Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Ethics Statement
4.3. Patients, Clinicians, and Implants
4.4. Study Objectives and Variables
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of literature. Int. J. Oral Maxillofac. Implants 1999, 14, 473–490. [Google Scholar] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective and longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prosthesis. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical and esthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23, 2–21. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prosthesis (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Zembic, A.; Kim, S.; Zwahlen, M.; Kelly, J.R. A systematic review of the survival rate and incidence of biologic, technical and esthetic complications of single implant abutments supporting fixed prosthesis. Int. J. Oral Maxillofac. Implants 2014, 29, 43–54. [Google Scholar] [CrossRef]
- Kern, J.S.; Kern, T.; Wolfart, S.; Heussen, N. A systematic review and meta-analysis of removable and fixed implant-supported prosthesis in edentulous jaws: Post-loading implant loss. Clin. Oral Implants Res. 2016, 27, 174–195. [Google Scholar] [CrossRef]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine-to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef]
- Derks, J.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and late implant loss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef]
- Baelum, V.; Ellegaard, B. Implant survival in periodontally compromised patients. J. Periodontol. 2004, 75, 1404–1412. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Cho, S.C.; Elian, N.; Jalbout, Z.N.; Froum, S.; Evian, C.I. A comparison of characteristics of implant failure and survival in periodontally compromised and periodontally healthy patients: A clinical report. Int. J. Oral Maxillofac. Implants 2004, 19, 873–879. [Google Scholar] [PubMed]
