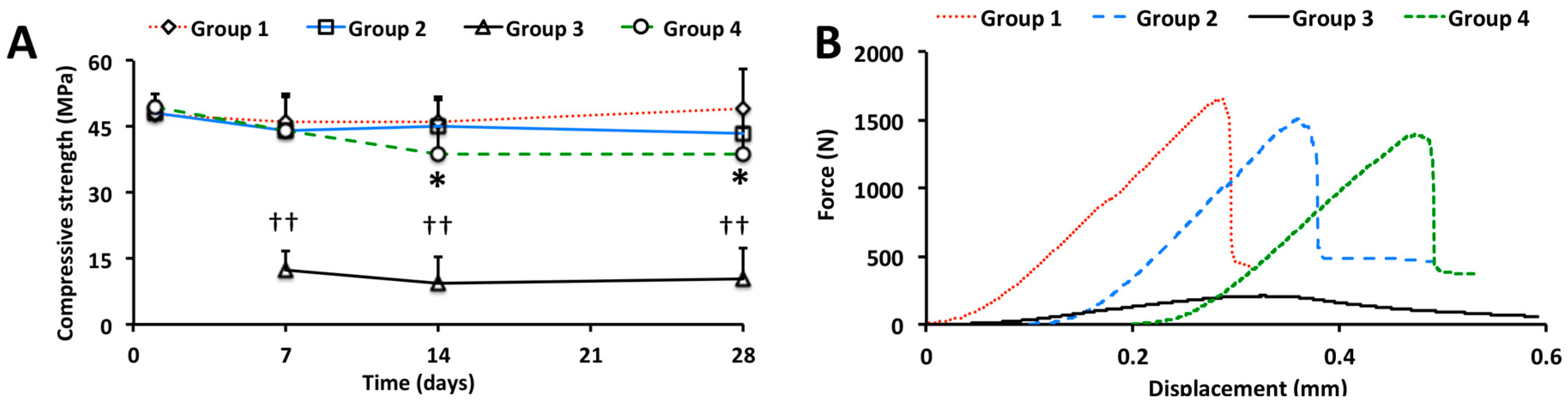
2.1.2. Compressive Strength
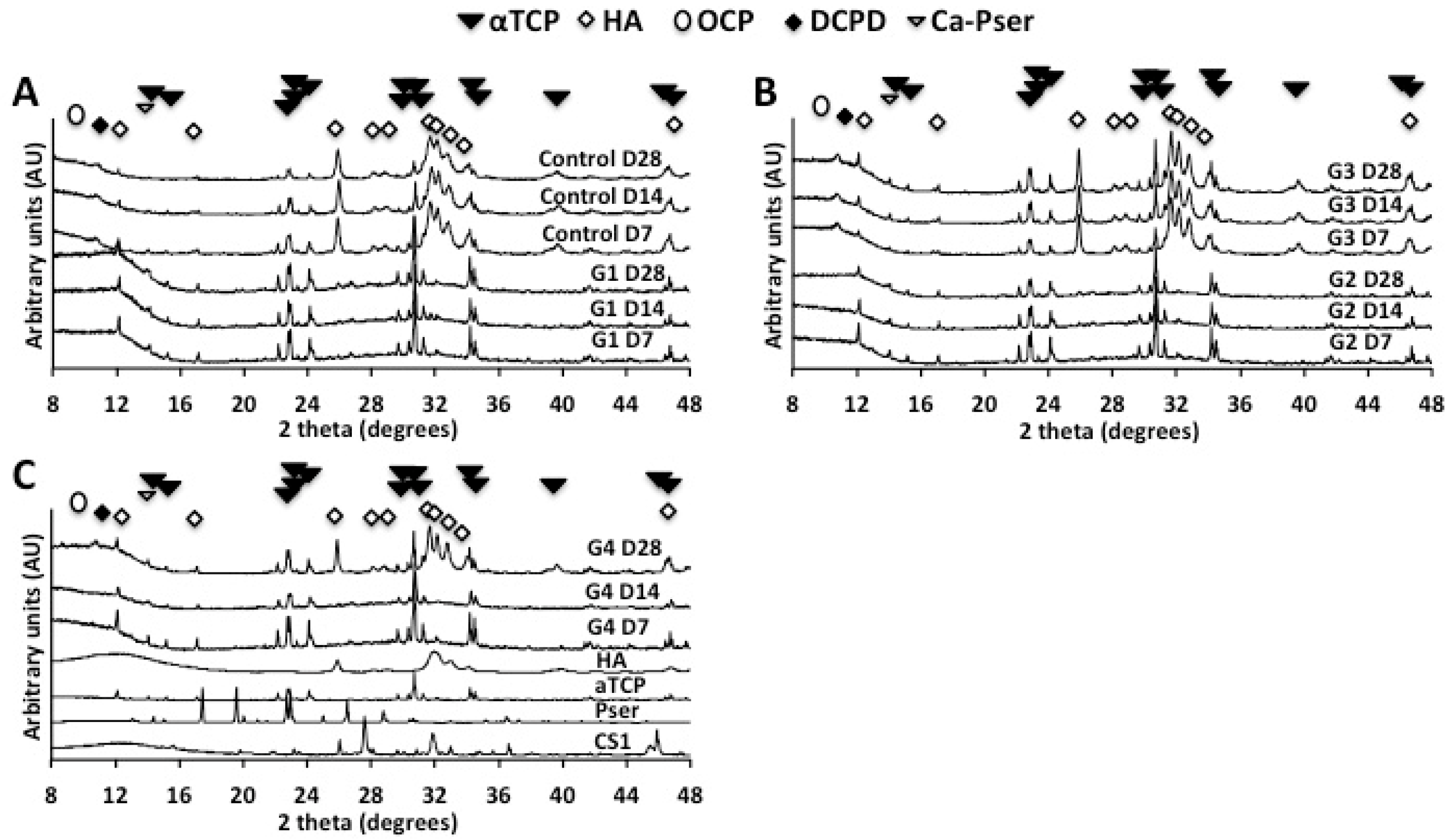
2.1.3. X-Ray Diffraction (XRD)
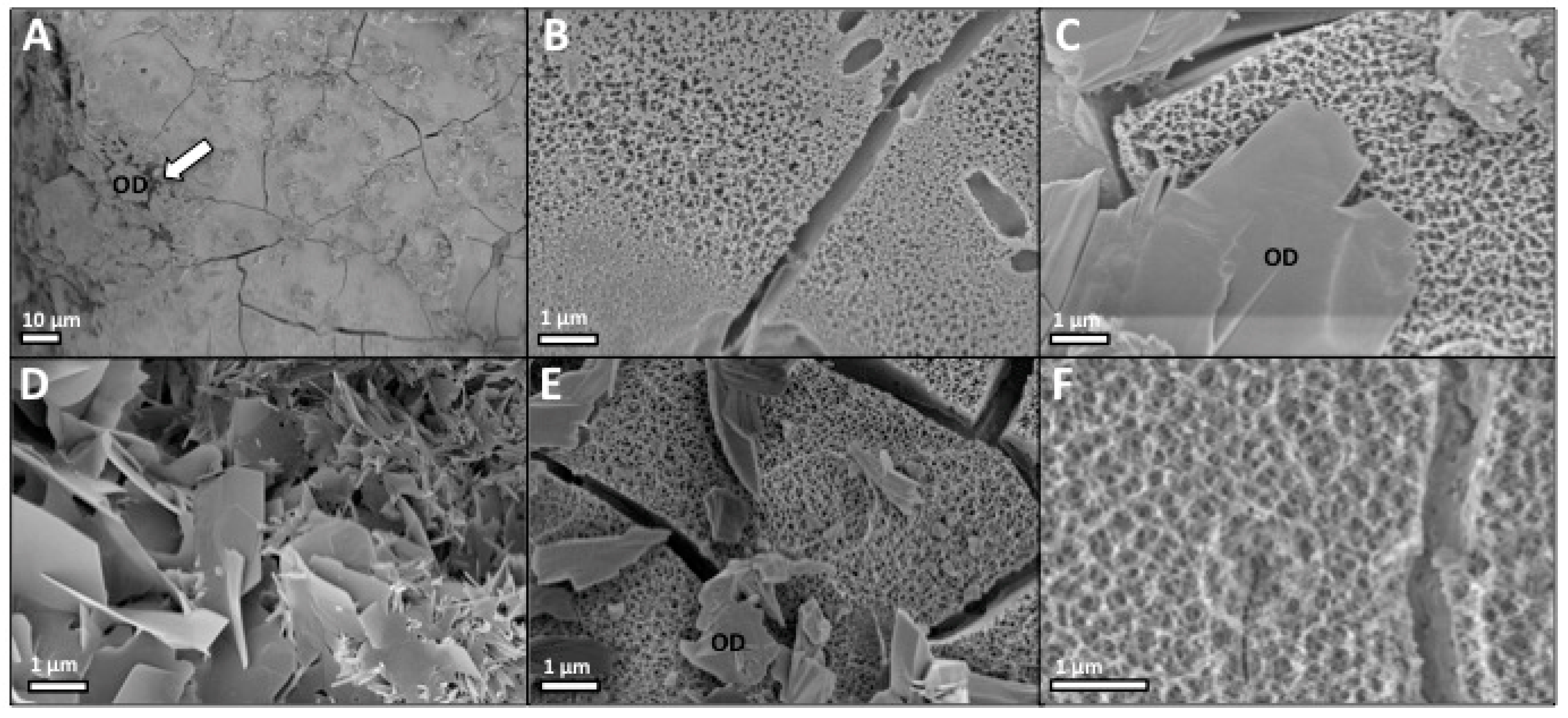
2.1.4. Scanning Electron Microscopy (SEM)
2.2. Dissolution and Reprecipitation on PMC Discs
2.2.1. Dissolution in Varied Surface Area to Volume Ratios (SVR)
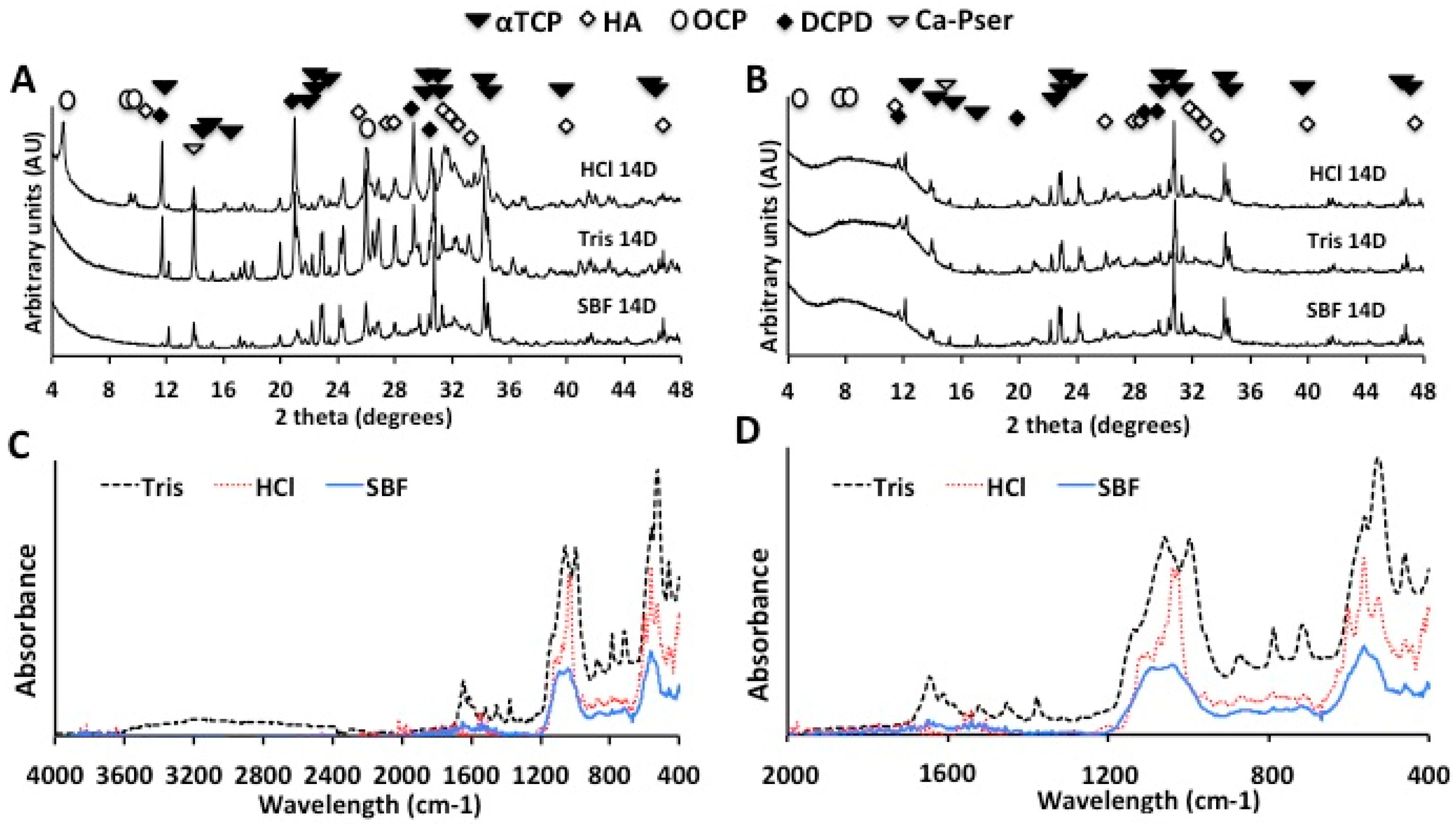
2.2.2. X-Ray Diffraction and Infrared Spectroscopy (FTIR-ATR) Analysis
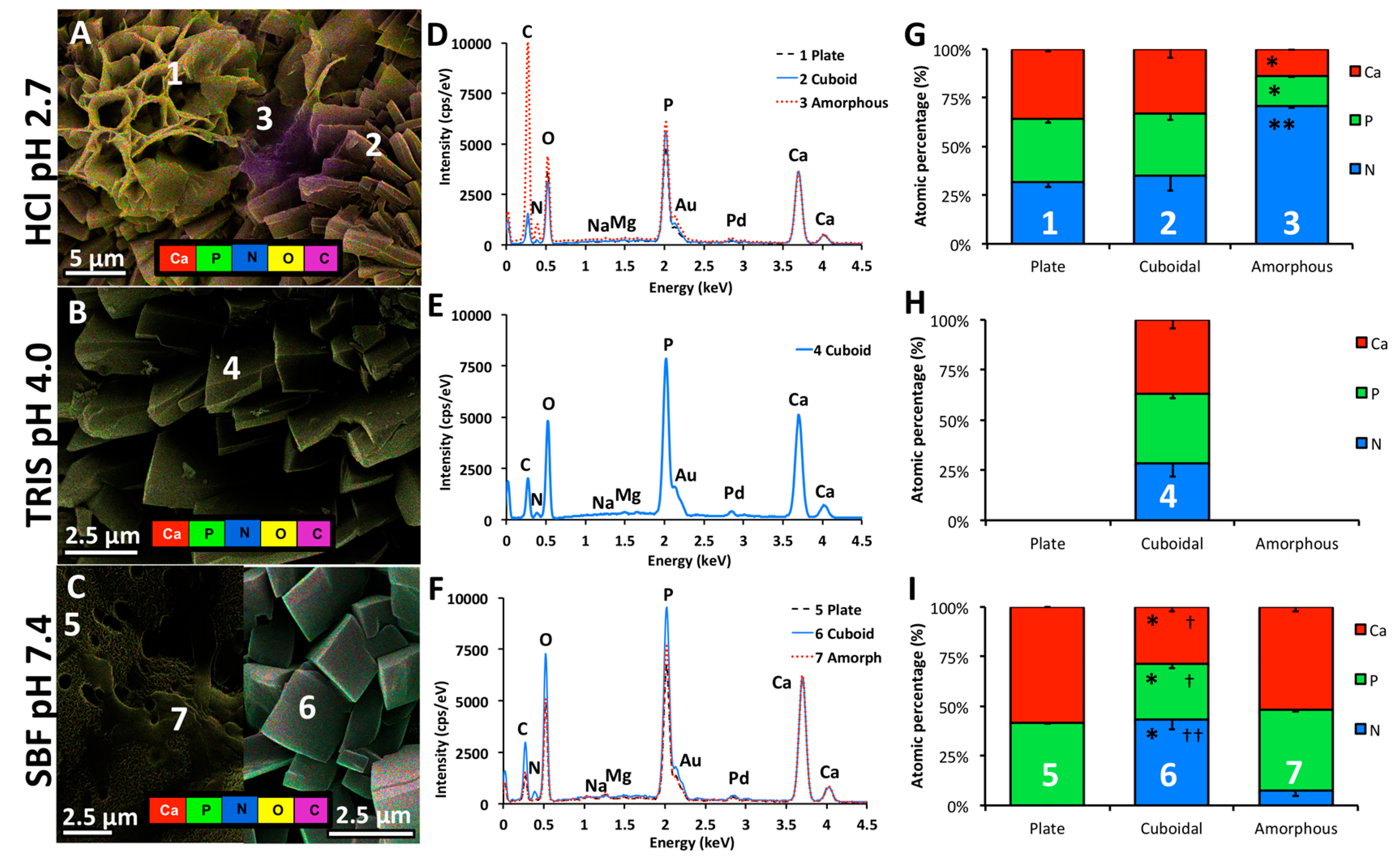
2.2.3. Scanning Electron Microscopy
2.2.4. Energy Dispersive X-Ray Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Material Synthesis and Milling
4.3. Material Chracterization
4.4. Sample Preparation
4.5. Mechanical Testing
4.6. Dissolution Testing
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Luo, J.; Faivre, J.; Engqvist, H.; Persson, C. The Addition of Poly(Vinyl Alcohol) Fibers to Apatitic Calcium Phosphate Cement Can Improve Its Toughness. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Sergey, V.D. Calcium Orthophosphate (CaPO4) Scaffolds for Bone Tissue Engineering Applications. J. Biotechnol. Biomed. Sci. 2018, 1, 25–93. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lin, A.Y.M.; Lin, Y.S.; Seki, Y.; Stokes, A.G.; Peyras, J.; Olevsky, E.A.; Meyers, M.A.; McKittrick, J. Structure and mechanical properties of selected biological materials. J. Mech. Behav. Biomed. Mater. 2008, 1, 208–226. [Google Scholar] [CrossRef]
- Libonati, F.; Buehler, M.J. Advanced Structural Materials by Bioinspiration. Adv. Eng. Mater. 2017, 19, 1600787. [Google Scholar] [CrossRef]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C.G. Interactions Between Inorganic and Organic Phases in Bone Tissue as a Source of Inspiration for Design of Novel Nanocomposites. Tissue Eng. Part B Rev. 2013, 20, 173–188. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Wise, E.R.; Maltsev, S.; Davies, M.E.; Duer, M.J.; Jaeger, C.; Loveridge, N.; Murray, R.C.; Reid, D.G. The Organic−Mineral Interface in Bone Is Predominantly Polysaccharide. Chem. Mater. 2007, 19, 5055–5057. [Google Scholar] [CrossRef]
- Currey, J.D. The structure and mechanics of bone. J. Mater. Sci. 2012, 47, 41–54. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning hardness in calcite by incorporation of amino acids. Nat. Mater. 2016, 15, 903. [Google Scholar] [CrossRef]
