Smart Contracts, Blockchain, and Health Policies: Past, Present, and Future
Abstract
1. Introduction
2. Background
2.1. State of the Art on Blockchain-Based Smart Contracts in Health Management
2.2. Conceptual Framework: Social Solidarity in Health Data Governance
3. Methods
3.1. Study Design
3.2. Information Sources and Search Strategy
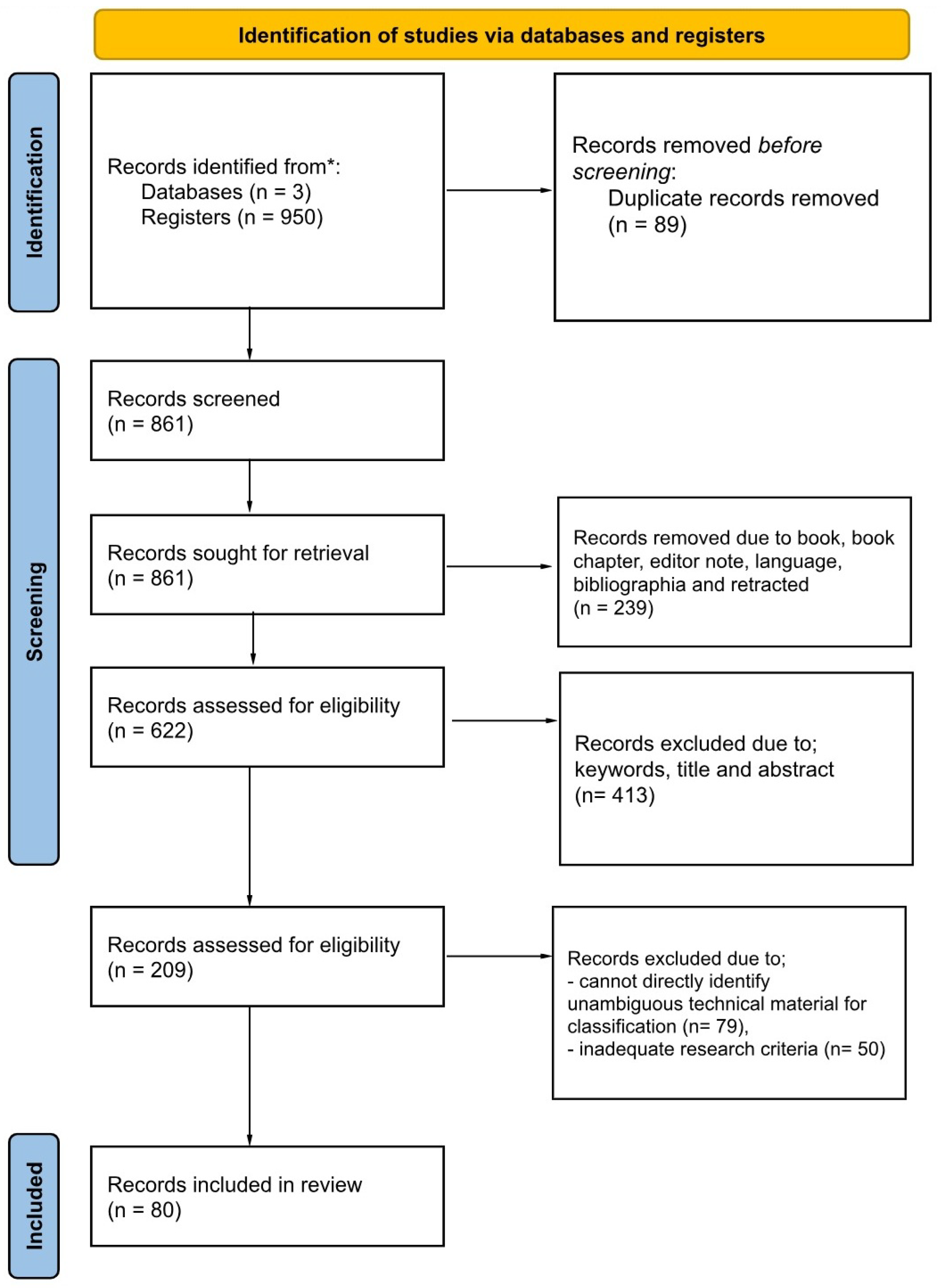
3.3. Study Selection
- Selection Breakdown:
- 950 articles identified;
- 89 duplicates removed;
- 239 excluded due to language, etc.;
- 413 excluded due to keywords, title, and abstract;
- 129 excluded due to ambiguous technical material and inadequate research, leaving 80.
3.4. Research Questions
3.5. Search Strategy
3.6. Data Collection Process
3.7. Quality Assessment
- research aims and contextualization;
- literature review and methodology;
- findings and policy relevance.
3.8. Data Items
3.9. Risk-of-Bias Assessment
3.10. Taxonomy Construction and Decision Rules
3.11. Effect Measures and Synthesis Methods
3.12. Heterogeneity and Sensitivity
3.13. Reporting Bias and Certainty of Evidence
3.14. Certainty Assessment
3.15. Performance Considerations in Healthcare Contexts
3.16. Critical Vulnerabilities of AI-Augmented Blockchain Security
- Data Imbalance: Healthcare datasets exhibit severe class imbalances, leading to high false positive rates.
- Model Interpretability: Black-box AI models conflict with healthcare’s explainability requirements.
- Adversarial Vulnerability: AI models can be compromised through input manipulation attacks.
- Privacy Conflicts: AI training requirements may violate HIPAA/GDPR patient privacy regulations.
