Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Groups
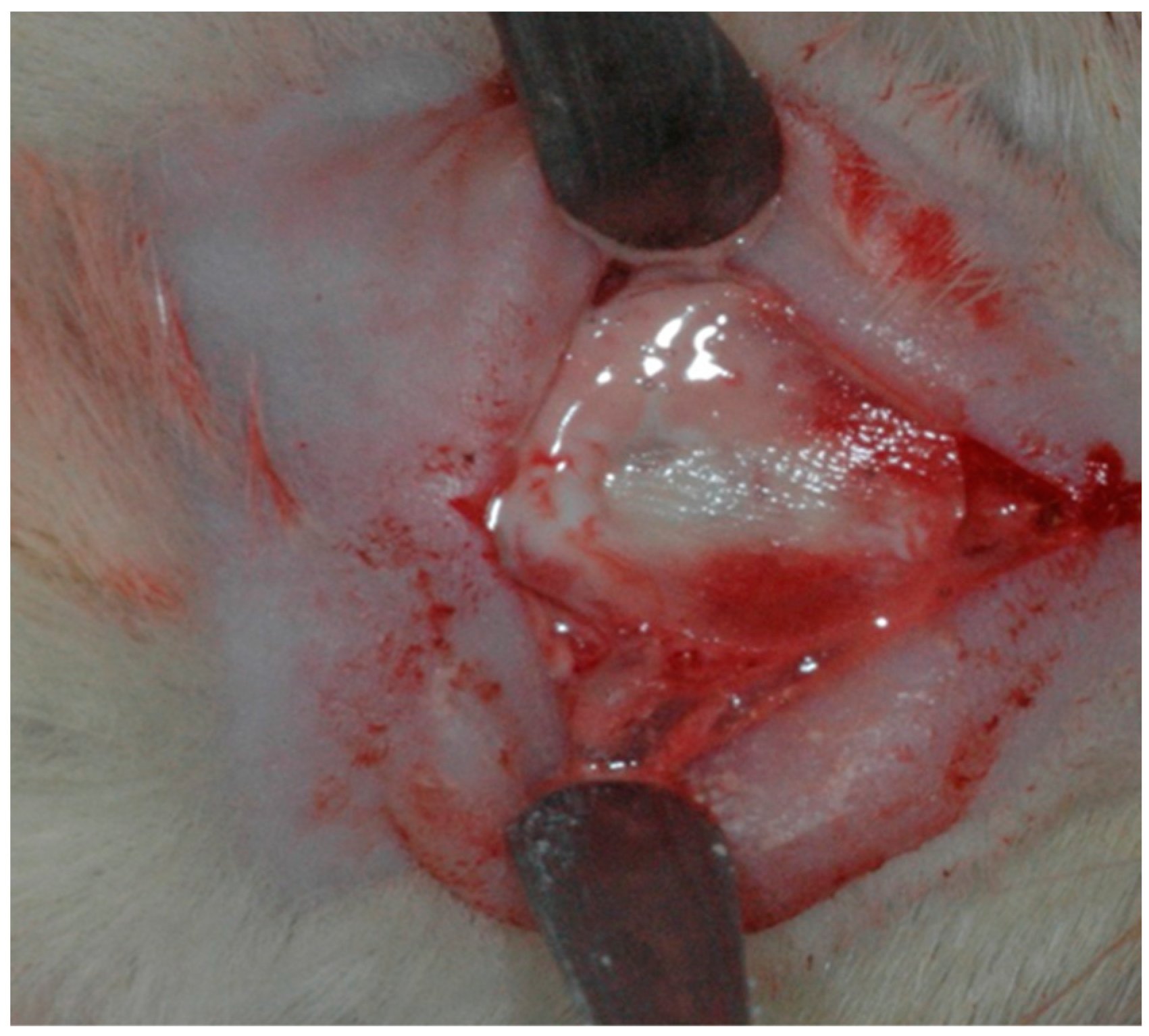
2.2. Surgical Procedure
2.3. Post-Operative Care
2.4. Euthanasia and Material Collection
2.5. Histometric Analysis
2.6. Statistical Analysis
3. Results
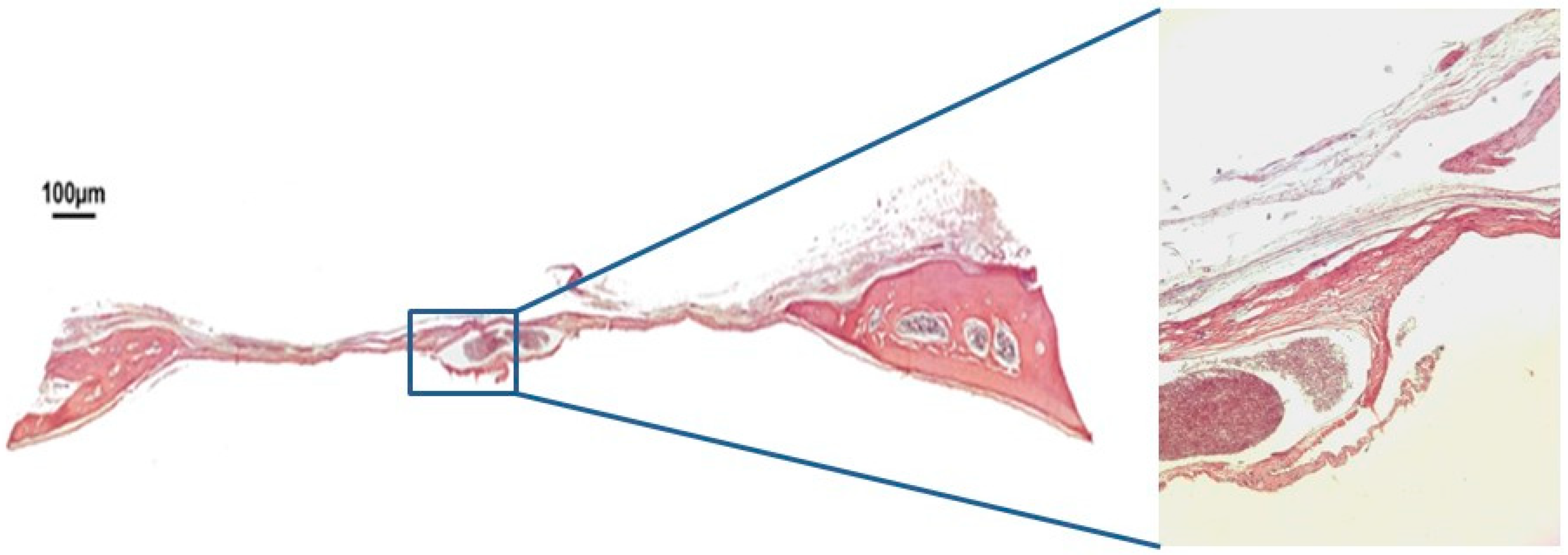
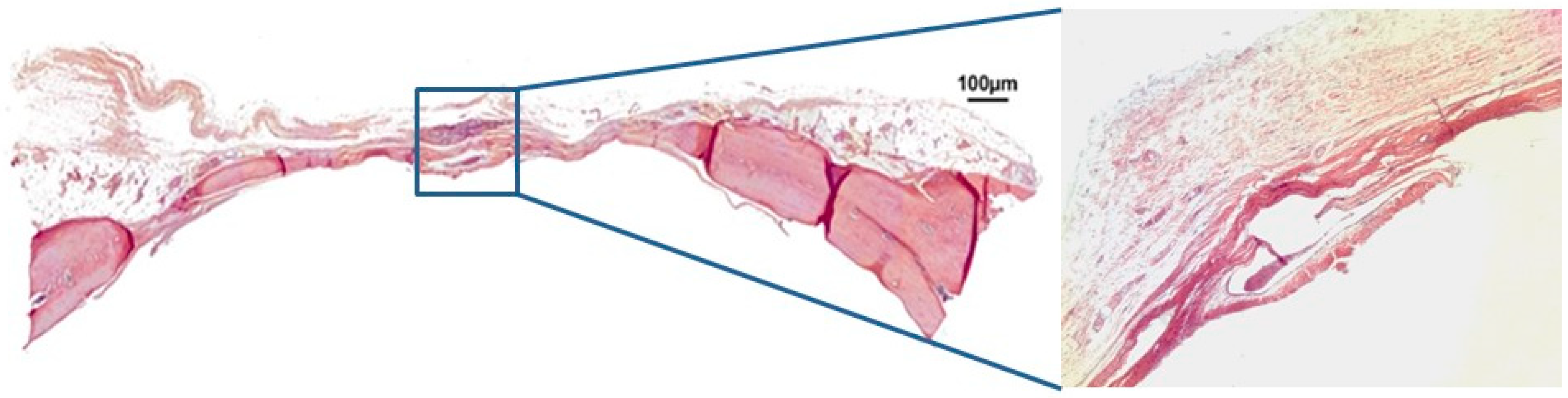
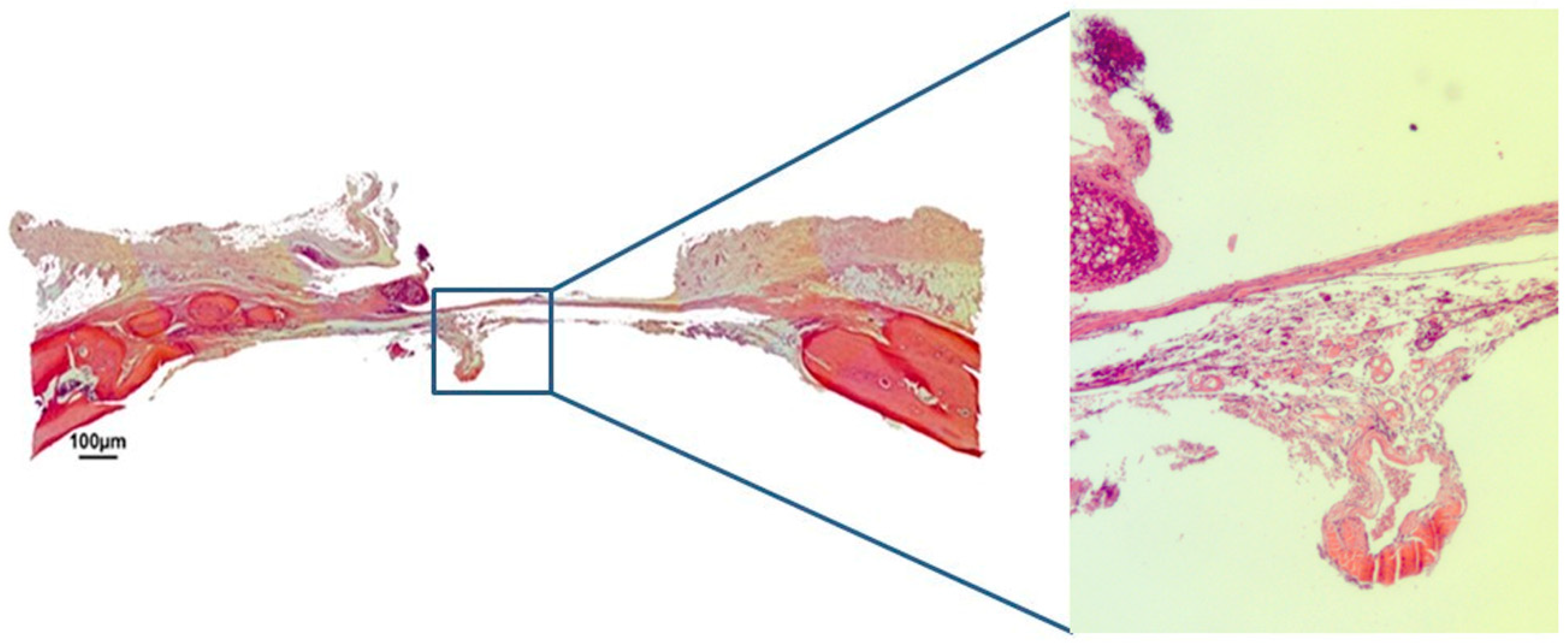
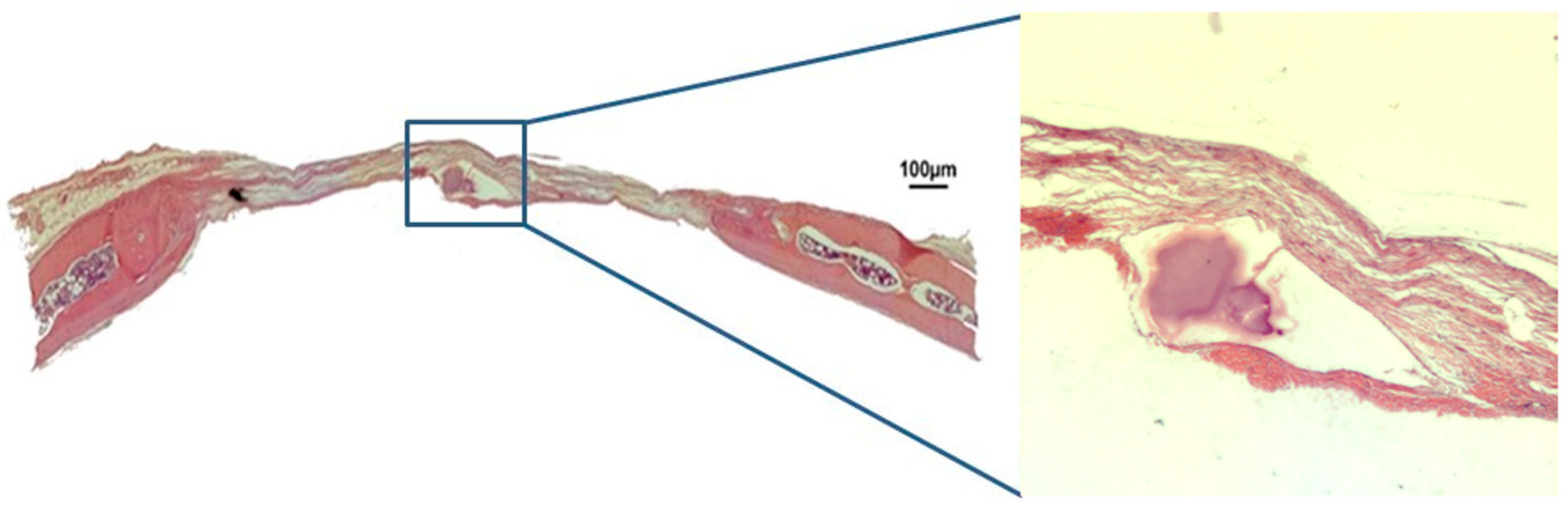
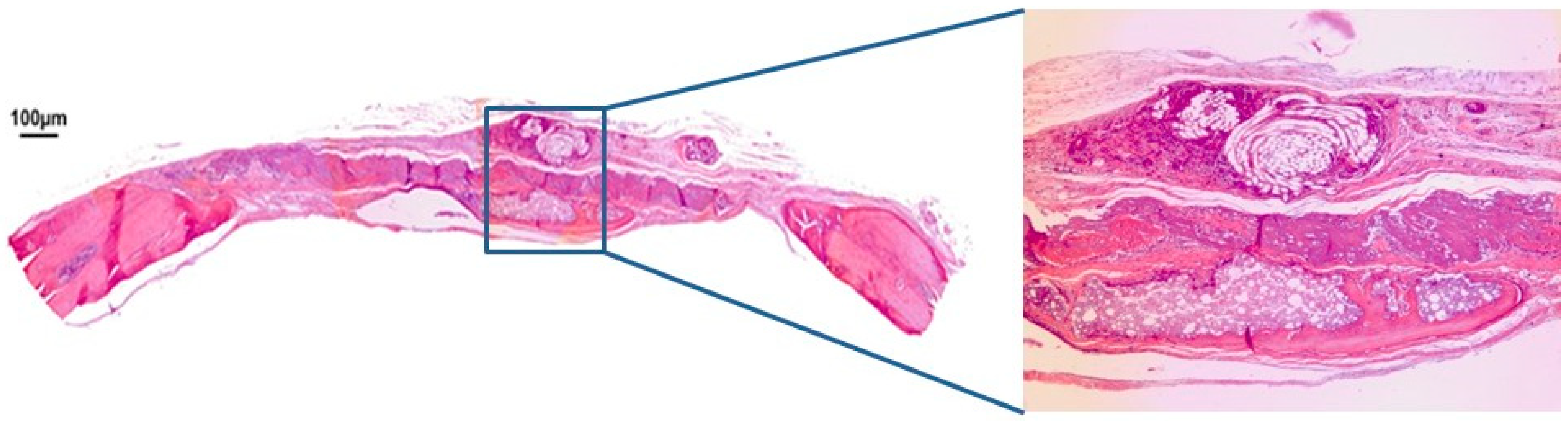
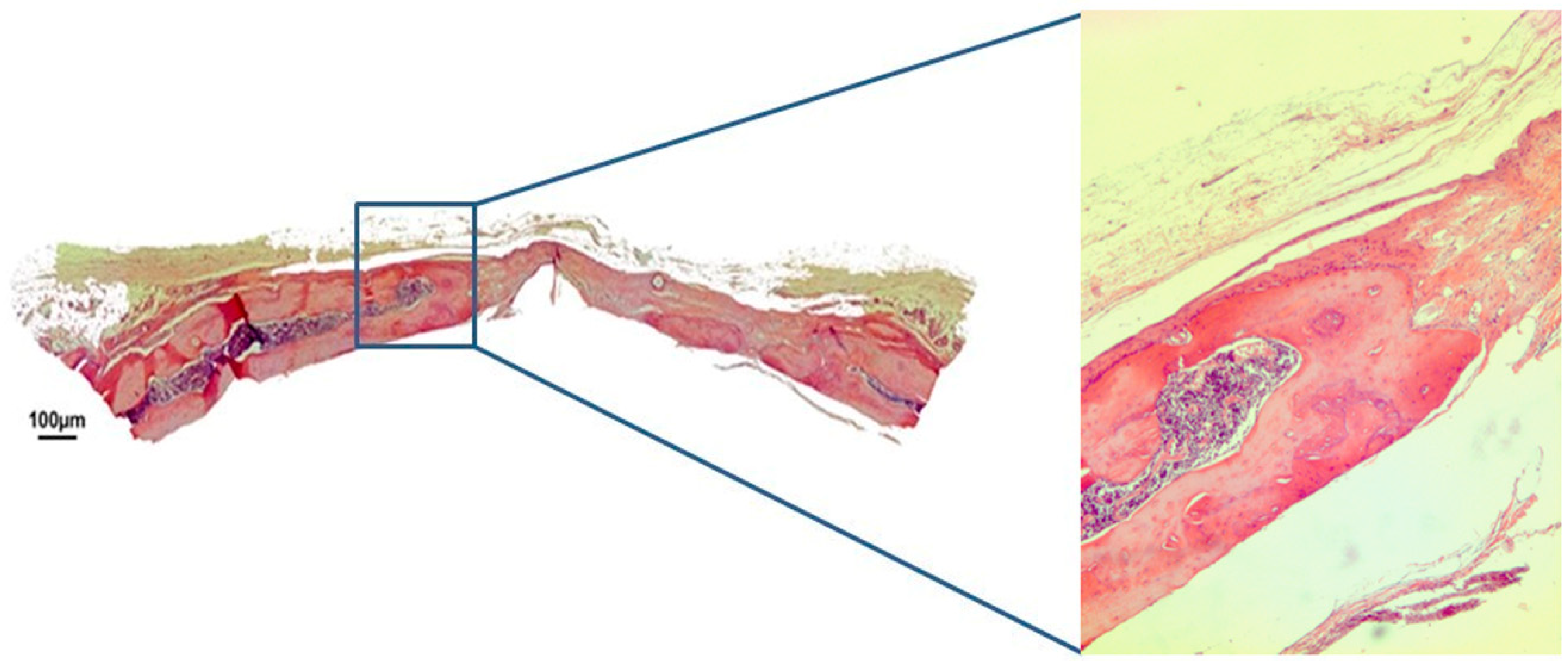
3.1. Qualitative Histological Analysis
3.2. Histometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quiles, J.C.; Souza, F.A.; Bassi, A.P.F.; Garcia, I.R., Jr.; França, M.T.; Carvalho, P.S.P. Survival rate of osseointegrated implants in atrophic maxillae grafted with