- Wennström, J.L.; Ekestubbe, A.; Gröndahl, K.; Karlsson, S.; Lindhe, J. Oral rehabilitation with implant-supported fixed partial dentures in periodontitis-susceptible subjects. A 5-year prospective study. J. Clin. Periodontol. 2004, 31, 713–724. [Google Scholar] [CrossRef]
- Mengel, R.; Flores-de-Jacoby, L. Implants in patients treated for generalized aggressive and chronic periodontitis: A 3-year prospective longitudinal study. J. Periodontol. 2005, 76, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Schou, S.; Holmstrup, P.; Worthington, H.V.; Esposito, M. Outcome of implant therapy in patients with previous tooth loss due to periodontitis. Clin. Oral Implants Res. 2006, 17, 104–123. [Google Scholar] [CrossRef]
- Karoussis, I.K.; Kotsovilis, S.; Fourmousis, I. A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin. Oral Implants Res. 2007, 18, 669–679. [Google Scholar] [CrossRef] [PubMed]
- García-Bellosta, S.; Bravo, M.; Subirá, C.; Echeverría, J.J. Retrospective study of the long-term survival of 980 implants placed in a periodontal practice. Int. J. Oral Maxillofac. Implants 2010, 25, 613–619. [Google Scholar] [PubMed]
- Bhatavadekar, N. Helping the clinician makes evidence-based implant selections. A systematic review and qualitative analysis of dental implant studies over a 20-year period. Int. Dent. J. 2010, 60, 359–369. [Google Scholar]
- Berglund, T.; Giannobile, W.V. Investigational Clinical Research in Implant Dentistry. J. Dent. Res. 2013, 92, 1075–1085. [Google Scholar] [CrossRef]
- Tallarico, M.; Meloni, S.M. Open-cohort prospective study on early implant failure and physiological marginal remodeling expected using sandblasted and acid-etched bone level implants featuring an 11° Morse taper connection within one year after loading. J. Oral Sci. Rehabil. 2017, 3, 68–79. [Google Scholar]