- Addadi, L.; Gal, A.; Faivre, D.; Scheffel, A.; Weiner, S. Control of Biogenic Nanocrystal Formation in Biomineralization. Israel J. Chem. 2016, 56, 227–241. [Google Scholar] [CrossRef]
- Barralet, J.E.; Tremayne, M.; Lilley, K.J.; Gbureck, U. Modification of Calcium Phosphate Cement with α-Hydroxy Acids and Their Salts. Chem. Mater. 2005, 17, 1313–1319. [Google Scholar] [CrossRef]
- Bohner, M.; Merkle, H.P.; Landuyt, P.V.; Trophardy, G.; Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci. Mater. Med. 2000, 11, 111–116. [Google Scholar] [CrossRef]
- Jang, J.-H.; Shin, S.; Kim, H.-J.; Jeong, J.; Jin, H.-E.; Desai, M.S.; Lee, S.-W.; Kim, S.-Y. Improvement of physical properties of calcium phosphate cement by elastin-like polypeptide supplementation. Sci. Rep. 2018, 8, 5216. [Google Scholar] [CrossRef]
- Mirtchi, A.A.; Lemaître, J.; Hunting, E. Calcium phosphate cements: Action of setting regulators on the properties of the β-tricalcium phosphate-monocalcium phosphate cements. Biomaterials 1989, 10, 634–638. [Google Scholar] [CrossRef]
- Barati, D.; Walters, J.D.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Jabbari, E. Effect of Organic Acids on Calcium Phosphate Nucleation and Osteogenic Differentiation of Human Mesenchymal Stem Cells on Peptide Functionalized Nanofibers. Langmuir 2015, 31, 5130–5140. [Google Scholar] [CrossRef]
- Chung, J.; Granja, I.; Taylor, M.G.; Mpourmpakis, G.; Asplin, J.R.; Rimer, J.D. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 2016, 536, 446. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Nat. Acad. Sci. USA 2010, 107, 22425. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Wang, L.; Wang, B.; Putnis, C.V. Mechanisms of Modulation of Calcium Phosphate Pathological Mineralization by Mobile and Immobile Small-Molecule Inhibitors. J. Phys. Chem. B 2018, 122, 1580–1587. [Google Scholar] [CrossRef]
- Pastero, L.; Bruno, M.; Aquilano, D. Habit Change of Monoclinic Hydroxyapatite Crystals Growing from Aqueous Solution in the Presence of Citrate Ions: The Role of 2D Epitaxy. Crystals 2018, 8. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; De Oliveira, L.C.; Rigo, E.C.S.; Carrodeguas, R.G.; Boschi, A.O.; De Arruda, A.C.F. Influence of polymeric additives on the mechanical properties of α-tricalcium phosphate cement. Bone 1999, 25, 99S–102S. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Sun, J.-Y.; Kim, H.-E. Dual-Crosslinking of Hyaluronic Acid–Calcium Phosphate Nanocomposite Hydrogels for Enhanced Mechanical Properties and Biological Performance. Macromol. Mater. Eng. 2017, 302, 1700160. [Google Scholar] [CrossRef]
