Withangulatin A Identified as a Covalent Binder to Zap70 Kinase by Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Structures and Software
2.2. In Silico Molecular Docking Procedure
2.3. DFT Calculations and Predicted Physicochemical and ADMET Properties
3. Results
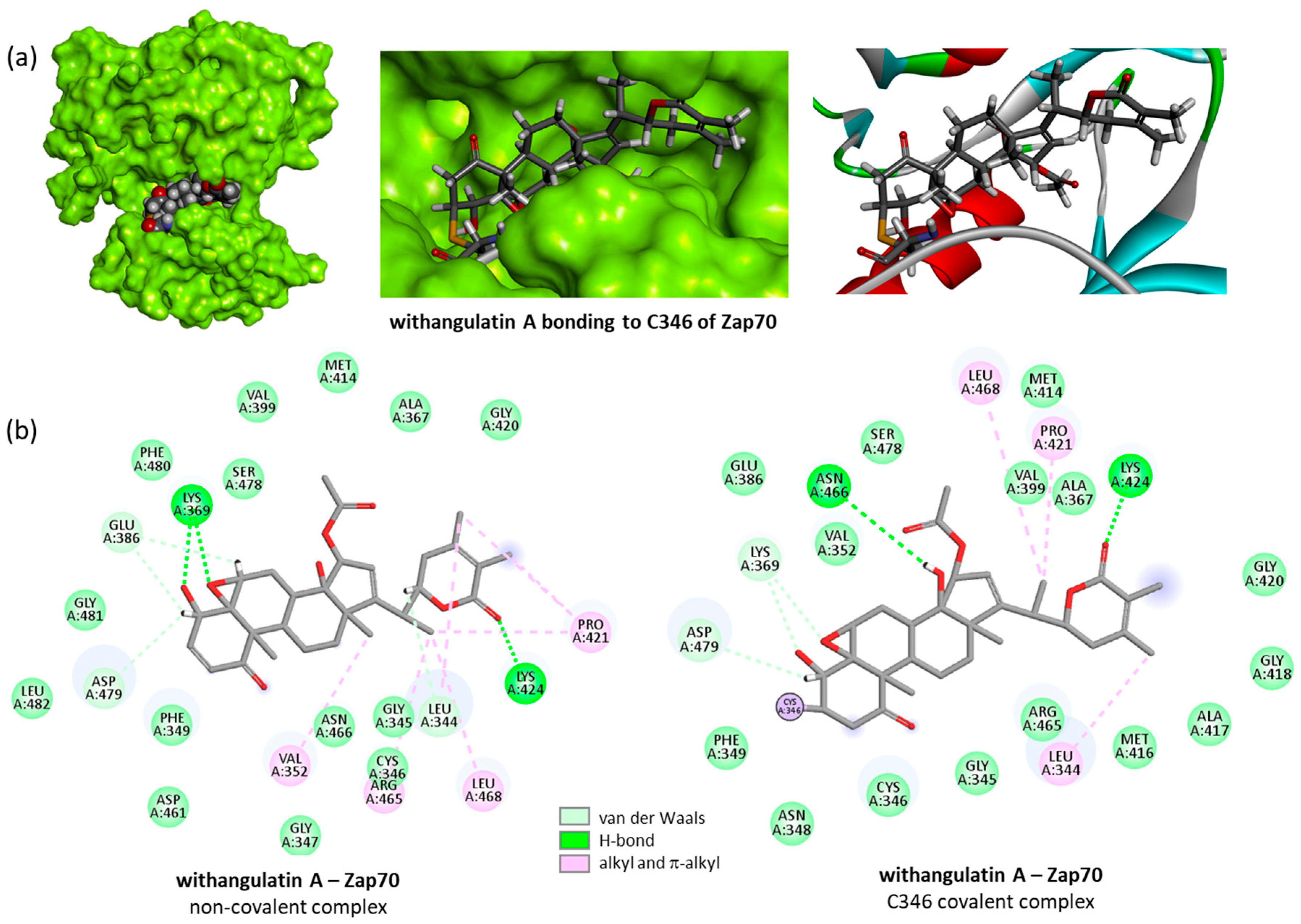
3.1. Protein Structure and Binding Site Analysis
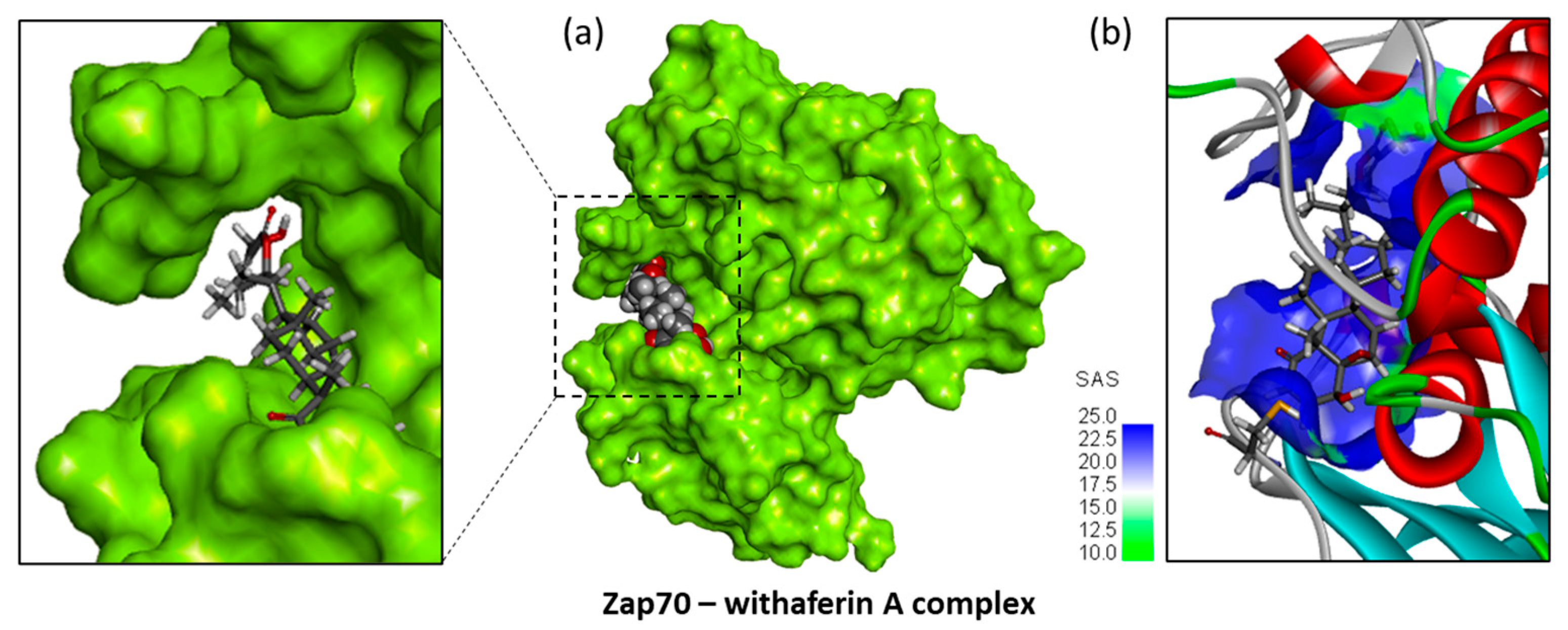
3.2. Covalent Binding of WFA to Zap70
3.3. Comparative Molecular Docking of Withanolides to Zap70
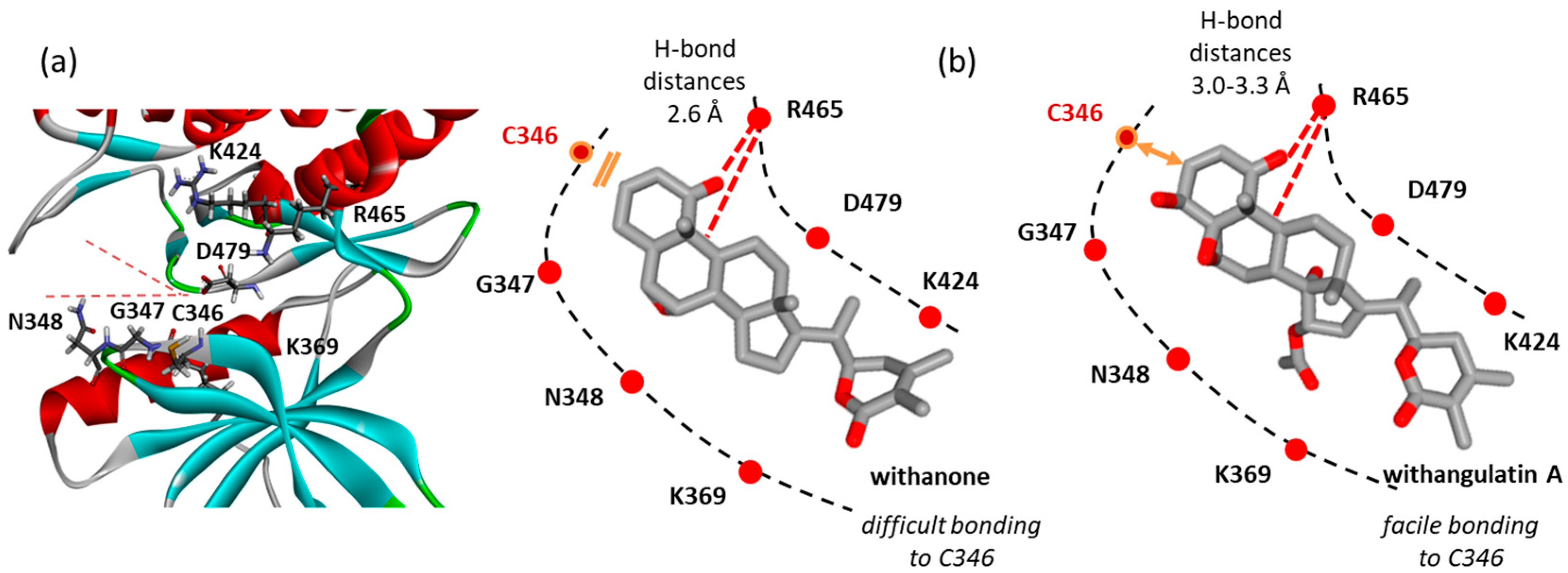
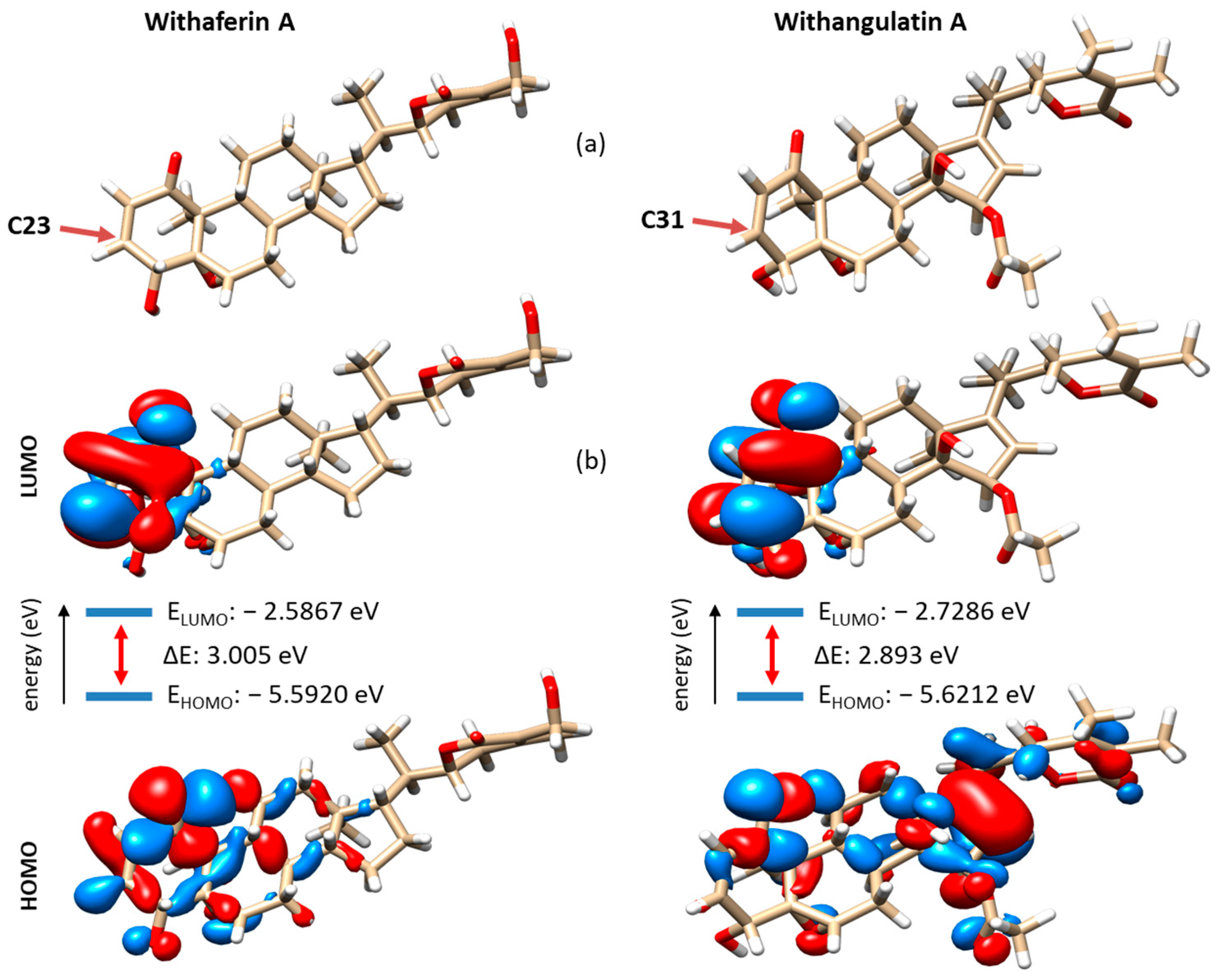
3.4. Comparative Reactivity and Druggability of Withangulatin A and WFA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLL | Chronic lymphocytic leukemia |
| DFT | Density functional theory |
| 4β-HWNE | 4β-hydroxywithanolide E |
| WFA | Withaferin A |
| Zap70 | Zeta-chain associated protein kinase 70 kDa |
References
- Ashouri, J.F.; Lo, W.L.; Nguyen, T.T.T.; Shen, L.; Weiss, A. ZAP70, too little, too much can lead to autoimmunity. Immunol. Rev. 2022, 307, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, B.B.; Shah, N.H.; Shen, L.; Weiss, A. ZAP-70 in Signaling, Biology, and Disease. Annu. Rev. Immunol. 2018, 36, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Moore, A.; Ringshausen, I. ZAP-70 Shapes the Immune Microenvironment in B Cell Malignancies. Front. Oncol. 2020, 10, 595832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, J.; Bi, Y.; Wang, H. ZAP-70 in chronic lymphocytic leukemia: A meta-analysis. Clin. Chim. Acta 2018, 483, 82–88. [Google Scholar] [CrossRef]
- Demir, M.; Cizmecioglu, O. ZAP70 Activation Compensates for Loss of Class IA PI3K Isoforms Through Activation of the JAK-STAT3 Pathway. Cancer Diagn. Progn. 2022, 2, 391–404. [Google Scholar] [CrossRef]
- Ren, L.; Li, P.; Li, Z.; Chen, Q. AQP9 and ZAP70 as immune-related prognostic biomarkers suppress proliferation, migration and invasion of laryngeal cancer cells. BMC Cancer 2022, 22, 465. [Google Scholar] [CrossRef]
- Yang, M.L.; Lam, T.T.; Kanyo, J.; Kang, I.; Zhou, Z.S.; Clarke, S.G.; Mamula, M.J. Natural isoaspartyl protein modification of ZAP70 alters T cell responses in lupus. Autoimmunity 2023, 56, 2282945. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, M.; Silakari, O. Insight into the therapeutic aspects of ‘Zeta-Chain Associated Protein Kinase 70 kDa’ inhibitors: A review. Cell Signal. 2014, 26, 2481–2492. [Google Scholar] [CrossRef]