- French, D.; Larjava, H.; Tallarico, M. Retrospective Study of 1087 Anodized Implants Placed in Private Practice: Risk Indicators Associated With Implant Failure and Relationship Between Bone Levels and Soft Tissue Health. Implant Dent. 2018, 27, 177–187. [Google Scholar] [CrossRef]
- Tallarico, M.; Meloni, S. Retrospective Analysis on Survival Rate, Template-Related Complications, and Prevalence of Peri-implantitis of 694 Anodized Implants Placed Using Computer-Guided Surgery: Results Between 1 and 10 Years of Follow-Up. Int. J. Oral Maxillofac. Implants 2017, 32, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef] [PubMed]
- Binon, P.P. Implants and components: Entering the new millennium. Int. J. Oral Maxillofac. Implants 2000, 15, 76–94. [Google Scholar] [PubMed]
- Aparicio, C.; Gil, F.J.; Planell, J.A. Human-osteoblast proliferation and differentiation on grit-blasted and bioactive titanium for dental applications. J. Mater. Sci. Mater. Med. 2002, 13, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2014, 7, CD003815. [Google Scholar] [CrossRef] [PubMed]
- Santander, S.; Alcaine, C.; Lyahyai, J.; PÉREZ, M.A.; Rodellar, C.; Doblare, M.; Ochoa, I. In vitro osteoinduction of human mesenchymal stem cells in biomimetic surface modified titanium alloy implants. Dent. Mater. J. 2012, 31, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Derks, J. Clinical research of peri-implant diseases—Quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J. Clin. Periodontol. 2012, 39, 207–223. [Google Scholar] [CrossRef]
- Esposito, M.; Cannizzaro, G.; Bozzoli, P.; Checchi, L.; Ferri, V.; Landriani, S.; Leone, M.; Todisco, M.; Torchio, C.; Testori, T.; et al. Effectiveness of prophylactic antibiotics at placement of dental implants: A pragmatic multicentre placebo-controlled randomized clinical trial. Eur. J. Oral Implantol. 2010, 3, 135–143. [Google Scholar]
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.P.; Buser, D. A retrospective analysis of patients referred for implant placement to a specialty clinic: Indications, surgical procedures, and early failures. Int. J. Oral Maxillofac. Implants 2008, 23, 1109–1116. [Google Scholar]