- Kozłowska, J.; Sionkowska, A. Effects of different crosslinking methods on the properties of collagen–calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.-W.; Ginebra, M.-P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3. [Google Scholar] [CrossRef]
- Bohner, M.; Brunner, T.J.; Stark, W.J. Controlling the reactivity of calcium phosphate cements. J. Mater. Chem. 2008, 18, 5669–5675. [Google Scholar] [CrossRef]
- Bohner, M.; Malsy, A.K.; Camiré, C.L.; Gbureck, U. Combining particle size distribution and isothermal calorimetry data to determine the reaction kinetics of α-tricalcium phosphate–water mixtures. Acta Biomater. 2006, 2, 343–348. [Google Scholar] [CrossRef]
- Unosson, J.; Engqvist, H. Development of a Resorbable Calcium Phosphate Cement with Load Bearing Capacity. Bioceram. Dev. Appl. 2014, 4. [Google Scholar] [CrossRef]
- Bohner, M. Reactivity of calcium phosphate cements. J. Mater. Chem. 2007, 17, 3980–3986. [Google Scholar] [CrossRef]
- Witek, L.; Smay, J.; Silva, N.R.F.A.; Guda, T.; Ong, J.L.; Coelho, P.G. Sintering effects on chemical and physical properties of bioactive ceramics. J. Adv. Ceram. 2013, 2, 274–284. [Google Scholar] [CrossRef]
- Khashaba, R.M.; Moussa, M.M.; Mettenburg, D.J.; Rueggeberg, F.A.; Chutkan, N.B.; Borke, J.L. Polymeric-Calcium Phosphate Cement Composites-Material Properties: In Vitro and In Vivo Investigations. Int. J. Biomater. 2010, 2010, 14. [Google Scholar] [CrossRef]
- Mickiewicz, R.A.; Mayes, A.M.; Knaack, D. Polymer—Calcium phosphate cement composites for bone substitutes. J. Biomed. Mater. Res. 2002, 61, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bartolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and beta-tri-Calcium Phosphate. Materials 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Victor, S.P.; Komeri, R.; Selvam, S.; Muthu, J. Covalently cross-linked hydroxyapatite–citric acid–based biomimetic polymeric composites for bone applications. J. Bioactive Comp. Polym. 2015, 30, 524–540. [Google Scholar] [CrossRef]
- Jahromi, M.T.; Yao, G.; Cerruti, M. The importance of amino acid interactions in the crystallization of hydroxyapatite. J. R. Soc. Interface 2013, 10, 20120906. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pacella, M.S.; Athanasiadou, D.; Nelea, V.; Vali, H.; Hazen, R.M.; Gray, J.J.; McKee, M.D. Chiral acidic amino acids induce chiral hierarchical structure in calcium carbonate. Nat. Commun. 2017, 8, 15066. [Google Scholar] [CrossRef]
