The Role of In Vitro Immune Response Assessment for Biomaterials
Abstract
1. Introduction
2. Current In Vitro Testing of Biomaterials
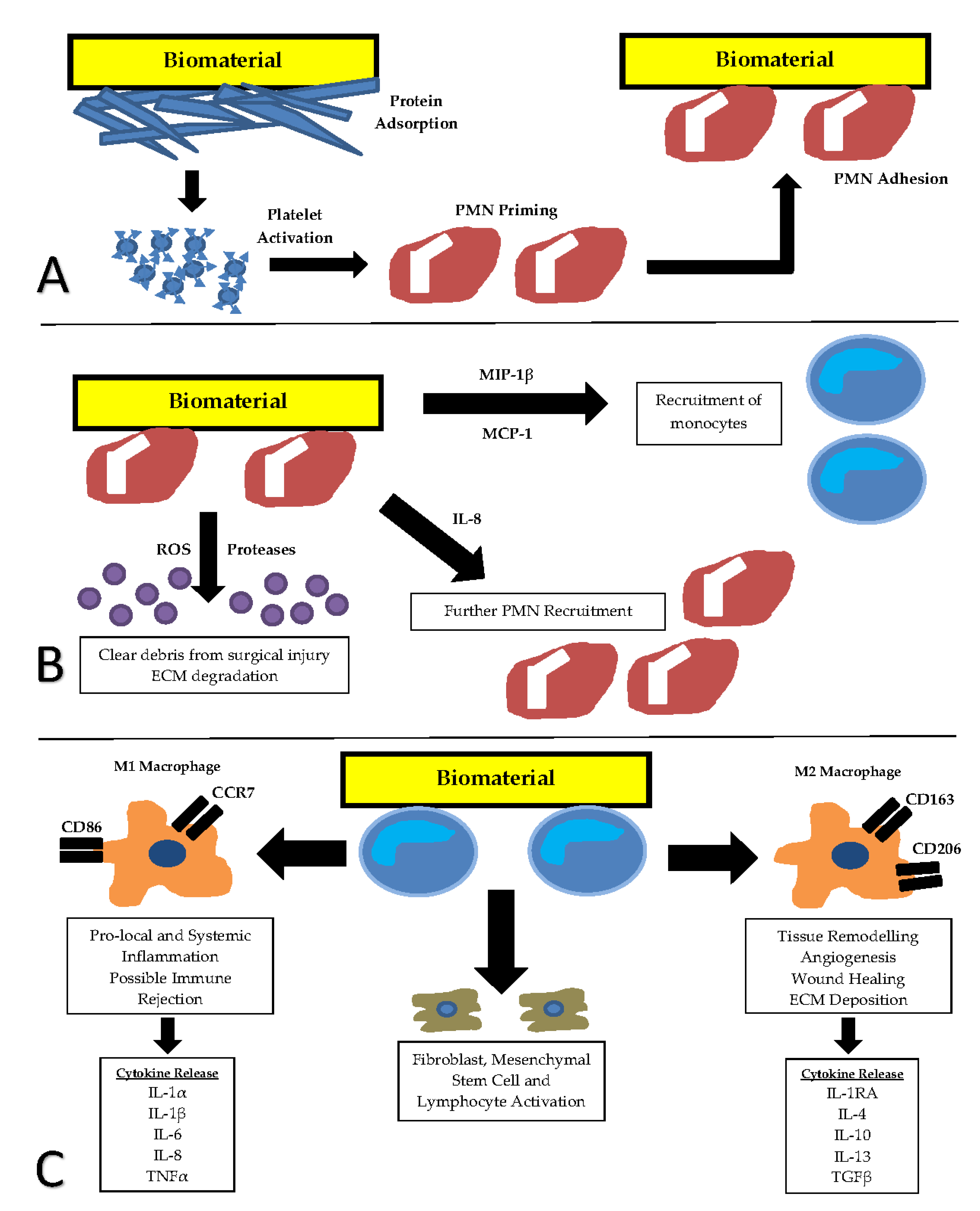
3. Foreign Body Response to Implanted Biomaterials
4. In Vitro Immunogenic Evaluation
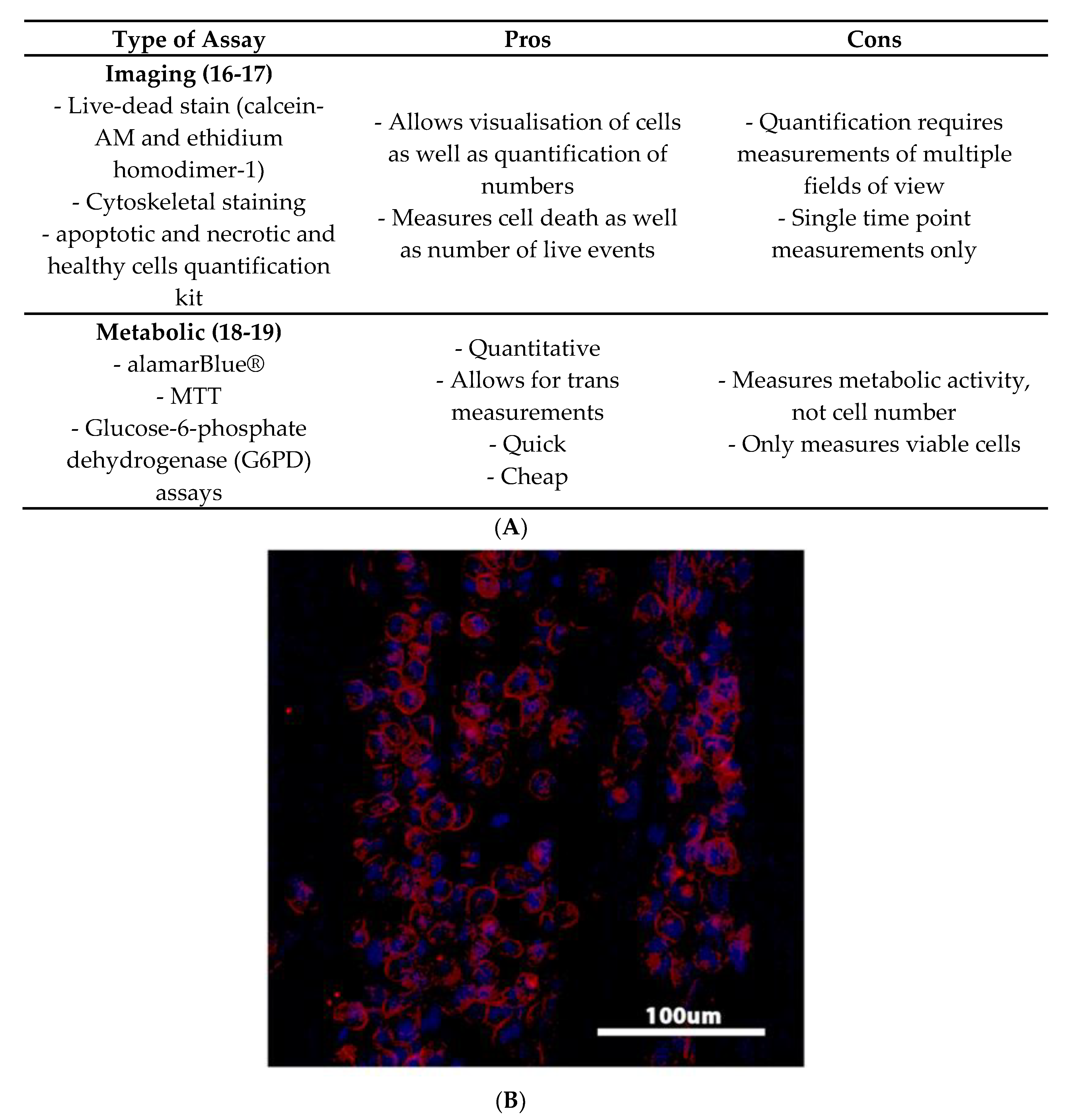
4.1. Viability Studies
4.2. Maturation Studies
4.3. Activation Studies
4.4. Protein-Based Assays
4.5. Relative Gene Expression-Based Assays
4.6. Functional Assays
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Almaiman, M.; Al-Bargi, H.H.; Manson, P. Complication of anterior iliac bone graft harvesting in 372 adult patients from May 2006 to May 2011 and a literature review. Craniomaxillofac. Trauma Reconstr. 2013, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D.; Giachelli, C.M.; Ratner, B.D. Engineering biomaterials to integrate and heal: The biocompatibility paradigm shifts. Biotechnol. Bioeng. 2012, 109, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18–45. [Google Scholar] [CrossRef]
- Manufacturer and User Facility Device Experience Database—(MAUDE). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm (accessed on 1 August 2018).