calvarial bone: A retrospective study. Int. J. Oral. Maxillofac. Surg. 2015, 44, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.S.P.; Pellizzer, E.P. Fundamentos em Implantodontia: Uma Visão Contemporânea; Quintessence Editora Ltda.: São Paulo, Brazil, 2011; (In Português). [Google Scholar]
- Cordaro, L. Bilateral simultaneous augmentation of the maxillary sinus floor with particulated mandible. Report of a technique and preliminary results. Clin. Oral Implant 2003, 14, 201–206. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Review Zirconia: Established Facts and Perspectives for a Biomaterial in Dental Implantology. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Wilson, J. Dental implants placed in extraction sites implanted with bioactive glass: Human histology and clinical outcome. Int. J. Oral Implant. 2002, 17, 249–257. [Google Scholar]
- Velnar, T.; Bunc, G.; Klobucar, R.; Gradišnik, L. Biomaterials and host versus graft response: A short review. Bosn. J. Basic Med Sci. 2016, 16, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Brown, W.E.; Chow, L.C. A new calcium phosphate setting cement. J. Dent. Res. 1983, 62, 672–679. [Google Scholar]
- Bucholz, R.W. Non allograft osteoconductive bone graft substitutes. Clin. Orthop. Relat. Res. 2002, 395, 44–52. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Boltong, M.G.; De Maeyer, E.A.P.; Vercruysse, C.W.J.; Wenz, R.; Verbeeck, R.M.H. Bioceramics (Proceedings of the 11th International Symposium on Ceramics in Medicine); Le Geros, R.Z., LeGeros, J.P., Eds.; World Scientific Publishin: New York City, NY, USA, 1998; Volume 11, pp. 231–233. [Google Scholar]