- Manoli, F.; Kanakis, J.; Malkaj, P.; Dalas, E. The effect of aminoacids on the crystal growth of calcium carbonate. J. Cryst. Growth 2002, 236, 363–370. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Zhao, W.; Sahai, N. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 2015, 3, 9157–9167. [Google Scholar] [CrossRef]
- Mai, R.; Lux, R.; Proff, P.; Lauer, G.; Pradel, W.; Leonhardt, H.; Reinstorf, A.; Gelinsky, M.; Jung, R.; Eckelt, U.; et al. O-phospho-l-serine: A modulator of bone healing in calcium-phosphate cements. Biomed. Eng. 2008, 53, 229. [Google Scholar] [CrossRef]
- Reinstorf, A.; Ruhnow, M.; Gelinsky, M.; Pompe, W.; Hempel, U.; Wenzel, K.W.; Simon, P. Phosphoserine—A convenient compound for modification of calcium phosphate bone cement collagen composites. J. Mater. Sci. Mater. Med. 2004, 15, 451–455. [Google Scholar] [CrossRef]
- Chatzipanagis, K.; Iafisco, M.; Roncal-Herrero, T.; Bilton, M.; Tampieri, A.; Kröger, R.; Delgado-López, J.M. Crystallization of citrate-stabilized amorphous calcium phosphate to nanocrystalline apatite: A surface-mediated transformation. Cryst. Eng. Comm. 2016, 18, 3170–3173. [Google Scholar] [CrossRef]
- Rubini, K.; Boanini, E.; Bigi, A. Role of Aspartic and Polyaspartic Acid on the Synthesis and Hydrolysis of Brushite. J. Funct. Biomater. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bentov, S.; Weil, S.; Glazer, L.; Sagi, A.; Berman, A. Stabilization of amorphous calcium carbonate by phosphate rich organic matrix proteins and by single phosphoamino acids. J. Struct. Biol. 2010, 171, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Ajili, W.; Chan-Chang, T.-H.-C.; Delices, A.; Laurent, G.; Babonneau, F.; Nassif, N.; Azaïs, T. Amorphous surface layer versus transient amorphous precursor phase in bone—A case study investigated by solid-state NMR spectroscopy. Acta Biomater. 2017, 59, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.-M.; et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.P.; Aikawa, E. Role of Extracellular Vesicles in De Novo Mineralization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1753–1758. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Thurner, P.J.; Lam, S.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. Localization of Phosphorylated Serine, Osteopontin, and Bone Sialoprotein on Bone Fracture Surfaces. J. Adh. 2009, 85, 526–545. [Google Scholar] [CrossRef]