3.17. Regulatory Compliance Analysis
3.18. AI-Model Vulnerability Appraisal
4. Findings
4.1. Comprehensive Literature Review and Analysis
4.2. Proceeding with Article Selection
4.3. Conducting the Quality Evaluation
5. Discussion
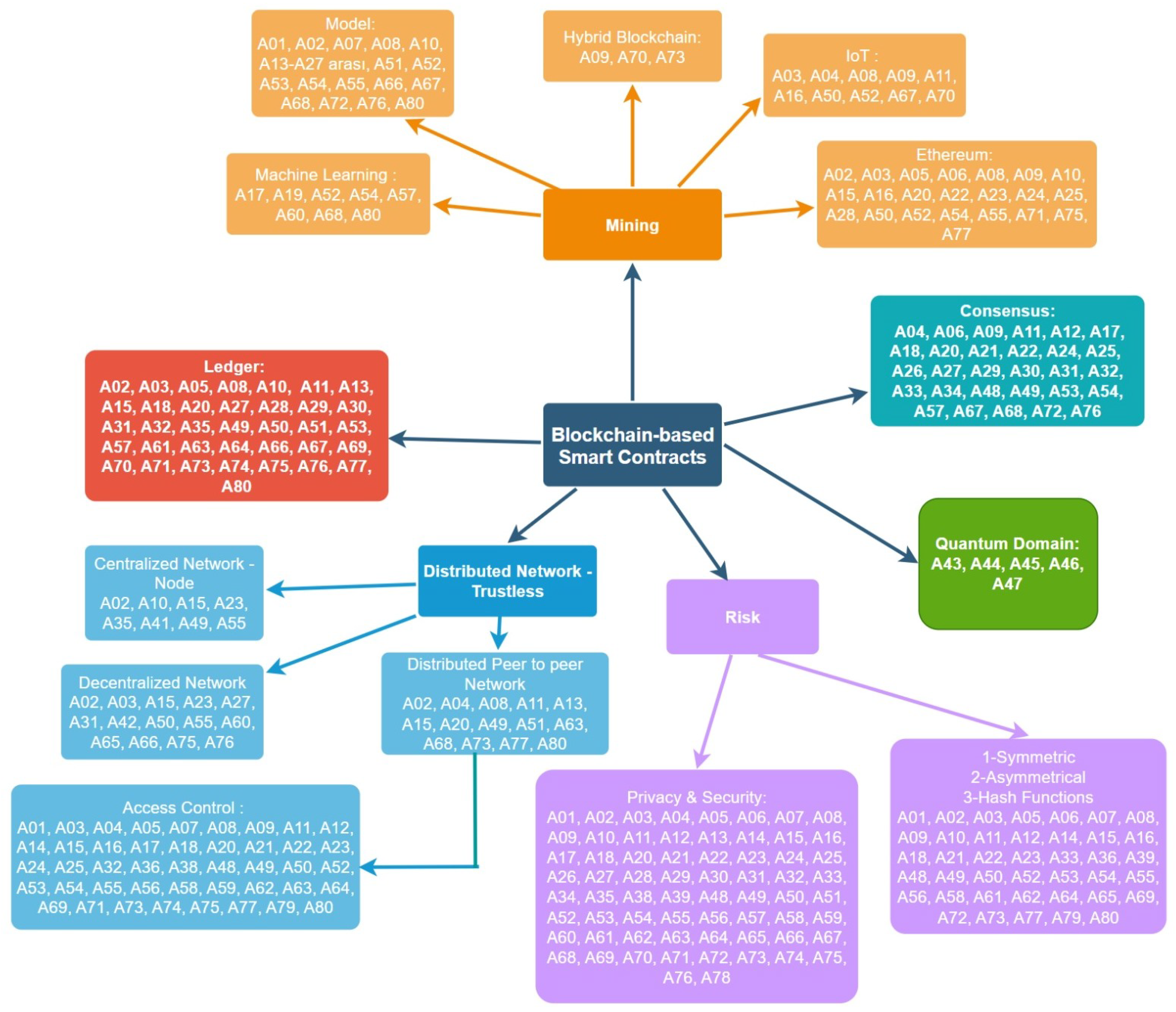
5.1. Mining
5.2. Consensus Mechanisms
5.3. Security and Encryption
5.4. Distributed Network
5.5. Ledger
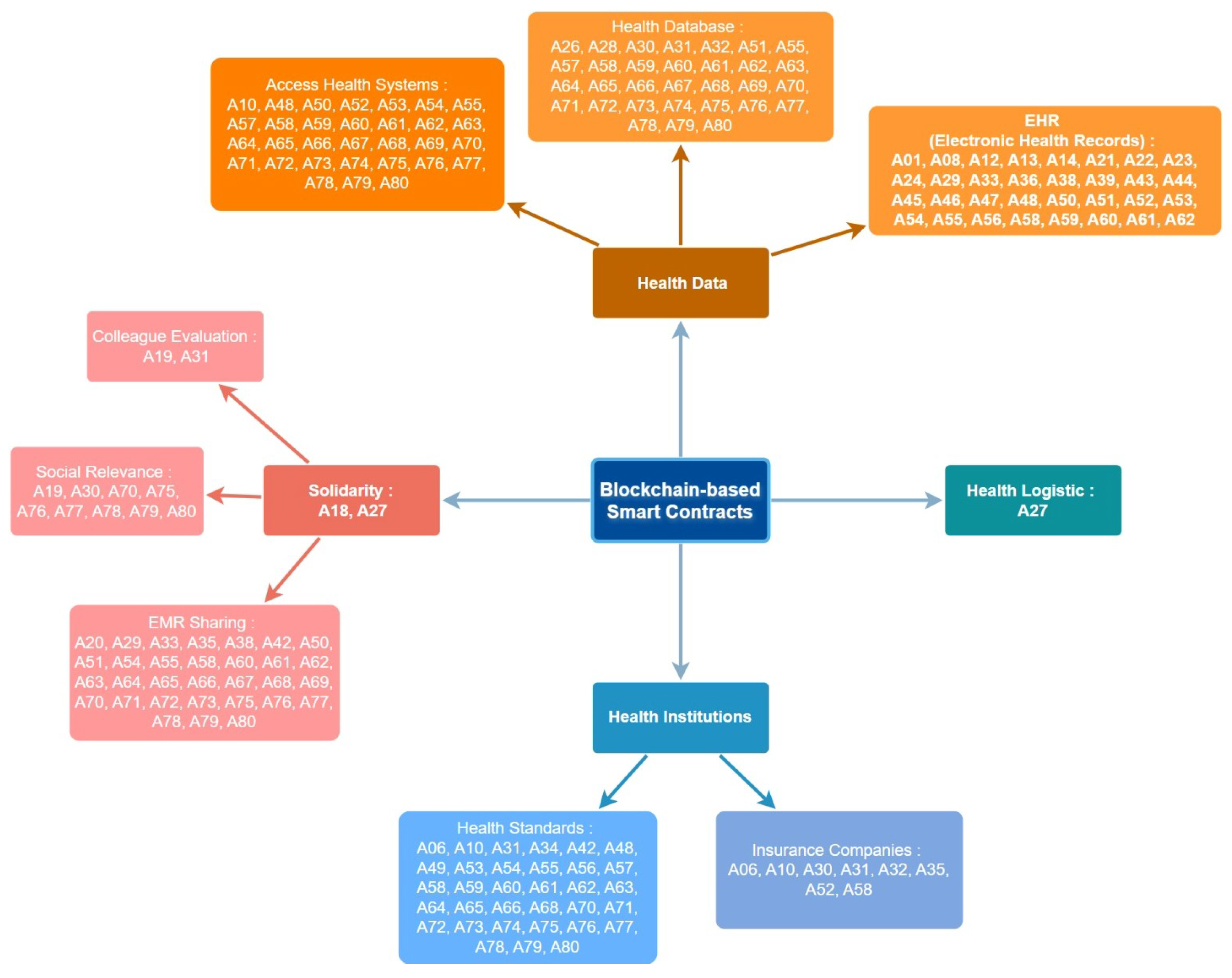
5.6. Solidarity
5.7. Health Institutions
5.8. Participants
5.9. Doctors
5.10. Insurance Companies
5.11. Quantum Domain
Linking Quantum Proposals to Healthcare Regulatory Imperatives
- Quantum key distribution (QKD) frameworks directly strengthen cross-border data transfer security and tamper-proof communication, addressing the security of processing.
- Quantum digital signatures and quantum-resistant hash functions provide forward-secure audit trails, aligning with the HIPAA requirements for verifiable access and breach notification.
- Quantum consultative trust models and reconciliation mechanisms operationalize patient consent and autonomy, which are critical pillars of healthcare policy and bioethics.
- Entangled medical record protocols and quantum authentication schemes enhance privacy protection in Internet of Medical Things (IoMT) networks, ensuring compliance with both HIPAA and GDPR consent and minimization principles.
5.12. Cross-Domain and Emerging Applications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABE | Attribute-Based Encryption |
| EHR | Electronic Health Record |
| EMR | Electronic Medical Record |
| BERT | Bidirectional Encoder Representations from Transformers |
| BFT | Byzantine Fault Tolerance |
| CA | Certificate Authorization |
| CPT-ABE | Ciphertext-Policy Attribute-Based Encryption |
| FHIR | Fast Healthcare Interoperability Resources |
| GQ | Research Guidance Question |
| HE | Homomorphic Encryption |
| HIPAA | Health Insurance Portability and Accountability Act |
| IoT | Internet of Things |
| NIH | National Institute of Health |
| NN | Neural Network |
| PHR | Personal Health Record |
| PoW | Proof of Work |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RAFT | Reliable, Replicated, Redundant, and Fault-Tolerant |
| RF | Random Forest |
| SAI | The Science and Information Organization |
| SVM | Support Vector Machine |
| WoS | Web of Science |
| ZKP | Zero-Knowledge Proof |
References
- Sun, J.; Ren, L.; Wang, S.; Yao, X. A blockchain-based framework for electronic medical records sharing with fine-grained access control. PLoS ONE 2020, 15, e0239946. [Google Scholar] [CrossRef]
- McGhin, T.; Choo, K.K.R.; Liu, C.Z.; He, D. Blockchain in healthcare applications: Research challenges and opportunities. J. Netw. Comput. Appl. 2019, 135, 62–75. [Google Scholar] [CrossRef]