- Walton, J.R.; Bowman, N.K.; Khatib, Y.; Linklater, L.; Murrell, G.A.C. Restore Orthobiologic Implant: Not Recommended for Augmentation of Rotator Cuff Repairs. J. Bone Joint Surg. Am. 2007, 89, 786–791. [Google Scholar] [CrossRef]
- Yaremchuk, K.L.; Toma, M.S.; Somers, M.L.; Peterson, E. Acute Airway Obstruction in Cervical Spinal Procedures with Bone Morphogenetic Proteins. Laryngoscope 2010, 120, 1954–1957. [Google Scholar] [CrossRef]
- Mroz, E.T.; Wang, J.C.; Hashimoto, R.; Norvell, D.C. Complications Related to Osteobiologics Use in Spine Surgery: A Systematic Review. Spine 2010, 35, S86–S104. [Google Scholar] [CrossRef]
- ISO Editor. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Hulsart-Billstrom, G.; Dawson, J.I.; Hofmann, S.; Müller, R.; Stoddart, M.J.; Alini, M.; Redl, H.; El Haj, A.; Brown, R.; Salih, V.; et al. A surprisingly poor correlation between in vitro and in vivo testing of biomaterials for bone regeneration: Results of a multicentre analysis. Eur. Cells Mater. 2016, 31, 312–322. [Google Scholar] [CrossRef]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef]
- Kaplan, S.S.; Basford, R.E.; Jeong, M.H.; Simmons, R.L. Mechanisms of biomaterial-induced superoxide release by neutrophils. J. Biomed. Mater. Res. 1994, 28, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.J.; Parisi-Amon, A.; Reichert, W.M. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J. Biomed. Mater. Res. Part A 2009, 88, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA-and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef]
- Yahyouche, A.; Zhidao, X.; Czernuszka, J.T.; Clover, A.J.P. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater. 2011, 7, 278–286. [Google Scholar] [CrossRef]
- Kou, P.M.; Pallassana, N.; Bowden, R.; Cunningham, B.; Joy, A.; Kohn, J.; Babensee, J.E. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 2012, 33, 1699–1713. [Google Scholar] [CrossRef]
- Smith, M.J.; White, K.L., Jr.; Smith, D.C.; Bowlin, G.L. In vitro evaluations of innate and acquired immune responses to electrospun polydioxanone–elastin blends. Biomaterials 2009, 30, 149–159. [Google Scholar] [CrossRef]
- Smith, M.J.; Smith, D.C.; Bowlin, G.L.; White, K.L., Jr. Modulation of murine innate and acquired immune responses following in vitro exposure to electrospun blends of collagen and polydioxanone. J. Biomed. Mater. Res. 2010, 93A, 793–806. [Google Scholar]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Fearing, B.V.; Van Dyke, M.E. in vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater. 2014, 10, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Bonner, W.A.; Hulett, H.R.; Sweet, R.G.; Herzenberg, L.A. Fluorescence activated cell sorting. Rev. Sci. Instrum. 1972, 43, 404–409. [Google Scholar] [CrossRef]
- Park, J.; Babensee, J.E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012, 8, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Musson, D.S.; Naot, D.; Chhana, A.; Matthews, B.G.; McIntosh, J.D.; Lin, S.T.C.; Choi, A.J.; Callon, K.E.; Dunbar, R.; Lesage, S.; et al. In vitro evaluation of a novel non-mulberry silk scaffold for use in tendon regeneration. Tissue Eng. Part A 2015, 21, 1539–1551. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.M.; Gao, R.; Naot, D.; Coleman, B.; Cornish, J.; Musson, D.S. Induction of immune gene expression and inflammatory mediator release by commonly used surgical suture materials: An experimental in vitro study. Patient Saf. Surg. 2017, 11, 16. [Google Scholar] [CrossRef]
- Fotticchia, A.; Musson, D.; Lenardi, C.; Demirci, E.; Liu, Y. Anisotropic cytocompatible electrospun scaffold for tendon tissue engineering elicits limited inflammatory response in vitro. J. Biomater. Appl. 2018, 33, 127–139. [Google Scholar] [CrossRef]
- Butler, J.E. Enzyme-linked immunosorbent assay. J. Immunoass. 2000, 21, 165–209. [Google Scholar] [CrossRef]