- Merolli, A.; Santin, M. Role of Phosphatidyl-Serine in Bone Repair and Its Technological Exploitation. Molecules 2009, 14. [Google Scholar] [CrossRef]
- Aoba, T.; Moreno, E.C.; Hay, D.I. Inhibition of apatite crystal growth by the amino-terminal segment of human salivary acidic proline-rich proteins. Calcif. Tissue Int. 1984, 36, 651–658. [Google Scholar] [CrossRef]
- Valente, M.T.; Moffa, E.B.; Crosara, K.T.B.; Xiao, Y.; de Oliveira, T.M.; Machado, M.A.d.A.M.; Siqueira, W.L. Acquired Enamel Pellicle Engineered Peptides: Effects on Hydroxyapatite Crystal Growth. Sci. Rep. 2018, 8, 3766. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, M.M.; Arêas, J.A.G. Dependence of the Interfacial Behavior of β-Casein on Phosphoserine Residues. J. Dairy Sci. 2003, 86, 3876–3880. [Google Scholar] [CrossRef]
- Hindmarsh, J.P.; Watkinson, P. Experimental evidence for previously unclassified calcium phosphate structures in the casein micelle. J. Dairy Sci. 2017, 100, 6938–6948. [Google Scholar] [CrossRef] [PubMed]
- Benaziz, L.; Barroug, A.; Legrouri, A.; Rey, C.; Lebugle, A. Adsorption of O-Phospho-L-Serine and L-Serine onto Poorly Crystalline Apatite. J. Colloid Interfaces Sci. 2001, 238, 48–53. [Google Scholar] [CrossRef]
- Shimabayashi, S.; Hashimoto, N.; Kawamura, H.; Uno, T. Formation of Hydroxyapatite in the Presence of Phosphorylated Polyvinylalcohol as a Simplified Model Compound for Mineralization Regulator Phosphoproteins. Chem. Pharmaceut. Bull. 1990, 38, 1810–1814. [Google Scholar] [CrossRef]
- Nelea, V.; Chien, Y.-C.; Paquette, J.; McKee, M.D. Effects of Full-Length Phosphorylated Osteopontin and Constituent Acidic Peptides and Amino Acids on Calcite Dissolution. Cryst. Growth Des. 2014, 14, 979–987. [Google Scholar] [CrossRef]
- Raj, P.A.; Johnsson, M.; Levine, M.J.; Nancollas, G.H. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J. Biol. Chem. 1992, 267, 5968–5976. [Google Scholar]