- Ante, L. Smart contracts on the blockchain–A bibliometric analysis and review. Telemat. Inform. 2021, 57, 101519. [Google Scholar] [CrossRef]
- Christidis, K.; Devetsikiotis, M. Blockchains and smart contracts for the internet of things. IEEE Access 2016, 4, 2292–2303. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Wu, Q.; Mu, Y.; Rezaeibagha, F. Secure decentralized attribute-based sharing of personal health records with blockchain. IEEE Internet Things J. 2021, 9, 12482–12496. [Google Scholar] [CrossRef]
- Elhence, A.; Goyal, A.; Chamola, V.; Sikdar, B. A blockchain and ML-based framework for fast and cost-effective health insurance industry operations. IEEE Trans. Comput. Soc. Syst. 2022, 10, 1642–1653. [Google Scholar] [CrossRef]
- Wang, W.; Hoang, D.T.; Hu, P.; Xiong, Z.; Niyato, D.; Wang, P.; Wen, Y.; Kim, D.I. A survey on consensus mechanisms and mining strategy management in blockchain networks. IEEE Access 2019, 7, 22328–22370. [Google Scholar] [CrossRef]
- Alammary, A.S. Building a sustainable digital infrastructure for higher education: A blockchain-based solution for cross-institutional enrollment. Sustainability 2024, 17, 194. [Google Scholar] [CrossRef]
- Kumar, C.S.; Padhy, A.B.; Singh, A.P.; Reddy, K.H.K. A Dynamic Trading Approach Based on Walrasian Equilibrium in a Blockchain-Based NFT Framework for Sustainable Waste Management. Mathematics 2025, 13, 521. [Google Scholar] [CrossRef]
- Kaushal, R.K.; Kumar, N.; Kukreja, V.; Boonchieng, E. Hyperledger fabric based remote patient monitoring solution and performance evaluation. Peer-to-Peer Netw. Appl. 2025, 18, 105. [Google Scholar] [CrossRef]
- Prajapat, S.; Kumar, P.; Kumar, D.; Das, A.K.; Hossain, M.S.; Rodrigues, J.J. Quantum secure authentication scheme for internet of medical things using blockchain. IEEE Internet Things J. 2024, 11, 38496–38507. [Google Scholar] [CrossRef]
- Khan, S.; Khan, M.; Khan, M.A.; Khan, M.A.; Wang, L.; Wu, K. A blockchain-enabled AI-driven secure searchable encryption framework for medical IoT systems. IEEE J. Biomed. Health Inform. 2025. early access. [Google Scholar] [CrossRef]
- Kapadiya, K.; Patel, U.; Gupta, R.; Alshehri, M.D.; Tanwar, S.; Sharma, G.; Bokoro, P.N. Blockchain and AI-empowered healthcare insurance fraud detection: An analysis, architecture, and future prospects. IEEE Access 2022, 10, 79606–79627. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, N.; Gupta, B.B. A survey on scalable consensus algorithms for blockchain technology. Cyber Secur. Appl. 2025, 3, 100065. [Google Scholar] [CrossRef]
- Yadav, J.; Shevkar, R. Performance-based analysis of blockchain scalability metric. Teh. Glas. 2021, 15, 133–142. [Google Scholar] [CrossRef]