- Visperas, P.R.; Wilson, C.G.; Winger, J.A.; Yan, Q.; Lin, K.; Arkin, M.R.; Weiss, A.; Kuriyan, J. Identification of Inhibitors of the Association of ZAP-70 with the T Cell Receptor by High-Throughput Screen. SLAS Discov. 2017, 22, 324–331. [Google Scholar] [CrossRef]
- Rao, D.; Li, H.; Ren, X.; Sun, Y.; Wen, C.; Zheng, M.; Huang, H.; Tang, W.; Xu, S. Discovery of a potent, selective, and covalent ZAP-70 kinase inhibitor. Eur. J. Med. Chem. 2021, 219, 113393. [Google Scholar] [CrossRef]
- Masip, V.; Lirio, Á.; Sánchez-López, A.; Cuenca, A.B.; Puig de la Bellacasa, R.; Abrisqueta, P.; Teixidó, J.; Borrell, J.I.; Gibert, A.; Estrada-Tejedor, R. Expanding the Diversity at the C-4 Position of Pyrido[2,3-d]pyrimidin-7(8H)-ones to Achieve Biological Activity against ZAP-70. Pharmaceuticals 2021, 14, 1311. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T Cell Receptor Activation by Semi-Synthetic Sesquiterpene Lactone Derivatives and Molecular Modeling of Their Interaction with Glutathione and Tyrosine Kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef] [PubMed]
- Deindl, S.; Kadlecek, T.A.; Brdicka, T.; Cao, X.; Weiss, A.; Kuriyan, J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 2007, 129, 735–746. [Google Scholar] [CrossRef]
- Jin, L.; Pluskey, S.; Petrella, E.C.; Cantin, S.M.; Gorga, J.C.; Rynkiewicz, M.J.; Pandey, P.; Strickler, J.E.; Babine, R.E.; Weaver, D.T.; et al. The three-dimensional structure of the ZAP-70 kinase domain in complex with staurosporine: Implications for the design of selective inhibitors. J. Biol. Chem. 2004, 279, 42818–42825. [Google Scholar] [CrossRef]
- Huber, R.G.; Fan, H.; Bond, P.J. The Structural Basis for Activation and Inhibition of ZAP-70 Kinase Domain. PLoS Comput. Biol. 2015, 11, e1004560. [Google Scholar] [CrossRef]
- Visperas, P.R.; Winger, J.A.; Horton, T.M.; Shah, N.H.; Aum, D.J.; Tao, A.; Barros, T.; Yan, Q.; Wilson, C.G.; Arkin, M.R.; et al. Modification by covalent reaction or oxidation of cysteine residues in the tandem-SH2 domains of ZAP-70 and Syk can block phosphopeptide binding. Biochem. J. 2015, 465, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Schnurra, M.; El-Bizri, A.; Woessner, N.M.; Hartmann, S.; Hartig, R.; Minguet, S.; Schraven, B.; Simeoni, L. A Cysteine Residue within the Kinase Domain of Zap70 Regulates Lck Activity and Proximal TCR Signaling. Cells 2022, 11, 2723. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Shayahati, B.; Fan, Y.; Akimzhanov, A.M. T cell receptor-dependent S-acylation of ZAP-70 controls activation of T cells. J. Biol. Chem. 2021, 296, 100311. [Google Scholar] [CrossRef]