- Strassburger, C.; Kerschbaum, T.; Heydecke, G. Influence of implant and conventional prostheses on satisfaction and quality of life: A literature review. Part 2: Qualitative analysis and evaluation of the studies. Int. J. Prosthodont. 2006, 19, 339–348. [Google Scholar]
- Lee, C.T.; Chen, Y.W.; Starr, J.R.; Chuang, S.K. Survival analysis of wide dental implant: Systematic review and meta-analysis. Clin. Oral Implants Res. 2016, 27, 1251–1264. [Google Scholar] [CrossRef]
- Lazzara, R.J.; Porter, S.S.; Testori, T.; Galante, J.; Zetterqvist, L. A prospective multicenter study evaluating loading of Osseotite implants two months after placement: One-year results. J. Esthet Dent. 1998, 10, 280–289. [Google Scholar] [CrossRef]
- Vinardell, A.M. Caracterización química y biológica de la superficie Biomimetic Advanced Surface. Rev. Int. Prot. Estomat. 2008, 10, 293–298. [Google Scholar]
- Cochran, D.L.; Bosshardt, D.D.; Grize, L.; Higginbottom, F.L.; Jones, A.A.; Jung, R.E.; Wieland, M.; Dard, M. Bone response to loaded implants with non-matching implant-abutment diameters in the canine mandible. J. Periodontol. 2009, 80, 609–617. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G. Patient selection and preparation. In Tissue-Integrated Protheses. Osseointegration in Clinical Dentistry; Brånemark, P.I., Zarb, G., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Pagano, M.; Gauvreau, K. Principles of Biostatistics, 2nd ed.; Duxbury Press: Pacific Grove, CA, USA, 2000; pp. 488–494. [Google Scholar]
- Laney, W.R. Glossary of Oral and Maxillofacial Implants; Laney, W.R., Ed.; Quintessence Publishing Co.: Chicago, IL, USA, 2007; pp. 187–188, ISBN 10 3938947004, ISBN 13 9783938947005. [Google Scholar]
- Rocuzzo, M. Editorial: Before you prepare another systematic review. Int. J. Periodontics Restor. Dent. 2016, 36, 463. [Google Scholar] [CrossRef]