- Azaria, A.; Ekblaw, A.; Vieira, T.; Lippman, A. Medrec: Using blockchain for medical data access and permission management. In Proceedings of the 2016 2nd International Conference on Open and Big Data (OBD), Vienna, Austria, 22–24 August 2016; pp. 25–30. [Google Scholar] [CrossRef]
- Ucbas, Y.; Eleyan, A.; Hammoudeh, M.; Alohaly, M. Performance and scalability analysis of ethereum and hyperledger fabric. IEEE Access 2023, 11, 67156–67167. [Google Scholar] [CrossRef]
- Mnasri, S.; Salah, D.; Idoudi, H. A hybrid blockchain and federated learning attention-based BERT transformer framework for medical records management. J. Supercomput. 2025, 81, 317. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, G.; Feng, B.; Li, Y. A Cross-Chain Medical Data Sharing Scheme Integrating Ring Signature. In Proceedings of the 2024 4th International Conference on Computer Science and Blockchain (CCSB), Shenzhen, China, 6–8 September 2024; pp. 338–342. [Google Scholar] [CrossRef]
- Qiao, Y.; Xue, Y.; Zhai, Y.; Zhang, D.; Vasilakos, A.V.; Hossain, M.S.; Mumtaz, S. A Controllable and Efficient Sharing Scheme for Medical IoT Data Based on Consortium Blockchain. In Proceedings of the 2024 IEEE International Conference on E-health Networking, Application & Services (HealthCom), Nara, Japan, 18–20 November 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Bezanjani, B.R.; Ghafouri, S.H.; Gholamrezaei, R. Privacy-preserving healthcare data in IoT: A synergistic approach with deep learning and blockchain. J. Supercomput. 2025, 81, 533. [Google Scholar] [CrossRef]
- Liu, Y.; Sharma, A.; Rani, S.; Yang, J. Supply Chain Security, Resilience and Agility in IoT-driven Healthcare. IEEE Internet Things J. 2025. early access. [Google Scholar] [CrossRef]
- Prainsack, B.; Buyx, A. Solidarity in Biomedicine and Beyond; Cambridge University Press: Cambridge, UK, 2017; Volume 33. [Google Scholar]
- Beauchamp, T.; Childress, J. Principles of biomedical ethics: Marking its fortieth anniversary. Am. J. Bioeth. 2019, 19, 9–12. [Google Scholar] [CrossRef]
- Hess, C. Mapping the new commons. SSRN Electron. J. 2008. early access. [Google Scholar] [CrossRef]
- Ostrom, E. Governing the Commons: The Evolution of Institutions for Collective Action; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Daniels, N. Just Health: Meeting Health Needs Fairly; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, A.; Da Costa, C.A.; da Rosa Righi, R. OmniPHR: A distributed architecture model to integrate personal health records. J. Biomed. Inform. 2017, 71, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Keele, S. Guidelines for Performing Systematic Literature Reviews in Software Engineering; ver. 2.3 EBSE Technical Report; EBSE. 2007. Available online: https://legacyfileshare.elsevier.com/promis_misc/525444systematicreviewsguide.pdf (accessed on 20 June 2025).