- Christoffersen, J.; Christoffersen, M.R. Kinetics of dissolution of calcium hydroxyapatite: IV. The effect of some biologically important inhibitors. J. Cryst. Growth 1981, 53, 42–54. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyajima, K.; Nakagaki, M.; Shimabayashi, S. Incongruent dissolution of hydroxyapatite in the presence of phosphoserine. Colloid Polym. Sci. 1991, 269, 161–165. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Guo, H.; Wenner, D.; Autefage, H.; Spicer, D.C.; Stevens, M.M.; Omar, O.; Thomsen, P.; Edén, M.; Insley, G.; et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials 2018, 11. [Google Scholar] [CrossRef]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral-Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, 1800467. [Google Scholar] [CrossRef]
- Procter, P.; Pujari-Palmer, M.; Hulsart-Billström, G.; Wenner, D.; Insley, G.; Larsson, S.; Engqvist, H. A biomechanical test model for evaluating osseous and osteochondral tissue adhesives. BMC Biomed. Eng. 2019, 1, 11. [Google Scholar] [CrossRef]
- Liu, X.; Pujari-Palmer, M.; Wenner, D.; Procter, P.; Insley, G.; Engqvist, H. Adhesive Cements That Bond Soft Tissue Ex Vivo. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.M.; Uskoković, V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro? Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Sogo, Y.; Yamazaki, A.; Aizawa, M.; Osaka, A.; Hayakawa, S.; Kikuchi, M.; Yamashita, K.; Tanaka, Y.; Tadokoro, M.; et al. Interlaboratory studies on in vitro test methods for estimating in vivo resorption of calcium phosphate ceramics. Acta Biomater. 2015, 25, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Newe, C.; Cunningham, E.; Buchanan, F.; Walker, G.; Prendergast, P.; Lennon, A.; Dunne, N.J. Static and Dynamic Degradation of Sintered Calcium Phosphate Ceramics. Key Eng. Mater. 2012, 493–494, 861–865. [Google Scholar] [CrossRef]
- Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H. Composition of Alfa-Tcp, Silicate and Phosphorylated Amino Acid. European Patent Application EP2017074551, 2017. [Google Scholar]
- Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H. Soft Tissue Adhesive Composition of Alfa-Tcp and Phosphorylated Amino Acid. European Patent Application EP2017074553, 2017. [Google Scholar]
- Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H. Composition of a Calcium Phosphate and an Additive Compound Containing a Phosphate or Phosphonate Group. European Patent Application PCT/EP2018/083197, 2018. [Google Scholar]
- Brazda, L.; Rohanova, D.; Helebrant, A. Kinetics of dissolution of calcium phosphate (Ca-P) bioceramics. Process. Appl. Ceram. 2008, 2, 57–62. [Google Scholar] [CrossRef]