- Alghamdi, H.S. Methods to Improve Osseointegration of Dental Implants in Low Quality (Type-IV) Bone: An Overview. J. Funct. Biomater. 2018, 9, E7. [Google Scholar] [CrossRef]
- Sachdeva, A.; Dhawan, P.; Sindwani, S. Assessment of implant stability: Methods and recent advances. Br. J. Med. Med. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef]
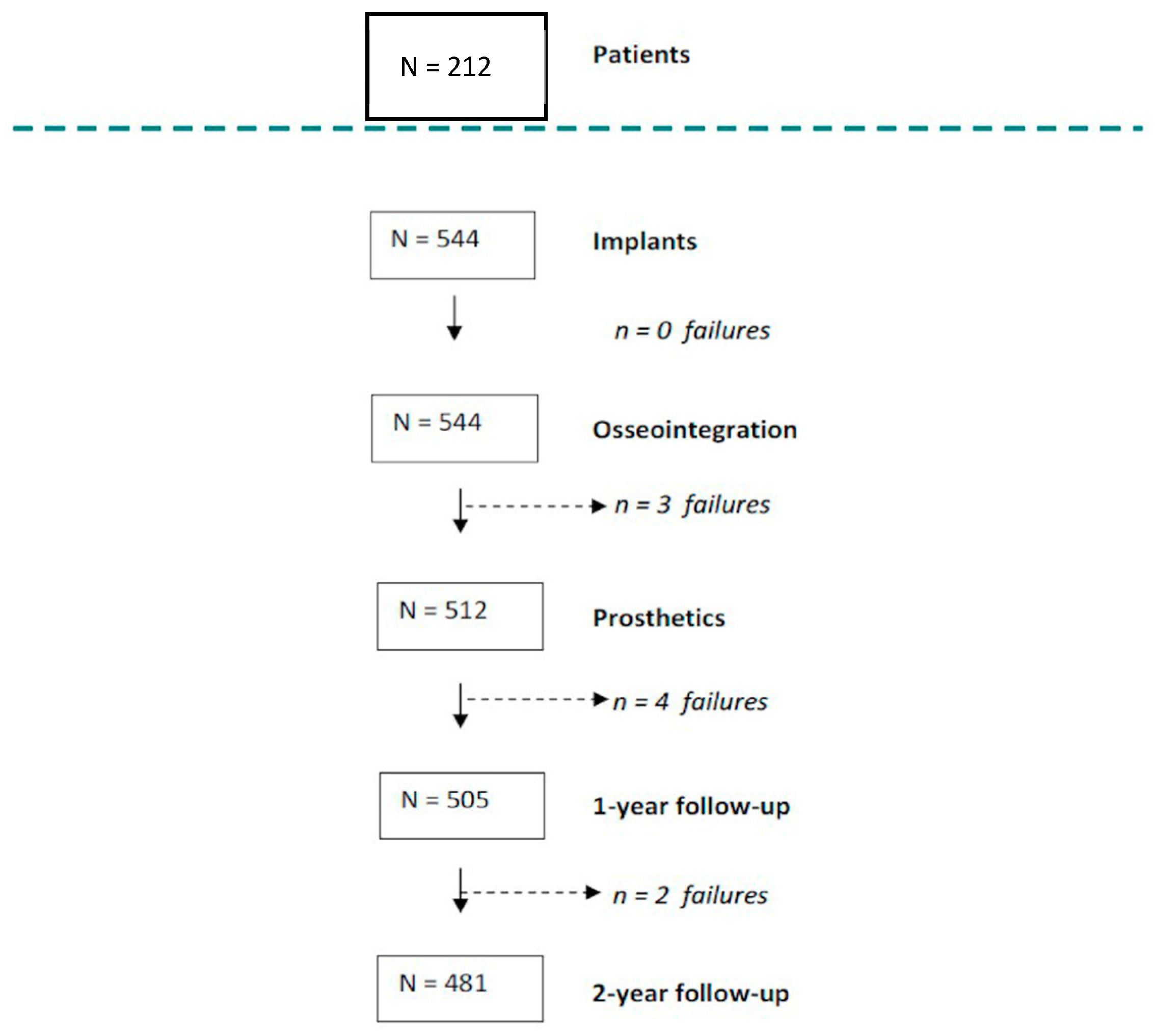
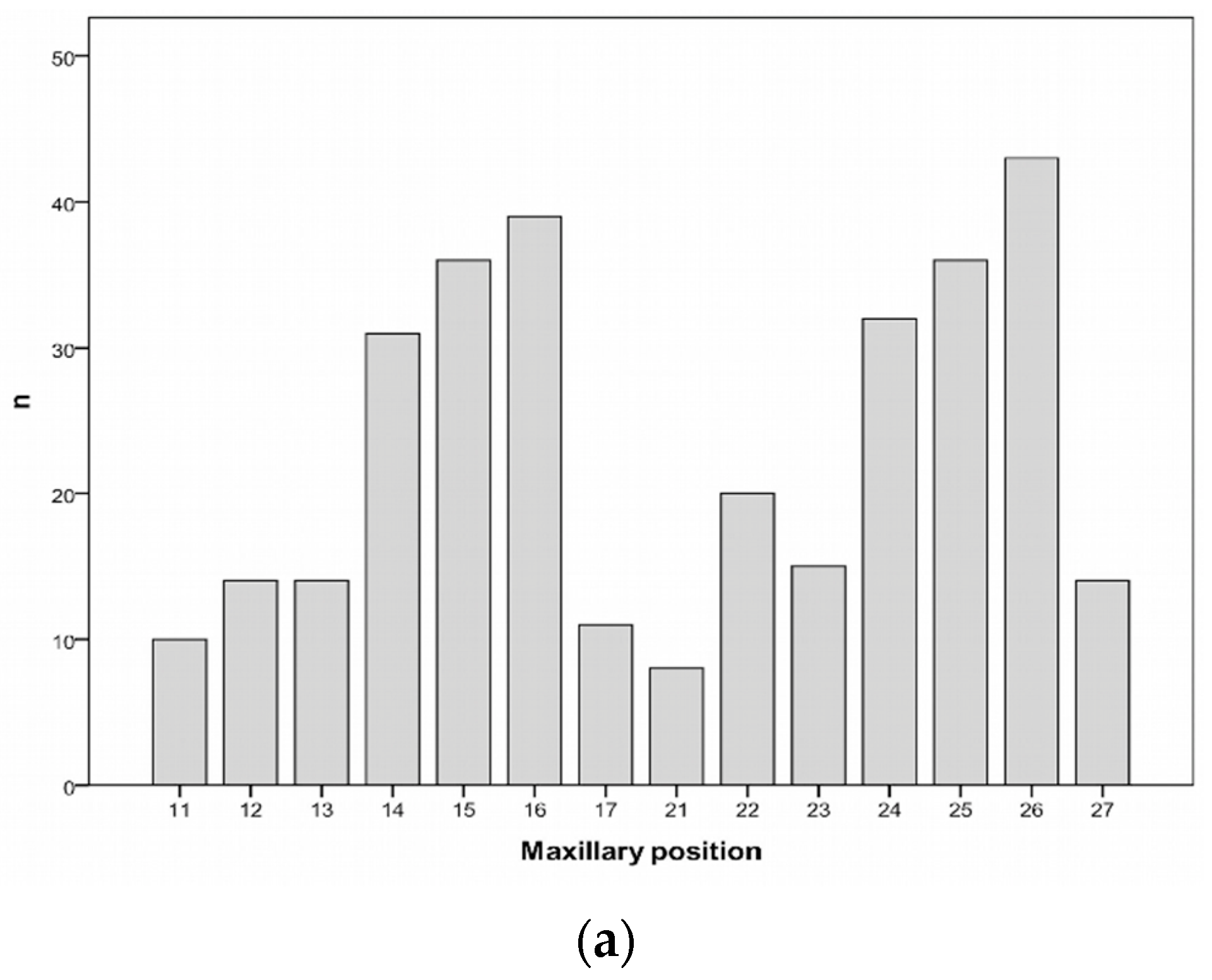
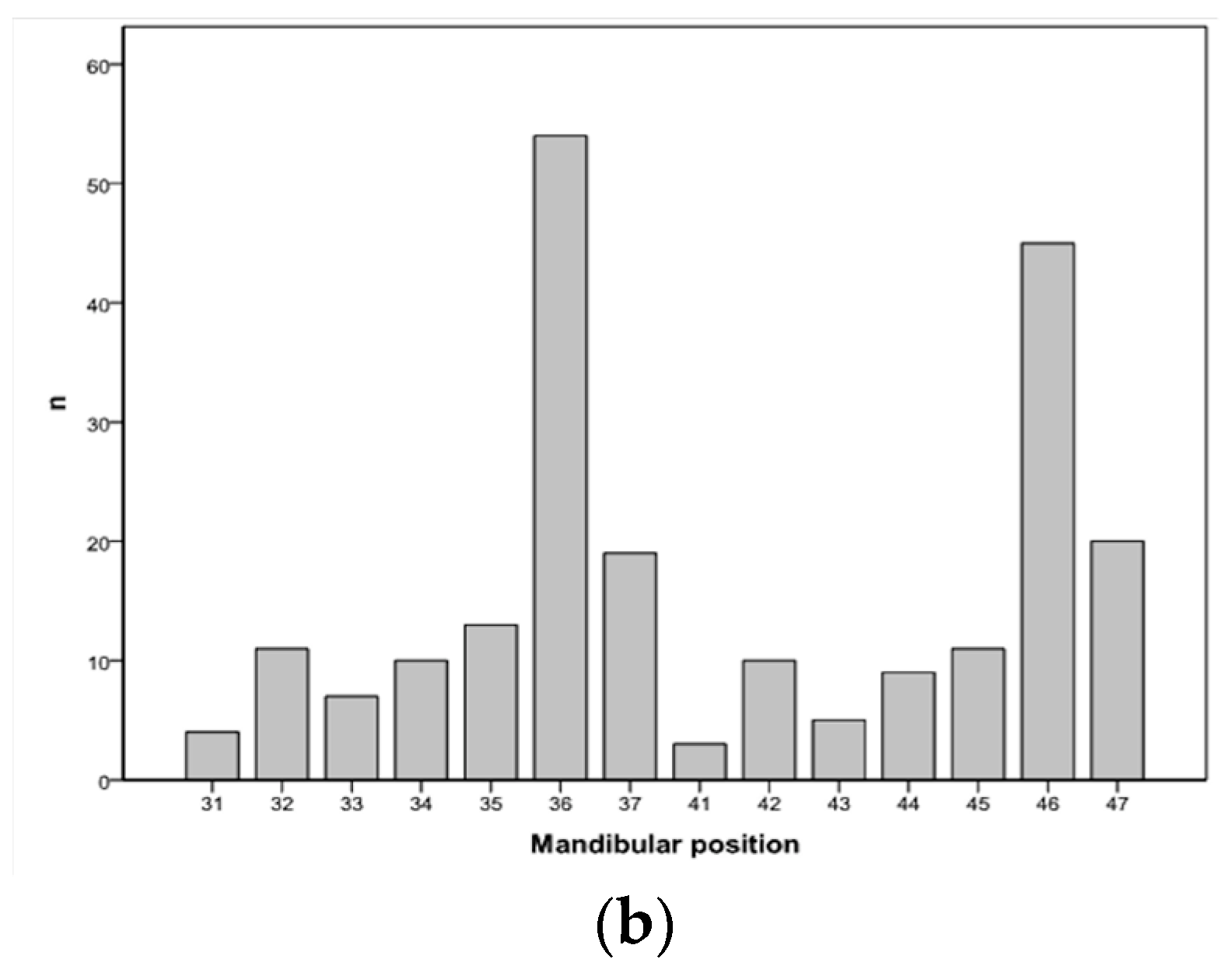
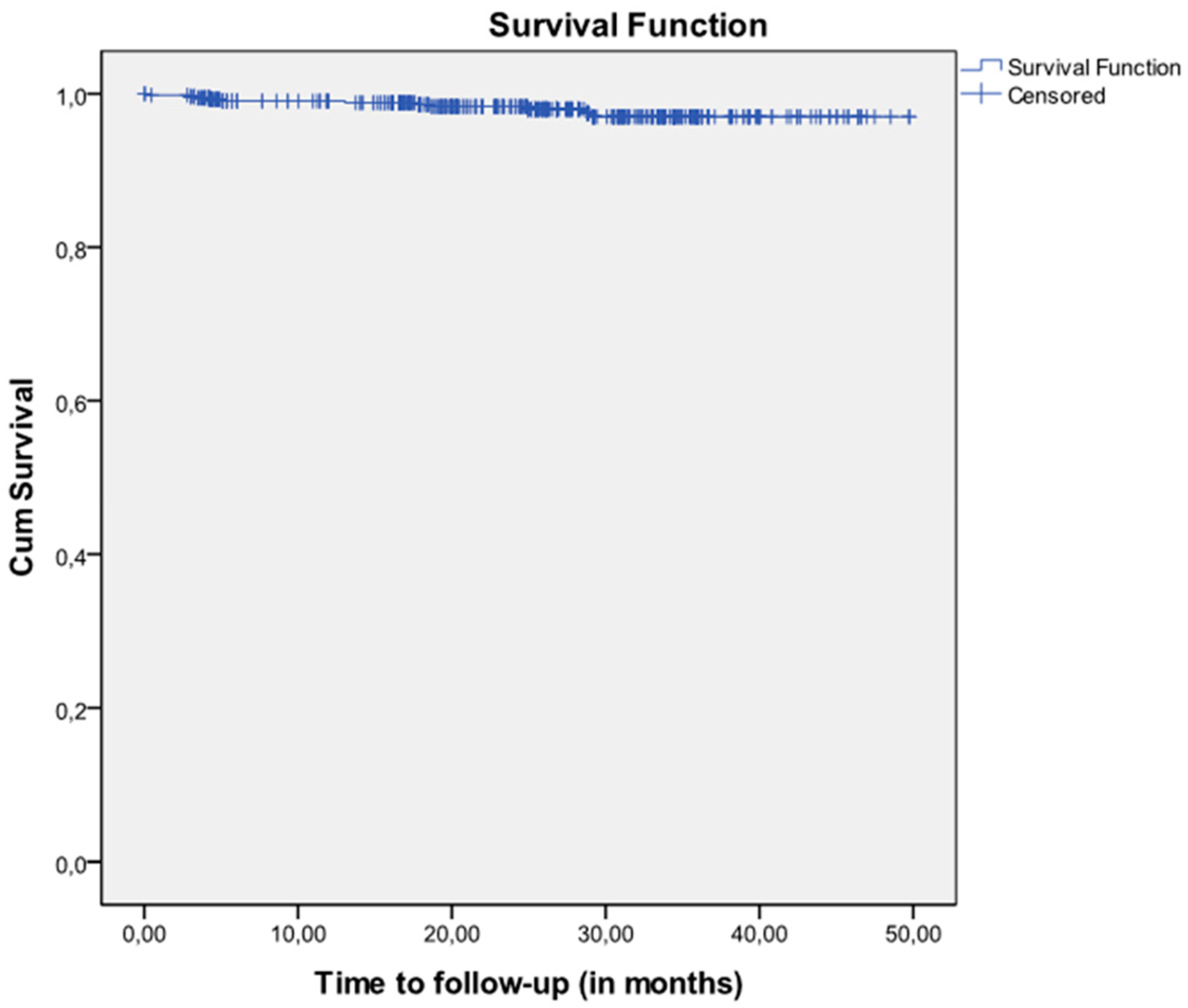
| Patients’ Characteristics | n = 212 | % | |
|---|---|---|---|
| Age, mean (SD) | 51.2 | (11.9) | |
| Gender, n (%) | Men | 109 | 51.5 |
| Women | 103 | 48.5 | |
| Implants per patient, median (IQR) | 2.0 | (2.0) | |
| Smoking status, n (%) | Smoker ≥ 10 cigarettes/day | 20 | 9.4 |
| Smoker < 10 cigarettes/day | 20 | 9.4 | |
| Never smoked | 172 | 81.1 | |
| Periodontal disease, n (%) | No | 148 | 69.8 |
| Yes; controlled | 64 | 30.2 | |
| Bruxism, n (%) | No | 136 | 64.2 |
| Yes | 76 | 35.8 | |
| Number of implants per patient, n (%) | 1 | 70 | 33.0 |
| 2 | 67 | 31.6 | |
| 3 | 27 | 12.7 | |
| 4 | 21 | 9.9 | |
| 5 | 12 | 5.7 | |
| 6 | 8 | 3.8 | |
| 7 | 2 | 0.9 | |
| 8 | 1 | 0.5 | |
| 9 | 1 | 0.5 | |
| 12 | 3 | 1.4 | |
| Characteristics of the Implants | n | % | |
|---|---|---|---|
| Connection, n (%) | External hexagon | 144 | 26.5 |
| Internal hexagon | 400 | 73.5 | |
| Diameter, n (%) | 3.5 mm | 153 | 28.1 |
| 4.0 mm | 185 | 34.0 | |
| 4.5 mm | 189 | 34.7 | |
| 5.0 mm | 17 | 3.1 | |
| Length, n (%) | 7.0 mm | 1 | 0.2 |
| 8.5 mm | 17 | 3.1 | |
| 10.0 mm | 155 | 28.5 | |
| 11.5 mm | 233 | 42.8 | |
| 13.0 mm | 138 | 25.4 | |
| Bone density, n (%) | Type I | 44 | 8.1 |
| Type II–III | 430 | 79.0 | |
| Type IV | 70 | 12.9 | |
| Bone characteristics, n (%) | Extraction site | 36 | 6.6 |
| Healed bone | 378 | 69.5 | |
| Regenerated bone | 130 | 23.9 | |
| Surgical stages, n (%) | 1 stage (healing abutment) | 89 | 16.4 |