- Driessens, F.C.M.; Planell, J.A.; Gil, F.J. Encyclopedic Handbook of Biomaterials and Bioengineering, Part B: Applications 4V Set; Marcel Dekker Inc.: New York City, NY, USA, 1995; p. 85. [Google Scholar]
- Ishikawa, K.; Miyamoto, Y.; Kon, M.; Nagayama, M.; Asaoka, K. Non-decay type fast-setting calcium phosphate cement: composite with sodium alginate. Biomaterials 1995, 16, 527–532. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001, 22, 2247–2255. [Google Scholar] [CrossRef]
- Xu, H.; Quinn, J.; Takagi, S.; Chow, L. Processing and Properties of Strong and Non-rigid Calcium Phosphate Cement. J. Dent. 2002, 81, 219–224. [Google Scholar]
- Tanaka, S.; Kishi, T.; Shimogoryo, R.; Matsuya, S.; Ishikawa, K. Biopex((r)) acquires anti-washout properties by adding sodium alginate into its liquid phase. Dent. Mater. J. 2003, 22, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Bracci, B.; Panzavolta, S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials 2004, 25, 2893–2899. [Google Scholar] [CrossRef]
- Barralet, J.E.; Tremayne, M.; Lilley, K.J.; Gbureck, U. Modification of calciumphosphate cement with a-hydroxy acids and their salts. Chem. Mater. 2005, 17, 1313–1319. [Google Scholar] [CrossRef]
- Tamimi-Mariño, F.; Mastio, J.; Rueda, C.; Blanco, L.; López-Cabarcos, E. Increase of the final setting time of brushite cements by using chondroitin 4-sulfate and silica gel. J. Mater. Sci. Mater. Med. 2007, 18, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.; Rueda, C.; Mariño, F.; Torres, J.; Jerez, L.; Gbureck, U.; Cabarcos, E. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Gruninger, S.E.; Siew, C.; Chow, L.C.; O’Young, A.; Ts’ao, N.K.; Brown, W.E. Evaluation of the biocompatibility of a new calcium-phosphate setting cement. J. Dent. Res. 1984, 63, 200. [Google Scholar]
- Yu, D.; Wong, J.; Matsuda, Y.; Fox, J.L.; Higuchi, W.I.; Otsuka, M. Self-setting hydroxyapatitecement: A novel skeletal drug delivery system for antibiotics. J. Pharm. Sci. 1992, 81, 529–531. [Google Scholar] [CrossRef]
- Santos, L.A.; Boschi, A.O.; Arruda, A.C.F. Double Setting Calcium Phosphate Cements; Pat. Require. PI0000760-9; National Institute of Industrial Property (INPI): Paris, France, 2000.