- Bonfield, T.L.; Colton, E.; Marchant, R.E.; Anderson, J.M. Cytokine and growth factor production by monocytes/macrophages on protein preadsorbed polymers. J. Biomed. Mater. Res. 1992, 26, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.A.; Simmons, R.L.; Kaplan, S.S. TNF and IL-1 generation by human monocytes in response to biomaterials. J. Biomed. Mater. Res. 1992, 26, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Zhao, T.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. A novel method to in vitro evaluate biocompatibility of nanoscaled scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Ginalska, G. in vitro evaluation of the risk of inflammatory response after chitosan/HA and chitosan/β-1, 3-glucan/HA bone scaffold implantation. Mater. Sci. Eng. C 2016, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fivenson, D.P.; Faria, D.T.; Nickoloff, B.J.; Poverini, P.J.; Kunkel, S.; Burdick, M.; Strieter, R.M. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen. 1997, 5, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef]
- Templin, M.F.; Stoll, D.; Schrenk, M.; Traub, P.C.; Vöhringer, C.F.; Joos, T.O. Protein microarray technology. Drug Discov. Today 2002, 7, 815–822. [Google Scholar] [CrossRef]
- Chang, D.T.; Jones, J.A.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Lymphocyte/macrophage interactions: Biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J. Biomed. Mater. Res. Part A 2008, 87, 676–687. [Google Scholar] [CrossRef]
- Morgan, E.; Varro, R.; Sepulveda, H.; Ember, J.A.; Apgar, J.; Wilson, J.; Lowe, L.; Chen, R.; Shivraj, L.; Agadir, A.; et al. Cytometric bead array: A multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 2004, 110, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, N.; Kops, N.; Bayon, Y.; Deerenberg, E.B.; Mulder, I.M.; van Osch, G.J.V.M.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. in vitro model to study the biomaterial-dependent reaction of macrophages in an inflammatory environment. Br. J. Surg. 2014, 101, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Nakayama, Y.; Matsuda, T.; Colton, E.; Ziats, N.P.; Anderson, J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Debret, R.; Antonicelli, F.; Theill, A.; Hornebeck, W.; Bernard, P.; Guenounou, M.; Le Naour, R. Elastin-derived peptides induce a T-helper type 1 polarization of human blood lymphocytes. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1353–1358. [Google Scholar] [CrossRef]
- Jin, L.; Wu, J.; Yuan, G.; Chen, T. In vitro study of the inflammatory cells response to biodegradable Mg-based alloy extract. PLoS ONE 2018, 13, e0193276. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Araújo-Gomes, N.; García-Arnáez, I.; Martínez-Ramos, C.; Elortza, F.; Azkargorta, M.; Iloro, I.; Gurruchaga, M.; Suay, J.; Goni, I. The effect of strontium incorporation into sol-gel biomaterials on their protein adsorption and cell interactions. Colloids Surf. B Biointerfaces 2019, 174, 9–16. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic andinflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184. [Google Scholar] [CrossRef]
- van Putten, S.M.; Ploeger, D.T.; Popa, E.R.; Bank, R.A. Macrophage phenotypes in the collagen-induced foreign body reaction in rats. Acta Biomater. 2013, 9, 6502–6510. [Google Scholar] [CrossRef]
- Chu, C.; Liu, L.; Wang, Y.; Yang, R.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Evaluation of epigallocatechin-3-gallate (EGCG)-modified scaffold determines macrophage recruitment. Mater. Sci. Eng. C 2019, 100, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zenni, G.C.; Ellinger, J.; Lam, T.M.; Greisler, H.P. Biomaterial-induced macrophage activation and monokine release. J. Investig. Surg. 1994, 7, 135–141. [Google Scholar] [CrossRef]