- Rao, D.; Yang, T.; Feng, H.; An, Q.; Zhang, S.; Yu, J.; Ren, X.; Diao, X.; Huang, H.; Tang, W.; et al. Discovery and Structural Optimization of Covalent ZAP-70 Kinase Inhibitors against Psoriasis. J. Med. Chem. 2023, 66, 12018–12032. [Google Scholar] [CrossRef]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef]
- Yadav, N.; Tripathi, S.; Sangwan, N.S. Phyto-therapeutic potential of Withania somnifera: Molecular mechanism and health implications. Phytother. Res. 2024, 38, 1695–1714. [Google Scholar] [CrossRef]
- Xing, Z.; Su, A.; Mi, L.; Zhang, Y.; He, T.; Qiu, Y.; Wei, T.; Li, Z.; Zhu, J.; Wu, W. Withaferin A: A Dietary Supplement with Promising Potential as an Anti-Tumor Therapeutic for Cancer Treatment—Pharmacology and Mechanisms. Drug Des. Devel Ther. 2023, 17, 2909–2929. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cao, S.; Kang, N.; Qiu, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Banik, S.P.; Goel, A.; Chakraborty, S.; Bagchi, M.; Bagchi, D. Revisiting the Multifaceted Therapeutic Potential of Withaferin A (WA), a Novel Steroidal Lactone, W-ferinAmax Ashwagandha, from Withania somnifera (L) Dunal. J. Am. Nutr. Assoc. 2024, 43, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Abeesh, P.; Guruvayoorappan, C. The Therapeutic Effects of Withaferin A against Cancer: Overview and Updates. Curr. Mol. Med. 2024, 24, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Covalent binding of withanolides to cysteines of protein targets. Biochem. Pharmacol. 2024, 226, 116405. [Google Scholar] [CrossRef]
- Fazil, M.H.U.T.; Chirumamilla, C.S.; Perez-Novo, C.; Wong, B.H.S.; Kumar, S.; Sze, S.K.; Vanden Berghe, W.; Verma, N.K. The steroidal lactone withaferin A impedes T-cell motility by inhibiting the kinase ZAP70 and subsequent kinome signaling. J. Biol. Chem. 2021, 297, 101377. [Google Scholar] [CrossRef]
- Fuska, J.; Fusková, A.; Rosazza, J.P.; Nicholas, A.W. Novel cytotoxic and antitumor agents. IV. Withaferin A: Relation of its structure to the in vitro cytotoxic effects on P388 cells. Neoplasma 1984, 31, 31–36. [Google Scholar]
- Nicholas, A.W.; Rosazza, J.P. Reactions of withaferin-A with model biological nucleophiles. Bioorg Chem. 1976, 5, 367–372. [Google Scholar] [CrossRef]
- Rabhi, C.; Arcile, G.; Le Goff, G.; Da Costa Noble, C.; Ouazzani, J. Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana. Molecules 2019, 24, 4599. [Google Scholar] [CrossRef]