- Bohner, M.; Galea, L.; Doebelin, N. Calcium phosphate bone graft substitutes: Failures and hopes. J. Eur. Ceram. Soc 2012, 32, 2663–2671. [Google Scholar] [CrossRef]
- Puttini, I.D.O.; Poli, P.P.; Maiorana, C.; De Vasconcelos, I.R.; Schmidt, L.E.; Colombo, L.T.; Hadad, H.; Dos Santos, G.M.; De Carvalho, P.S.P.; Souza, F.Á.; et al. Evaluation of Osteoconduction of Biphasic Calcium Phosphate Ceramic in the Calvaria of Rats: Microscopic and Histometric Analysis. J. Funct. Biomater. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Furlaneto, F.A.C.; Nagata, M.J.H.; Fucini, S.E.; Deliberador, T.M.; Okamoto, T.; Messora, M.R. Bone healing in critical-size defects treated with bioactive glass/calcium sulfate: A histologic and histometric study in rat calvaria. Clin. Oral Implant 2007, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.; Momesso, G.A.C.; Ramalho-Ferreira, G.; Consolaro, R.B.; De Carvalho, P.S.P.; Faverani, L.P.; Bassi, A.P.F. Bone Healing Evaluation in Critical-Size Defects Treated with Xenogenous Bone Plus Porcine Collagen. Implant Dent. 2017, 26, 296–302. [Google Scholar] [CrossRef]
- Donos, N.; Kostopoulos, L.; Karring, T. Alveolar ridge augmentation using a resorbable copolymer membrane and autogenous bone grafts. An experimental study in the rat. Clin. Oral Implant. 2002, 13, 203–213. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided Bone Regeneration for the Reconstruction of Alveolar Bone Defects. Ann. Surg. 2017, 7, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Levandowski Junior, N.; Pfeifer, A.B.; Paza, A.O.; Valiati, R.; Silva, M.R. Utilização do osso alógeno em bloco para aumento de rebordo alveolar: Revisão da literatura. Implant News 2008, 5, 51–57. [Google Scholar]
- Reddi, A. Morphogenesis and Tissue Engineering of Bone and Cartilage: Inductive Signals, Stem Cells, and Biomimetic Biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Montufar, E.; Perez, R.; Mestres, G.; Montufar, E.; Perez, R. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef]
- Pagano, S.; Chieruzzi, M.; Balloni, S.; Lombardo, G.; Torre, L.; Bodo, M.; Cianetti, S.; Marinucci, L. Biological, thermal and mechanical characterization of modified glass ionomer cements: the role of nanohydroxyapatite, ciprofloxacin and zinc l-carnosine. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Calcium Orthophosphates in Medicine: from Ceramics to Calcium Phosphate Cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Xia, L.; Xu, Y.; Wei, J.; Zeng, D.; Ye, D.; Liu, C.; Zhang, Z.; Jiang, X. Maxillare Sinus Floor Elevation Using a Tissue-Engineered Bone with rhBMP-2-Loaded Porous Calcium Phosphate Cement Scaffold and Bone Marrow Strmal Cells in Rabbits. Cells Tissues Organs 2011, 194, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Ooms, E.; Verdonschot, N.; Wolke, J. Injectable calcium phosphate cement for bone repair and implant fixation. Orthop. Clin. N.a. 2005, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of Pore Size on the Osteoconductivity and Mechanical Properties of Calcium PhosphateCement in a Rabbit Model. Artif. Organs 2017, 41, 199–204. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Shuang, Y.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Chandad, F.; Zhang, Y. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin. Oral Implants Res. 2016, 27, 668–675. [Google Scholar] [CrossRef]