- Mahmoudzadeh, A.; Mohsenifar, A.; Rahmani-Cherati, T. Collagen-chitosan 3D nano-scaffolds effects on macrophage phagocytosis and pro-inflammatory cytokine release. J. Immunotoxicol. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Chłopek, J.; Czajkowska, B.; Szaraniec, B.; Frackowiak, E.; Szostak, K.; Beguin, F. In vitro studies of carbon nanotubes biocompatibility. Carbon 2006, 44, 1106–1111. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Melendez-Ortiz, H.I.; Bucio, E.; Alves, P.T.; Lima, M.I.; Goulart, L.R.; Mathor, M.B.; Varca, G.H.C.; Lugao, A.B. Current Methods Applied to Biomaterials –Characterization Approaches, Safety Assessment and Biological International Standards. Curr. Top. Med. Chem. 2018, 18, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J. Animal welfare and ISO—The International Organisation for Standardization. AATEX Spec. Issue 2007, 14, 723–726. [Google Scholar]
- Musson, D.S.; McIntosh, J.; Callon, K.E.; Chhana, A.; Dunbar, P.R.; Naot, D.; Cornish, J. The need for thorough in vitro testing of biomaterial scaffolds: Two case studies. Procedia Eng. 2013, 59, 138–143. [Google Scholar] [CrossRef][Green Version]
- Evans, C.H. Barriers to the clinical translation of orthopaedic tissue engineering. Tissue Eng. Part B 2011, 17, 437–441. [Google Scholar] [CrossRef]
- Hollister, S.J.; Murphy, W.L. Scaffold translation: Barriers between concept and clinic. Tissue Eng. Part B 2011, 17, 459–474. [Google Scholar] [CrossRef]
- Wolf, M.T.; Vodovotz, Y.; Tottey, S.; Brown, B.N.; Badylak, S.F. Predicting in vivo responses to biomaterials via combined in vitro and in silico analysis. Tissue Eng. Part C Methods 2014, 21, 148–159. [Google Scholar] [CrossRef]
- Jannasch, M.; Weigel, T.; Engelhardt, L.; Wiezoreck, J.; Gaetzner, S.; Walles, H.; Schmitz, T.; Hansmann, J. In Vitro Chemotaxis and Tissue Remodeling Assays Quantitatively Characterize Foreign Body Reaction. ALTEX Altern. Anim. Exp. 2017, 34, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, M.; Gaetzner, S.; Weigel, T.; Walles, H.; Schmitz, T.; Hansmann, J. A comparative multi-parametric in vitro model identifies the power of test conditions to predict the fibrotic tendency of a biomaterial. Sci. Rep. 2017, 7, 1689. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Espanol, M.; Bonany, M.; Lu, X.; Persson, C.; Ginebra, M.P. Heparinization of Beta Tricalcium Phosphate: Osteo-immunomodulatory Effects. Adv. Healthc. Mater. 2018, 7, 1700867. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Quabius, S.; Kewitz, T.; Hansen, L.; Becker, G.; Kern, M.; Kerston, H.; Harder, S. In vitro proinflammatory gene expression changes in human whole blood after contact with plasma-treated implant surfaces. J. Cranio-Maxillofac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
| Pro-Inflammatory Cytokines Assessed Associated with Immune Rejection | Anti-Inflammatory Cytokines Assessed Associated with Immune Acceptance |
|---|---|
| IL-1α | IL-1RA |
| IL-1β | IL-4 |
| IL-6 | IL-10 |
| IL-8 | IL-13 |
| IL-17A | TGFβ |
| CXCL10 | |
| TNFα |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lock, A.; Cornish, J.; Musson, D.S. The Role of In Vitro Immune Response Assessment for Biomaterials. J. Funct. Biomater. 2019, 10, 31. https://doi.org/10.3390/jfb10030031
Lock A, Cornish J, Musson DS. The Role of In Vitro Immune Response Assessment for Biomaterials. Journal of Functional Biomaterials. 2019; 10(3):31. https://doi.org/10.3390/jfb10030031
Chicago/Turabian StyleLock, Alistair, Jillian Cornish, and David S. Musson. 2019. "The Role of In Vitro Immune Response Assessment for Biomaterials" Journal of Functional Biomaterials 10, no. 3: 31. https://doi.org/10.3390/jfb10030031
APA StyleLock, A., Cornish, J., & Musson, D. S. (2019). The Role of In Vitro Immune Response Assessment for Biomaterials. Journal of Functional Biomaterials, 10(3), 31. https://doi.org/10.3390/jfb10030031