- Goode, K.M.; Petrov, D.P.; Vickman, R.E.; Crist, S.A.; Pascuzzi, P.E.; Ratliff, T.L.; Davisson, V.J.; Hazbun, T.R. Targeting the Hsp90 C-terminal domain to induce allosteric inhibition and selective client downregulation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1992–2006. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chang, F.R.; Huang, Z.Y.; Chen, J.H.; Wu, Y.C.; Wu, C.C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J. Biol. Chem. 2008, 283, 17184–17193. [Google Scholar] [CrossRef]
- Yang, W.J.; Chen, X.M.; Wang, S.Q.; Hu, H.X.; Cheng, X.P.; Xu, L.T.; Ren, D.M.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; et al. 4β-Hydroxywithanolide E from Goldenberry (Whole Fruits of Physalis peruviana L.) as a Promising Agent against Chronic Obstructive Pulmonary Disease. J. Nat. Prod. 2020, 83, 1217–1228. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, T.; Liu, X.; Zhu, D.; Zhang, Y.; Wu, S.; Han, C.; Zhang, H.; Luo, J.; Kong, L. Identification of a novel PHGDH covalent inhibitor by chemical proteomics and phenotypic profiling. Acta Pharm. Sin. B 2022, 12, 246–261. [Google Scholar] [CrossRef]
- Chen, C.; Gong, L.; Liu, X.; Zhu, T.; Zhou, W.; Kong, L.; Luo, J. Identification of peroxiredoxin 6 as a direct target of withangulatin A by quantitative chemical proteomics in non-small cell lung cancer. Redox Biol. 2021, 46, 102130. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, C.; Wang, S.; Zhang, Y.; Zhu, D.; Li, L.; Luo, J.; Kong, L. Cellular target identification of Withangulatin A using fluorescent analogues and subsequent chemical proteomics. Chem. Commun. 2019, 55, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.J.; Cheng, Y.B.; Lin, L.C.; Tsai, Y.H.; Yao, B.Y.; Tang, J.Y.; Chang, F.R.; Yen, C.H.; Ou-Yang, F.; Chang, H.W. Physalis peruviana-Derived Physapruin A (PHA) Inhibits Breast Cancer Cell Proliferation and Induces Oxidative-Stress-Mediated Apoptosis and DNA Damage. Antioxidants 2021, 10, 393. [Google Scholar] [CrossRef]
- Yu, T.J.; Shiau, J.P.; Tang, J.Y.; Yen, C.H.; Hou, M.F.; Cheng, Y.B.; Shu, C.W.; Chang, H.W. Physapruin A Induces Reactive Oxygen Species to Trigger Cytoprotective Autophagy of Breast Cancer Cells. Antioxidants 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.J.; Yen, C.Y.; Cheng, Y.B.; Yen, C.H.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Physapruin A Enhances DNA Damage and Inhibits DNA Repair to Suppress Oral Cancer Cell Proliferation. Int. J. Mol. Sci. 2022, 23, 8839. [Google Scholar] [CrossRef]