- Sakai, S.; Anada, T.; Tsuchiya, K.; Yamazaki, H.; Margolis, H.C.; Suzuki, O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent. Mater. J. 2016, 35, 216–224. [Google Scholar] [CrossRef]
- Ajaxon, I.; Öhman, C.; Persson, C. Long-Term In Vitro Degradation of a High-Strength Brushite Cement in Water, PBS, and Serum Solution. BioMed. Res. Int. 2015, 2015, 17. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Ishikawa, K. Effect of Calcium and Phosphate on Compositional Conversion from Dicalcium Hydrogen Phosphate Dihydrate Blocks to Octacalcium Phosphate Blocks. Crystals 2018, 8. [Google Scholar] [CrossRef]
- Pan, H.B.; Darvell, B.W. Calcium Phosphate Solubility: The Need for Re-Evaluation. Cryst. Growth Des. 2009, 9, 639–645. [Google Scholar] [CrossRef]
- Li, H.; Gong, M.; Yang, A.; Ma, J.; Li, X.; Yan, Y. Degradable biocomposite of nano calcium-deficient hydroxyapatite-multi(amino acid) copolymer. Int. J. Nanomed. 2012, 7, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed. Res. Int. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous calcium phosphate materials: Formation, structure and thermal behaviour. J. Eur. Ceram. Soc. 2019, 39, 1642–1649. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Takeuchi, M.; Tanaka, H. Effect of amino acid on calcium phosphate phase transformation: Attenuated total reflectance-infrared spectroscopy and chemometrics. Colloid Polym. Sci. 2019, 297, 155–163. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy, Infrared Spectroscopy; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar]
- Dey, A.; Bomans, P.H.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J. Mater. Sci. Mater. Med. 2006, 17, 697–707. [Google Scholar] [CrossRef]
- Weng, J.; Liu, Q.; Wolke, J.G.C.; Zhang, D.; De Groot, K. The role of amorphous phase in nucleating bone-like apatite on plasma-sprayed hydroxyapatite coatings in simulated body fluid. J. Mater. Sci. Lett. 1997, 16, 335–337. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.-S.; Jung, H.S.; Hong, K.S. Influence of Ca/P ratios of starting solutions on the crystallization of amorphous calcium phosphate to hydroxyapatite. Metals Mater. Int. 2004, 10, 171. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; International Organization for Standards (ISO) 23317:2007; ISO: Geneva, Switzerland, 2017.
- Implants for Surgery—In Vitro Evaluation of Apatite-Forming Ability of Implant Materials; International Organization for Standards (ISO) 23317:2014; ISO: Geneva, Switzerland, 2014.