| 2 stages (cover screw) | 455 | 83.6 | |
| Type of loading, n (%) | Immediate (<48 h) | 32 | 5.9 |
| Early (8–12 weeks) | 33 | 6.0 | |
| Delayed (>12 weeks) | 479 | 88.1 | |
| Final position of the platform, n (%) | Subcrestal | 29 | 5.3 |
| Crestal | 510 | 93.8 | |
| Supracrestal | 5 | 0.9 | |
| Prosthesis Characteristics | n | % | |
|---|---|---|---|
| Material of the prosthesis, n (%) | Cast Cobalt-Chromium | 212 | 42.1 |
| Milled Cobalt-Chromium | 266 | 52.8 | |
| Milled Titanium | 6 | 1.1 | |
| Others | 20 | 4.0 | |
| Type of prosthetic restoration, n (%) | Screw-retained crown | 146 | 29.0 |
| Screw-retained bridge | 273 | 54.2 | |
| Cement-retained crown | 6 | 1.2 | |
| Cement-retained bridge | 24 | 4.8 | |
| Overdentures | 23 | 4.6 | |
| Others | 32 | 6.3 | |
| Design of the prosthesis, n (%) | CAD-CAM | 266 | 52.8 |
| Conventional | 238 | 47.2 |
| Failure | Position | Months of Follow-Up | Implant | Bone Density | Bone Characteristics | Periodontal Disease | Bruxism | Smoking | Age |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | 3 | 3.5 × 13 IH | Type II–III | Regenerated bone | Yes | No | Non smoker | 76 |
| 2 | 31 | 13.70 | 3.5 × 13 IH | Type II–III | Regenerated bone | No | Yes | Non smoker | 43 |
| 3 | 47 | 4.27 | 4.0 × 10 IH | Type II–III | Healed bone | No | No | Non smoker | 62 |
| 4 | 31 | 5 | 3.5 × 11.5 IH | Type II–III | Alveolar after extraction | No | Yes | Non smoker | 45 |
| 5 | 26 | 0.23 | 4.0 × 10 IH | Type II–III | Healed bone | No | No | Non smoker | 60 |
| 6 | 26 | 3.30 | 4.5 × 10 IH | Type II–III | Regenerated bone | Yes | No | Non smoker | 64 |
| 7 | 36 | 18.63 | 4.5 × 10 IH | Type II–III | Regenerated bone | Yes | No | Non smoker | 57 |
| 8 | 46 | 6.53 | 4.0 × 10 IH | Type II–III | Healed bone | No | Yes | Non smoker | 35 |
| 9 | 37 | 4.20 | 4.0 × 11.5 IH | Type II–III | Healed bone | Yes | Yes | Non smoker | 44 |