- Yu, T.J.; Shiau, J.P.; Tang, J.Y.; Farooqi, A.A.; Cheng, Y.B.; Hou, M.F.; Yen, C.H.; Chang, H.W. Physapruin A Exerts Endoplasmic Reticulum Stress to Trigger Breast Cancer Cell Apoptosis via Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 8853. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xu, W.; Liu, Y.; Zhang, J.H.; Yang, Y.Y.; Wang, Z.W.; Sun, D.J.; Li, H.; Liu, B.; Chen, L.X. Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int. J. Biol. Sci. 2023, 19, 2531–2550. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689–1700. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Meziane-Tani, M.; Lagant, P.; Semmoud, A.; Vergoten, G. The SPASIBA force field for chondroitin sulfate: Vibrational analysis of D-glucuronic and N-acetyl-D-galactosamine 4-sulfate sodium salts. J. Phys. Chem. A 2006, 110, 11359–11370. [Google Scholar] [CrossRef] [PubMed]
- Lagant, P.; Nolde, D.; Stote, R.; Vergoten, G.; Karplus, M. Increasing normal modes analysis accuracy: The SPASIBA spectroscopic force field introduced into the CHARMM program. J. Phys. Chem. A 2004, 108, 4019–4029. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Ulmschneider, J.P.; Tirado-Rives, J. Free energies of hydration from a generalized Born model and an ALL-atom force field. J. Phys. Chem. B 2004, 108, 16264–16270. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Molecular docking of cryptoconcatones to α-tubulin and related pironetin analogues. Plants 2023, 12, 296. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Interaction of microcolin cyanobacterial lipopeptides with phosphatidylinositol transfer protein (PITP)—Molecular docking analysis. Future Pharmacol. 2025, 5, 13. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Liu, Z.; Zubatiuk, T.; Roitberg, A.; Isayev, O. Auto3D: Automatic Generation of the Low-Energy 3D Structures with ANI Neural Network Potentials. J. Chem. Inf. Model. 2022, 62, 5373–5382. [Google Scholar] [CrossRef]
- Francoeur, P.G.; Koes, D.R. SolTranNet-A Machine Learning Tool for Fast Aqueous Solubility Prediction. J. Chem. Inf. Model. 2021, 61, 2530–2536, Erratum in J. Chem. Inf. Model. 2021, 61, 4120–4123. [Google Scholar] [CrossRef]
- Jang, W.D.; Jang, J.; Song, J.S.; Ahn, S.; Oh, K.S. PredPS: Attention-based graph neural network for predicting stability of compounds in human plasma. Comput. Struct. Biotechnol. J. 2023, 21, 3532–3539. [Google Scholar] [CrossRef]
- Lee, S.W.; Pan, M.H.; Chen, C.M.; Chen, Z.T. Withangulatin I, a new cytotoxic withanolide from Physalis angulata. Chem. Pharm. Bull. 2008, 56, 234–236. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, Z.T.; Hsieh, C.H.; Li, W.S.; Wen, S.Y. Withangulatin A, a new withanolide from Physalis angulata. Heterocycles 1990, 31, 1371–1375. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Richards, F.M. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef]