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; John Wiley & Sons: Hoboken, NJ, USA, 2008; Available online: https://fcsalud.ua.es/en/portal-de-investigacion/documentos/tools-for-the-bibliographic-research/guide-of-systematic-reviews-in-social-sciences.pdf (accessed on 20 June 2025).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Nickerson, R.C.; Varshney, U.; Muntermann, J. A method for taxonomy development and its application in information systems. Eur. J. Inf. Syst. 2013, 22, 336–359. [Google Scholar] [CrossRef]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, A.; Wu, S.; Chen, J. Blockchain-based multi-hop permission delegation scheme with controllable delegation depth for electronic health record sharing. High-Confid. Comput. 2022, 2, 100084. [Google Scholar] [CrossRef]
- Marino, C.A.; Diaz Paz, C. Smart Contracts and Shared Platforms in Sustainable Health Care: Systematic Review. JMIR Med. Inform. 2025, 13, e58575. [Google Scholar] [CrossRef] [PubMed]
- Sittig, D.F.; Singh, H. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. BMJ Qual. Saf. 2010, 19, i68–i74. [Google Scholar] [CrossRef]
- Jabarulla, M.Y.; Lee, H.N. Blockchain-based distributed patient-centric image management system. Appl. Sci. 2020, 11, 196. [Google Scholar] [CrossRef]
- Iqbal, N.; Jamil, F.; Ahmad, S.; Kim, D. A novel blockchain-based integrity and reliable veterinary clinic information management system using predictive analytics for provisioning of quality health services. IEEE Access 2021, 9, 8069–8098. [Google Scholar] [CrossRef]
- Mohsan, S.A.H.; Razzaq, A.; Ghayyur, S.A.K.; Alkahtani, H.K.; Al-Kahtani, N.; Mostafa, S.M. Decentralized patient-centric report and medical image management system based on blockchain technology and the inter-planetary file system. Int. J. Environ. Res. Public Health 2022, 19, 14641. [Google Scholar] [CrossRef]
- Ali, A.; Almaiah, M.A.; Hajjej, F.; Pasha, M.F.; Fang, O.H.; Khan, R.; Teo, J.; Zakarya, M. An industrial IoT-based blockchain-enabled secure searchable encryption approach for healthcare systems using neural network. Sensors 2022, 22, 572. [Google Scholar] [CrossRef]
- Hang, L.; Choi, E.; Kim, D.H. A novel EMR integrity management based on a medical blockchain platform in hospital. Electronics 2019, 8, 467. [Google Scholar] [CrossRef]
- Jamil, F.; Ahmad, S.; Iqbal, N.; Kim, D.H. Towards a remote monitoring of patient vital signs based on IoT-based blockchain integrity management platforms in smart hospitals. Sensors 2020, 20, 2195. [Google Scholar] [CrossRef]
- Dhillon, V. Blockchain based peer-review interfaces for digital medicine. Front. Blockchain 2020, 3, 8. [Google Scholar] [CrossRef]
- Malamas, V.; Kotzanikolaou, P.; Dasaklis, T.K.; Burmester, M. A hierarchical multi blockchain for fine grained access to medical data. IEEE Access 2020, 8, 134393–134412. [Google Scholar] [CrossRef]
- Gong, J.; Zhao, L. Blockchain application in healthcare service mode based on Health Data Bank. Front. Eng. Manag. 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Ali, A.; Rahim, H.A.; Ali, J.; Pasha, M.F.; Masud, M.; Rehman, A.U.; Chen, C.; Baz, M. A novel secure blockchain framework for accessing electronic health records using multiple certificate authority. Appl. Sci. 2021, 11, 9999. [Google Scholar] [CrossRef]
- Chondrogiannis, E.; Andronikou, V.; Karanastasis, E.; Litke, A.; Varvarigou, T. Using blockchain and semantic web technologies for the implementation of smart contracts between individuals and health insurance organizations. Blockchain Res. Appl. 2022, 3, 100049. [Google Scholar] [CrossRef]
- Su, J.; Zhang, L.; Mu, Y. BA-RMKABSE: Blockchain-aided ranked multi-keyword attribute-based searchable encryption with hiding policy for smart health system. Future Gener. Comput. Syst. 2022, 132, 299–309. [Google Scholar] [CrossRef]
- Sutanto, E.; Mulyana, R.; Arisgraha, F.C.S.; Escrivá-Escrivá, G. Integrating blockchain for health insurance in Indonesia with hash authentication. J. Theor. Appl. Electron. Commer. Res. 2022, 17, 1602–1615. [Google Scholar] [CrossRef]
- Careline, L.G.S.; Godhavari, T. Implementation of Electronic health record and health insurance management system using blockchain technology. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 668–673. [Google Scholar] [CrossRef]
- Salonikias, S.; Khair, M.; Mastoras, T.; Mavridis, I. Blockchain-based access control in a globalized healthcare provisioning ecosystem. Electronics 2022, 11, 2652. [Google Scholar] [CrossRef]
- De Oliveira, M.T.; Reis, L.H.A.; Verginadis, Y.; Mattos, D.M.F.; Olabarriaga, S.D. SmartAccess: Attribute-based access control system for medical records based on smart contracts. IEEE Access 2022, 10, 117836–117854. [Google Scholar] [CrossRef]
- Bhandawat, R.; Casucci, S.; Ramamurthy, B.; Walteros, J.L. Cooperative Blood Inventory Ledger (CoBIL): A decentralized decision-making framework for improving blood product management. Comput. Ind. Eng. 2022, 172, 108571. [Google Scholar] [CrossRef]