| No. of Implants at Risk | No. of Failures | No. of Lost Implants | Cumulative Survival | Lower Limit 95% CI | Upper Limit 95% CI | Interval Survival | Lower Limit 95% CI | Upper Limit 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| 0–3 months | 544 | 2 | 5 | 99.63 | 99.12 | 100.00 | 99.63 | 99.12 | 100.00 |
| 3–6 months | 537 | 4 | 64 | 99.04 | 98.22 | 99.87 | 98.89 | 98.00 | 99.78 |
| 6–9 months | 469 | 0 | 7 | 99.04 | 98.16 | 99.93 | 100.00 | -- | -- |
| 9–12 months | 462 | 0 | 12 | 99.04 | 98.16 | 99.93 | 100.00 | -- | -- |
| 12–15 months | 450 | 1 | 6 | 98.82 | 97.82 | 99.82 | 98.67 | 97.61 | 99.73 |
| 15–18 months | 443 | 1 | 53 | 98.57 | 97.47 | 99.67 | 98.44 | 97.29 | 99.59 |
| 18–21 months | 389 | 1 | 54 | 98.32 | 97.04 | 99.60 | 98.18 | 96.85 | 99.51 |
| 21–24 months | 334 | 0 | 30 | 98.32 | 96.94 | 99.70 | 100.00 | -- | -- |
| Patient Characteristics | Cumulative Survival at 24 Months (95% CI) | Log-Rank p-Value | |
|---|---|---|---|
| Age | <65 years | 98.14 (96.42–99.86) | 0.413 |
| ≥65 years | 99.02 (96.68–100.00) | ||
| Smoking Status | Never smoked | 97.86 (96.02–99.70) | 0.226 |
| Smoker < 10 cigarettes/day | 100.00 (-) | ||
| Smoker ≥ 10 cigarettes/day | 100.00 (-) | ||
| Periodontal disease | No | 98.49 (96.84–100.00) | 0.339 |
| Yes | 97.93 (95.04–100.00) | ||
| Bruxism | No | 98.57 (96.99–100.00) | 0.246 |
| Yes, with/without bite splint | 97.73 (94.60–100.00) | ||
| Position of the implant | Mandible | 97.35 (94.89–99.81) | 0.136 |
| Maxilla | 99.06 (97.63–100.00) | ||
| Implant connection | External hexagon | 99.26 (96.86–100.00) | 0.785 |
| Internal hexagon | 98.11 (96.44–99.78) | ||
| Bone density | Type I | 97.14 (90.73–100.00) | 0.911 |
| Type II-III | 98.16 (96.45–99.87) | ||
| Type IV | 100.00 (-) | ||
| Bone characteristics | Extraction site | 96.67 (88.81–100.00) | 0.160 |
| Healed bone | 99.11 (97.87–100.00) | ||
| Regenerated bone | 96.29 (91.70–100.00) | ||
| Surgical stages | One stage (healing abutment) | 95.72 (89.60–100.00) | 0.268 |