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Pujari-Palmer, M.; Robo, C.; Persson, C.; Procter, P.; Engqvist, H. Influence of cement compressive strength and porosity on augmentation performance in a model of orthopedic screw pull-out. J. Mech. Behav. Biomed. Mater. 2018, 77, 624–633. [Google Scholar] [CrossRef]
- Gallinetti, S.; Canal, C.; Ginebra, M.-P. Development and Characterization of Biphasic Hydroxyapatite/β-TCP Cements. J. Am. Ceram. Soc. 2014, 97, 1065–1073. [Google Scholar] [CrossRef]
- Biological evaluation of medical devices. In Part 12: Sample Preparation and Reference Materials; International organization for standards (ISO) 10993-12:2012; ISO: Geneva, Switzerland, 2012.
- Biological evaluation of medical devices. In Part 14: Identification and Quantification of Degradation Products from Ceramics; International organization for standards (ISO) 10993-13:2001; ISO: Geneva, Switzerland, 2001.
- Marcoline, F.V.; Ishida, Y.; Mindell, J.A.; Nayak, S.; Grabe, M. A mathematical model of osteoclast acidification during bone resorption. Bone 2016, 93, 167–180. [Google Scholar] [CrossRef]


| Group | Phosphoserine (%) | αTCP (%) | Portland (%) | CS1 (%) | CS Day 28 (MPa) |
|---|---|---|---|---|---|
| Control * | 0 | 100 | 0 | 0 | - |
| 1 | 31.0 | 79.0 | 0 | 0 | 49.2 |
| 2 | 31.0 | 67.8 | 1.2 | 0 | 43.5 |
| 3 | 0 | 98.3 | 1.7 | 0 | 10.4 |
| 4 | 31.0 | 67.8 | 0 | 1.2 | 38.6 |
| Group | Measured SVR | Thickness (mm) | Diameter (mm) |
|---|---|---|---|
| 2A | 8.98 | 3 | 18 |
| 2B | 16.67 | 2 | 6 |
| 2C | 24.06 | 1 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bystrom, J.L.; Pujari-Palmer, M. Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro. J. Funct. Biomater. 2019, 10, 54. https://doi.org/10.3390/jfb10040054
Bystrom JL, Pujari-Palmer M. Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro. Journal of Functional Biomaterials. 2019; 10(4):54. https://doi.org/10.3390/jfb10040054
Chicago/Turabian StyleBystrom, Joseph Lazraq, and Michael Pujari-Palmer. 2019. "Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro" Journal of Functional Biomaterials 10, no. 4: 54. https://doi.org/10.3390/jfb10040054
APA StyleBystrom, J. L., & Pujari-Palmer, M. (2019). Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro. Journal of Functional Biomaterials, 10(4), 54. https://doi.org/10.3390/jfb10040054