- Haritha, T.; Anitha, A. Multi-level security in healthcare by integrating lattice-based access control and blockchain-based smart contracts system. IEEE Access 2023, 11, 114322–114340. [Google Scholar] [CrossRef]
- Thantharate, P.; Thantharate, A. ZeroTrustBlock: Enhancing security, privacy, and interoperability of sensitive data through ZeroTrust permissioned blockchain. Big Data Cogn. Comput. 2023, 7, 165. [Google Scholar] [CrossRef]
- Abdelgalil, L.; Mejri, M. HealthBlock: A framework for a collaborative sharing of electronic health records based on blockchain. Future Internet 2023, 15, 87. [Google Scholar] [CrossRef]
- Chandini, A.; Basarkod, P.I. Patient centric pre-transaction signature verification assisted smart contract based blockchain for electronic healthcare records. J. Ambient Intell. Humaniz. Comput. 2023, 14, 4221–4235. [Google Scholar] [CrossRef]
- Karmakar, A.; Ghosh, P.; Banerjee, P.S.; De, D. ChainSure: Agent free insurance system using blockchain for healthcare 4.0. Intell. Syst. Appl. 2023, 17, 200177. [Google Scholar] [CrossRef]
- Selvarajan, S.; Mouratidis, H. A quantum trust and consultative transaction-based blockchain cybersecurity model for healthcare systems. Sci. Rep. 2023, 13, 7107. [Google Scholar] [CrossRef]
- Liu, A.; Chen, X.B.; Xu, G.; Wang, Z.; Feng, X.; Feng, H. Quantum-Enhanced Blockchain: A Secure and Practical Blockchain Scheme. Comput. Mater. Contin. 2023, 76, 259–277. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Surendiran, B. QUMA: Quantum unified medical architecture using blockchain. Informatics 2024, 11, 33. [Google Scholar] [CrossRef]
- Venkatesh, R.; Darandale, S. Enhancing Healthcare Security with Quantum Blockchain: Electronic Medical Records Protection. In Proceedings of the 2024 Second International Conference on Networks, Multimedia and Information Technology (NMITCON), Bengaluru, India, 9–10 August 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Pu, X.; Jiang, R.; Song, Z.; Liang, Z.; Yang, L. A medical big data access control model based on smart contracts and risk in the blockchain environment. Front. Public Health 2024, 12, 1358184. [Google Scholar] [CrossRef]
- Kaur, J.; Rani, R.; Kalra, N. Attribute-based access control scheme for secure storage and sharing of EHRs using blockchain and IPFS. Clust. Comput. 2024, 27, 1047–1061. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Chen, J.; Chen, F.; Xie, D.; Shen, H. Health data security sharing method based on hybrid blockchain. Future Gener. Comput. Syst. 2024, 153, 251–261. [Google Scholar] [CrossRef]
- Li, P.; Zhou, D.; Ma, H.; Lai, J. Flexible and secure access control for EHR sharing based on blockchain. J. Syst. Archit. 2024, 146, 103033. [Google Scholar] [CrossRef]
- Bobrova, P.; Perego, P.; Boiano, R. Design and Development of a Smart Fidget Toy Using Blockchain Technology to Improve Health Data Control. Sensors 2024, 24, 6582. [Google Scholar] [CrossRef]
- Igboanusi, I.S.; Nnadiekwe, C.A.; Ogbede, J.U.; Kim, D.S.; Lensky, A. BOMS: Blockchain-enabled organ matching system. Sci. Rep. 2024, 14, 16069. [Google Scholar] [CrossRef] [PubMed]
- Kaafarani, R.; Ismail, L.; Zahwe, O. Automatic Recommender System of Development Platforms for Smart Contract–Based Health Care Insurance Fraud Detection Solutions: Taxonomy and Performance Evaluation. J. Med Internet Res. 2024, 26, e50730. [Google Scholar] [CrossRef]
- Liang, X.; Alam, N.; Sultana, T.; Bandara, E.; Shetty, S. Designing A Blockchain-Empowered Telehealth Artifact for Decentralized Identity Management and Trustworthy Communication: Interdisciplinary Approach. J. Med Internet Res. 2024, 26, e46556. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Ullah, Z.; Battineni, G.; Babar, M.G.; Daood, U. The Telehealth chain: A framework for secure and transparent telemedicine transactions on the blockchain. Ir. J. Med. Sci. 2024, 193, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Zhihao, Y.; Omote, K. Emergency Medical Access Control System Based on Public Blockchain. J. Med. Syst. 2024, 48, 90. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Jiang, Z.; Li, G.; Wu, C.; Li, S. Efficient Use of Biological Data in the Web 3.0 Era by Applying Nonfungible Token Technology. J. Med. Internet Res. 2024, 26, e46160. [Google Scholar] [CrossRef]
- Yang, X.; Li, L. BPPKS: A blockchain-based privacy preserving and keyword-searchable scheme for medical data sharing. Peer-to-Peer Netw. Appl. 2024, 17, 4033–4048. [Google Scholar] [CrossRef]
- Duc, T.; Trung, P.H.T.; Trong, N.D.P.; Phuc, N.T.; Khoa, T.D.; Khiem, H.G.; Nam, B.T.; Bang, L.K. Developing a Patient-Centric Healthcare IoT Platform with Blockchain and Smart Contract Data Management. Int. J. Adv. Comput. Sci. Appl. 2024, 15, 1139–1146. [Google Scholar] [CrossRef]