| Two stages (cover screw) | 98.78 (97.45–100.00) | ||
| Type of loading | Immediate (<48 h) | 100.00 (-) | 0.255 |
| Early (8–12 weeks) | 96.77 (93.34–100.00) | ||
| Delayed (>12 weeks) | 98.37 (96.82–99.92) | ||
| Final position of the platform | Subcrestal | 100.00 (-) | 0.699 |
| Crestal | 98.20 (96.66–99.74) | ||
| Supracrestal | 100.00 (-) | ||
| Diameter of the implant (mm) | 3.5 | 97.90 (95.45–100.00) | 0.869 |
| 4.0 | 98.27 (96.22–100.00) | ||
| 4.5 | 98.51 (96.19–100.00) | ||
| 5.0 | 100.00 (-) | ||
| Length of the implant (mm) | 7.0 | 100.00 (-) | 0.322 |
| 8.5 | 100.00 (-) | ||
| 10.0 | 97.97 (95.60–100.00) | ||
| 11.5 | 99.54 (98.64–100.00) | ||
| 13.0 | 99.26 (97.81–100.00) | ||
| Number of implants | 1 | 93.26 (86.31–100.00) | 0.157 |
| >1 | 97.06 (93.92–100.00) | ||
| Number of implants | 1 | 93.26 (86.31–100.00) | 0.205 |
| 2–6 | 97.60 (94.01–100.00) | ||
| >6 | 87.50 (63.00–100.00) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subirà-Pifarré, C.; Masuet-Aumatell, C.; Rodado Alonso, C.; Medina Madrid, R.; Galletti, C. Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up. J. Funct. Biomater. 2019, 10, 5. https://doi.org/10.3390/jfb10010005
Subirà-Pifarré C, Masuet-Aumatell C, Rodado Alonso C, Medina Madrid R, Galletti C. Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up. Journal of Functional Biomaterials. 2019; 10(1):5. https://doi.org/10.3390/jfb10010005
Chicago/Turabian StyleSubirà-Pifarré, Carles, Cristina Masuet-Aumatell, Carlos Rodado Alonso, Ricardo Medina Madrid, and Cosimo Galletti. 2019. "Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up" Journal of Functional Biomaterials 10, no. 1: 5. https://doi.org/10.3390/jfb10010005
APA StyleSubirà-Pifarré, C., Masuet-Aumatell, C., Rodado Alonso, C., Medina Madrid, R., & Galletti, C. (2019). Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up. Journal of Functional Biomaterials, 10(1), 5. https://doi.org/10.3390/jfb10010005