- Aral, A.; Yalçιn, S.; Karabuda, Z.C.; Anιl, A.; Jansen, J.A.; Mutlu, Z. Injectable calcium phosphate cement as a graft material for maxillary sinus augmentation: An experimental pilot study. Clin. Oral Implant 2008, 19, 612–617. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Imbronito, A.V.; Todescan, J.H.; Carvalho, C.V.; E Arana-Chavez, V. Healing of alveolar bone in resorbable and non-resorbable membrane-protected defects. A histologic pilot study in dogs. Biomaterials 2002, 23, 4079–4086. [Google Scholar] [CrossRef]
- Cameron, J.A.; Milner, D.J.; Lee, J.S.; Cheng, J.; Fang, N.X.; Jasiuk, I.M. Employing the biology of successful fracture repair to heal critical size bone defects. In New Perspectives in Regeneration; Springer: Berlin/Heidelberg, Germay, 2012; pp. 113–132. [Google Scholar]
- Ginebra, M.-P.; Canal, C.; Español, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemmb, M.; Nies, B.U.; Gbureck, U. Ready-to-use injectable calcium phosphate bone cement pasteas drug carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef] [PubMed]
| Periods | (BC) | (BCM) | (HBS) | p Value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 30 days | 1.02 ± 0.97 A | 6.04 ± 1.69 B | 9.26 ± 4.82 B | 0.004 * |
| 60 days | 10.67 ± 5.57 A | 16.71 ± 5.00 AB | 55.11 ± 13.20 B | 0.002 * |
| P value | 0.016 * | 0.006 * | 0.003 * |
| Groups | R2 | β | p Value |
|---|---|---|---|
| Blood Clot Group (BC) | 0.57 | 0.32 | 0.004 * |
| Blood Clot Group-Membrane (BCM) | 0.73 | 0.35 | 0.001 * |
| Injectable β-TCP group (HBS) | 0.86 | 1.52 | <0.001 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, L.E.; Hadad, H.; Vasconcelos, I.R.d.; Colombo, L.T.; da Silva, R.C.; Santos, A.F.P.; Cervantes, L.C.C.; Poli, P.P.; Signorino, F.; Maiorana, C.; et al. Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement. J. Funct. Biomater. 2019, 10, 21. https://doi.org/10.3390/jfb10020021
Schmidt LE, Hadad H, Vasconcelos IRd, Colombo LT, da Silva RC, Santos AFP, Cervantes LCC, Poli PP, Signorino F, Maiorana C, et al. Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement. Journal of Functional Biomaterials. 2019; 10(2):21. https://doi.org/10.3390/jfb10020021
Chicago/Turabian StyleSchmidt, Luis Eduardo, Henrique Hadad, Igor Rodrigues de Vasconcelos, Luara Teixeira Colombo, Rodrigo Capalbo da Silva, Ana Flavia Piquera Santos, Lara Cristina Cunha Cervantes, Pier Paolo Poli, Fabrizio Signorino, Carlo Maiorana, and et al. 2019. "Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement" Journal of Functional Biomaterials 10, no. 2: 21. https://doi.org/10.3390/jfb10020021
APA StyleSchmidt, L. E., Hadad, H., Vasconcelos, I. R. d., Colombo, L. T., da Silva, R. C., Santos, A. F. P., Cervantes, L. C. C., Poli, P. P., Signorino, F., Maiorana, C., Carvalho, P. S. P. d., & Souza, F. Á. (2019). Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement. Journal of Functional Biomaterials, 10(2), 21. https://doi.org/10.3390/jfb10020021