- Guerra, K.; Koh, C.; Prybutok, V.; Johnson, V. A privacy perspective in adopting smart contract applications for healthcare. J. Comput. Inf. Syst. 2024, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Liang, W. A smart contract-driven access control scheme with integrity checking for electronic health records. Clust. Comput. 2024, 27, 11515–11535. [Google Scholar] [CrossRef]
- Rekik, S.; Alsulaiman, N.; Albadrani, N. A Health Record Management System Using Blockchain and Smart Contract. In Proceedings of the 2024 Seventh International Women in Data Science Conference at Prince Sultan University (WiDS PSU), Riyadh, Saudi Arabia, 3–4 March 2024; pp. 204–208. [Google Scholar] [CrossRef]
- Saha, S.; Das, A.K.; Wazid, M.; Park, Y.; Garg, S.; Alrashoud, M. Smart contract-based access control scheme for blockchain assisted 6G-enabled IoT-based big data driven healthcare cyber physical systems. IEEE Trans. Consum. Electron. 2024, 70, 6975–6986. [Google Scholar] [CrossRef]
- Vidhya, S.; Siva Raja, P.; Sumithra, R. Blockchain-Enabled Decentralized Healthcare Data Exchange: Leveraging Novel Encryption Scheme, Smart Contracts, and Ring Signatures for Enhanced Data Security and Patient Privacy. Int. J. Netw. Manag. 2024, 34, e2289. [Google Scholar] [CrossRef]
- Arabnouri, A.; Shafieinejad, A. BACASE-SH: Blockchain-based authenticated certificate-less asymmetric searchable encryption for smart healthcare. Peer-to-Peer Netw. Appl. 2024, 17, 2298–2314. [Google Scholar] [CrossRef]
- Bunia, S.; Campbell, O.; Carvalho, A.; Alluri, V. SCeFSTA: Smart Contract enabled Fair, Secure, and Transparent Auction for Healthcare Transportation. In Proceedings of the 2024 IEEE International Systems Conference (SysCon), Montreal, QC, Canada, 15–18 April 2024; pp. 1–8. [Google Scholar] [CrossRef]
- Bieniek, J.; Rahouti, M.; Xiong, K.; Ferreira Araujo, G. SecureCare: A blockchain-assisted wearable body area network for secure and scalable IoT healthcare services. Secur. Priv. 2024, 7, e431. [Google Scholar] [CrossRef]
- Alharbi, S.H.; Alzahrani, A.M.; Syed, T.A.; Alqahtany, S.S. Integrity and privacy assurance framework for remote healthcare monitoring based on IoT. Computers 2024, 13, 164. [Google Scholar] [CrossRef]
- Kumar, N.; Ali, R. A smart contract-based 6G-enabled authentication scheme for securing Internet of Nano Medical Things network. Ad Hoc Netw. 2024, 163, 103606. [Google Scholar] [CrossRef]
- Rohini, K.; Subramanian, R.; Soman, G. Improving Data Security and Scalability in Healthcare System using Blockchain Technology. Scalable Comput. Pract. Exp. 2024, 25, 3440–3452. [Google Scholar] [CrossRef]
- Li, M.; Xue, J.; Liu, Z.; Suo, Y.; Lei, T.; Wang, Y. DAMFSD: A decentralized authorization model with flexible and secure delegation. Internet Things 2024, 27, 101317. [Google Scholar] [CrossRef]
- Zhu, X.; Lai, T.; Li, H. Privacy-Preserving Byzantine-Resilient Swarm Learning for E-Healthcare. Appl. Sci. 2024, 14, 5247. [Google Scholar] [CrossRef]
- Kar, J.; Liu, X.; Li, F. LA-IMDCN: A Lightweight Authentication Scheme With Smart Contract in Implantable Medical Device Communication Networks. IEEE Access 2024, 12, 99694–99703. [Google Scholar] [CrossRef]
- Zhang, D.M.; Nie, C.; Zhang, J.Z.; Huang, H.W.; Huang, X. Consortium blockchain-based tunnel data bank for traceable sharing and treatment of structural health monitoring data. Autom. Constr. 2024, 167, 105720. [Google Scholar] [CrossRef]
- Abid, A.; Cheikhrouhou, S.; Kallel, S.; Tari, Z.; Jmaiel, M. A smart contract-based access control framework for smart healthcare systems. Comput. J. 2024, 67, 407–422. [Google Scholar] [CrossRef]
- Ahmed, H.; Gamal, A.; Abdelmouty, A. Optimizing Blockchain Platform Selection: A Decision-Making Approach Using LLMs, Type-2 Neutrosophic Numbers, CRITIC, and MAIRCA. Neutrosophic Sets Syst. 2025, 83, 25. [Google Scholar] [CrossRef]
- Ahmed, I.; Fumimoto, K.; Nakano, T.; Tran, T.H. Blockchain-empowered decentralized philanthropic charity for social good. Sustainability 2024, 16, 210. [Google Scholar] [CrossRef]
- Akhyani, J.; Patel, J.; Desai, V.; Gupta, R.; Tanwar, S.; Bhatia, J. GRACE: Blockchain and Game-Based Resource Allocation Scheme for SDN Controllers in ioT. In Proceedings of the 2024 IEEE International Conference on Communications Workshops (ICC Workshops), Denver, CO, USA, 9–13 June 2024; pp. 1431–1436. [Google Scholar] [CrossRef]
- Ansar, S.; Natarajan, P.; Guran, L.R.V.R. A New Encryption Scheme Using Blockchain for Secured Accessing of Sensitive Health Care Records. J. Adv. Inf. Technol. 2025, 16, 510–526. [Google Scholar] [CrossRef]
- Badidi, E.; Lamaazi, H.; El Harrouss, O. Toward a Secure Healthcare Ecosystem: A Convergence of Edge Analytics, Blockchain, and Federated Learning. In Proceedings of the 2024 20th International Conference on the Design of Reliable Communication Networks (DRCN), Montreal, QC, Canada, 6–9 May 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Basudan, S. IPFS-blockchain-based delegation model for internet of medical robotics things telesurgery system. Connect. Sci. 2024, 36, 2367549. [Google Scholar] [CrossRef]