- Khan, S.A.; Adhikari, A.; Ayub, K.; Farooq, A.; Mahar, S.; Qureshi, M.N.; Rauf, A.; Khan, S.B.; Ludwig, R.; Mahmood, T. Isolation, characterization and DFT studies of epoxy ring containing new withanolides from Withania coagulans Dunal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Abeesh, P.; Vishnu, W.K.; Guruvayoorappan, C. Preparation and characterization of withaferin A loaded pegylated nanoliposomal formulation with high loading efficacy: In vitro and in vivo anti-tumour study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112335. [Google Scholar] [CrossRef]
- Abeesh, P.; Guruvayoorappan, C. Withaferin A-Encapsulated PEGylated Nanoliposomes Induce Apoptosis in B16F10 Melanoma Cells by Regulating Bcl2 and Bcl xl Genes and Mitigates Murine Solid Tumor Development. J. Environ. Pathol. Toxicol. Oncol. 2024, 43, 29–42. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Lee, J.H.; Lee, B.H.; Song, J.S.; Ahn, S.; Oh, K.S. PredMS: A random forest model for predicting metabolic stability of drug candidates in human liver microsomes. Bioinformatics 2022, 38, 364–368. [Google Scholar] [CrossRef]
- Venkatraman, V. FP-ADMET: A compendium of fingerprint-based ADMET prediction models. J. Cheminform. 2021, 13, 75. [Google Scholar] [CrossRef]
- Philips, C.A.; Theruvath, A.H. A comprehensive review on the hepatotoxicity of herbs used in the Indian (Ayush) systems of alternative medicine. Medicine 2024, 103, e37903. [Google Scholar] [CrossRef]
- Lerose, V.; Ponticelli, M.; Benedetto, N.; Carlucci, V.; Lela, L.; Tzvetkov, N.T.; Milella, L. Withania somnifera (L.) Dunal, a Potential Source of Phytochemicals for Treating Neurodegenerative Diseases: A Systematic Review. Plants 2024, 13, 771. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Goel, A.; Chakraborty, S.; Bagchi, M.; Bagchi, D. A critical assessment of the whole plant-based phytotherapeutics from Withania somnifera (L.) Dunal with respect to safety and efficacy vis-a-vis leaf or root extract-based formulation. Toxicol. Mech. Methods 2023, 33, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.; Tune, B.X.J.; Wu, Y.S.; Batumalaie, K.; Sekar, M.; Sarker, M.M.R.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Gopinath, S.C.B. Withanone as an Emerging Anticancer Agent and Understanding Its Molecular Mechanisms: Experimental and Computational Evidence. Curr. Cancer Drug Targets 2025, 25, 574–585. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, L.; Chen, M.; Zhong, R.; Liu, J. Amelioration of systemic lupus erythematosus by Withangulatin A in MRL/lpr mice. J. Cell Biochem. 2011, 112, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Mosallam, A.M.; Ibrahim, A.O.A.; Badr, M.; Abdelmonsef, A.H. Novel 3-phenylquinazolin-2,4(1H,3H)-diones as dual VEGFR-2/c-Met-TK inhibitors: Design, synthesis, and biological evaluation. Sci. Rep. 2023, 13, 18567. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelhady, H.A.; Hassain, D.Z.H.; Abdelmonsef, A.H.; El-Naggar, M.; Elaasser, M.M.; Mahmoud, H.K. Thiazole-Based Thiosemicarbazones: Synthesis, Cytotoxicity Evaluation and Molecular Docking Study. Drug Des. Devel Ther. 2021, 15, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Hu, H.H.; Chang, F.R.; Tsai, J.Y.; Kuo, C.Y.; Wu, Y.C.; Wu, C.C. Different effects of 4beta-hydroxywithanolide E and withaferin A, two withanolides from Solanaceae plants, on the Akt signaling pathway in human breast cancer cells. Phytomedicine 2019, 53, 213–222. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.W.; Liu, P.; Yu, Y.J.; Ma, L.; Hu, L.H. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochem. 2011, 46, 482–488. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Wang, X.; Yu, L.; Hu, L.H.; Shen, X. Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways. Acta Pharmacol. Sin. 2010, 31, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.X.; Chen, C.; Liu, X.Q.; Li, Y.; Lin, Y.L.; Wu, X.T.; Kong, L.Y.; Luo, J.G. Discovery and optimization of withangulatin A derivatives as novel glutaminase 1 inhibitors for the treatment of triple-negative breast cancer. Eur. J. Med. Chem. 2021, 210, 112980. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, J.; Cui, D.; Li, J.; Yu, Y.; Ma, L.; Hu, L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J. Cell Biochem. 2010, 109, 532–541. [Google Scholar] [CrossRef]
- Sun, D.J.; Yang, Y.Y.; Liu, Y.; Ma, X.X.; Li, H.; Chen, L.X. Identification of ADP-ribosylation factor 6 as the cellular target of withangulatin A against TNBC cells by ferroptosis. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Klammt, C.; Novotná, L.; Li, D.T.; Wolf, M.; Blount, A.; Zhang, K.; Fitchett, J.R.; Lillemeier, B.F. T cell receptor dwell times control the kinase activity of Zap70. Nat. Immunol. 2015, 16, 961–969. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; Li, N.; Meng, H.; Li, Z.; Luo, J.; Qiu, Z. Hydrolytic Metabolism of Withangulatin A Mediated by Serum Albumin Instead of Common Esterases in Plasma. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.X.; Chen, C.; Liu, X.Q.; Li, Y.; Kong, L.Y.; Luo, J.G. Synthesis and biological evaluation of novel withangulatin A derivatives as potential anticancer agents. Bioorg. Chem. 2021, 108, 104690. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Zhao, J.; Yang, H.; Yin, F.; Ding, M.; Luo, J.; Wang, X.; Kong, L. Design and SAR of Withangulatin A Analogues that Act as Covalent TrxR Inhibitors through the Michael Addition Reaction Showing Potential in Cancer Treatment. J. Med. Chem. 2020, 63, 11195–11214. [Google Scholar] [CrossRef]
- Saghiri, K.; Daoud, I.; Melkemi, N.; Mesli, F. Molecular docking/dynamics simulations, MEP analysis, and pharmacokinetics prediction of some withangulatin A derivatives as allosteric glutaminase C inhibitors in breast cancer. Chem. Data Collect. 2023, 46, 101044. [Google Scholar] [CrossRef]
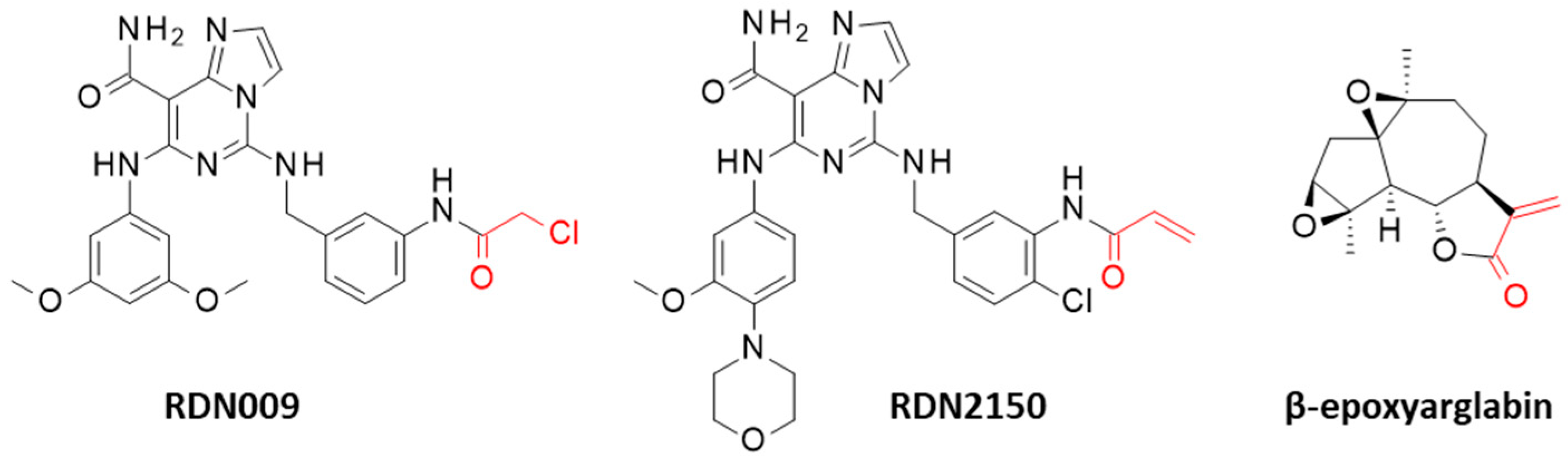
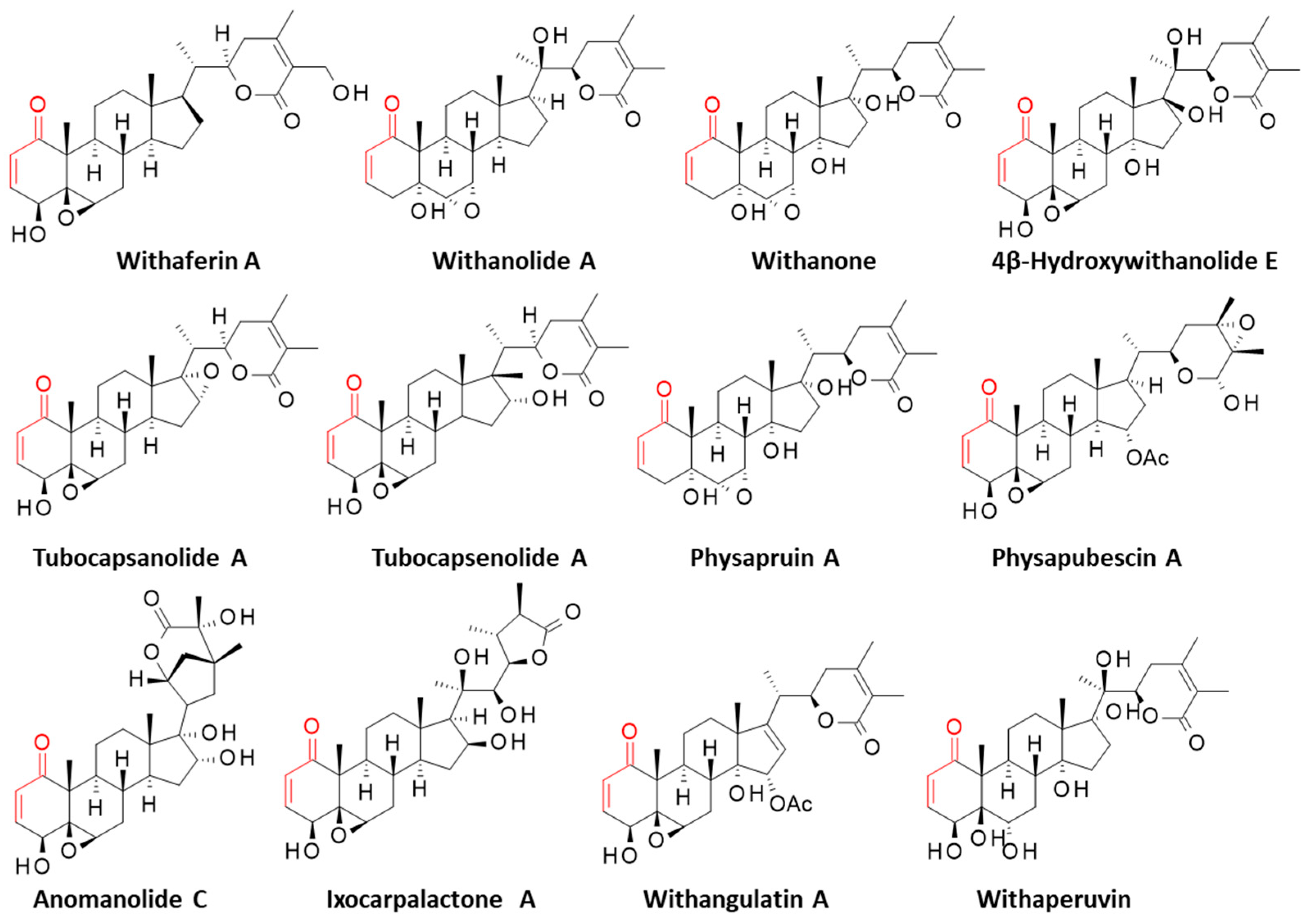




| Compounds | CID 1 | ΔE (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|
| Anomanolide C | 44423050 | −61.40 | −21.50 |
| 4β-Hydroxywithanolide E | 73621 | −75.50 | −24.30 |
| Ixocarpalactone A | 327287 | −75.50 | −24.85 |
| Physapruin A | 21607598 | −56.35 | −19.70 |
| Physapubescin | 78077011 | −72.00 | −22.40 |
| Tubocapsanolide A | 16680369 | −63.40 | −20.00 |
| Tubocapsenolide A | 16679812 | −70.20 | −23.90 |
| Withaferin A | 265237 | −69.30 | −19.10 |
| Withangulatin A | 147647 | −93.55 | −21.90 |
| Withanolide A | 11294368 | −60.15 | −19.90 |
| Withanone | 21679027 | −55.30 | −21.00 |
| Withaperuvin | 333470 | −75.35 | −20.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedart, C.; Vergoten, G.; Bailly, C. Withangulatin A Identified as a Covalent Binder to Zap70 Kinase by Molecular Docking. Computation 2025, 13, 207. https://doi.org/10.3390/computation13090207
Bedart C, Vergoten G, Bailly C. Withangulatin A Identified as a Covalent Binder to Zap70 Kinase by Molecular Docking. Computation. 2025; 13(9):207. https://doi.org/10.3390/computation13090207
Chicago/Turabian StyleBedart, Corentin, Gérard Vergoten, and Christian Bailly. 2025. "Withangulatin A Identified as a Covalent Binder to Zap70 Kinase by Molecular Docking" Computation 13, no. 9: 207. https://doi.org/10.3390/computation13090207
APA StyleBedart, C., Vergoten, G., & Bailly, C. (2025). Withangulatin A Identified as a Covalent Binder to Zap70 Kinase by Molecular Docking. Computation, 13(9), 207. https://doi.org/10.3390/computation13090207