- Chegenizadeh, M.; Tessone, C.J. PAVA: Privacy-Preserving Attribute-Based Verifiable Authentication in Healthcare using Smart Contracts. In Proceedings of the 2024 IEEE International Conference on Blockchain (Blockchain), Copenhagen, Denmark, 19–22 August 2024; pp. 346–353. [Google Scholar] [CrossRef]
- Devgun, T.; Kumar, G.; Conti, M. FASALKA: Offloaded Privacy Classification for Blockchain Smart Contracts. In Proceedings of the 2024 6th International Conference on Blockchain Computing and Applications (BCCA), Dubai, United Arab Emirates, 26–29 November 2024; pp. 204–210. [Google Scholar] [CrossRef]
- Kumari, D.; Parmar, A.S.; Goyal, H.S.; Mishra, K.; Panda, S. Healthrec-chain: Patient-centric blockchain enabled ipfs for privacy preserving scalable health data. Comput. Netw. 2024, 241, 110223. [Google Scholar] [CrossRef]
- Sun, L.; Liu, D.; Li, Y.; Zhou, D. A blockchain-based E-healthcare system with provenance awareness. IEEE Access 2024, 12, 110098–110112. [Google Scholar] [CrossRef]
- Rani, P.; Sachan, R.K.; Kukreja, S. Educert-chain: A secure and notarized educational certificate authentication and verification system using permissioned blockchain. Clust. Comput. 2024, 27, 10169–10196. [Google Scholar] [CrossRef]
- Riahi, A.; Erbad, A.; Bouras, A.; Mohamed, A. RL-Based Incentive Cooperative Data Learning Framework over Blockchain in Healthcare Applications (RL-ICDL-BC). In Proceedings of the 2024 International Wireless Communications and Mobile Computing (IWCMC), Ayia Napa, Cyprus, 27–31 May 2024; pp. 90–96. [Google Scholar] [CrossRef]
- Cihan, S.; Ozsoy, A.; Beyan, O.D. Managing Clinical Research on Blockchain Using FAIR Principles. Concurr. Comput. Pract. Exp. 2025, 37, e70005. [Google Scholar] [CrossRef]
- Aakanksha, A.; Sundaram, D. Optimizing Smart Ecosystems Using DAO: Collaborative Hospital Location Decision-Making. In Proceedings of the 58th Hawaii International Conference on System Sciences, Waikoloa Village, HI, USA, 7–10 January 2025; Available online: https://scholarspace.manoa.hawaii.edu/10.24251/HICSS.2025.147 (accessed on 18 June 2025).
- Ding, X.; Liu, Y.; Ning, J.; Chen, D. Blockchain-Enhanced Anonymous Data Sharing Scheme for 6G-Enabled Smart Healthcare with Distributed Key Generation and Policy Hiding. IEEE J. Biomed. Health Inform. 2025. [Google Scholar] [CrossRef]
- Abdunabi, R.; Al Amin, M.; Basnet, R. An authorization framework for body area network: A policy verification and smart contract-based integrity assurance approach. J. Comput. Secur. 2025, 33, 119–162. [Google Scholar] [CrossRef]
- Ahanger, T.A.; Ullah, I.; Algamdi, S.A.; Tariq, U. Machine learning-inspired intrusion detection system for IoT: Security issues and future challenges. Comput. Electr. Eng. 2025, 123, 110265. [Google Scholar] [CrossRef]
- Ahmed, W.; Iqbal, W.; Hassan, A.; Ahmad, A.; Ullah, F.; Srivastava, G. Elevating e-health excellence with IOTA distributed ledger technology: Sustaining data integrity in next-gen fog-driven systems. Future Gener. Comput. Syst. 2025, 168, 107755. [Google Scholar] [CrossRef]
- Ali, W.; Zhou, X.; Shao, J. Privacy-preserved and responsible recommenders: From conventional defense to federated learning and blockchain. ACM Comput. Surv. 2025, 57, 1–35. [Google Scholar] [CrossRef]
- Mishra, D.K.; Mehra, P.S. DiabeticChain: A novel blockchain approach for patient-centric diabetic data management. J. Supercomput. 2025, 81, 166. [Google Scholar] [CrossRef]
- Chaudhry, U.H.; Arshad, R.; Khalid, A.; Ray, I.G.; Hussain, M. zk-DASTARK: A quantum-resistant, data authentication and zero-knowledge proof scheme for protecting data feed to smart contracts. Comput. Electr. Eng. 2025, 123, 110089. [Google Scholar] [CrossRef]
- Guo, R.; Liao, S.; Zhu, J. CrowdBA: A Low-Cost Quality-Driven Crowdsourcing Architecture for Bounding Box Annotation Based on Blockchain. Electronics 2025, 14, 345. [Google Scholar] [CrossRef]
- Gupta, A.; Lakhwani, K. Enhancing blockchain quality-of-service: A comparative analysis and novel smart contract mechanism. Discov. Appl. Sci. 2025, 7, 807. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Cheng, Q.; Chen, X.; Luo, X. LB3AS: Lightweight Blockchain-Assisted Anonymous Authentication Scheme for Fog-Cloud-Based Internet of Medical Things. IEEE Internet Things J. 2025, 12, 18098–18114. [Google Scholar] [CrossRef]
- Huang, P.; Lin, C.; Ning, J.; Wu, W. Optimized Blockchain-Based EMR Sharing via Secure Channel-Free Universal Designated Verifier Signature Proofs. IEEE Internet Things J. 2025. [Google Scholar] [CrossRef]
- Jayachandran, P. The difference between public and private blockchain. Blockchain Unleashed: IBM Blockchain Blog 2017, 2017. Available online: https://www.ibm.com/blogs/blockchain/2017/05/the-difference-between-public-and-private-blockchain/ (accessed on 20 June 2025).
- Kamel Boulos, M.N.; Wilson, J.T.; Clauson, K.A. Geospatial blockchain: Promises, challenges, and scenarios in health and healthcare. Int. J. Health Geogr. 2018, 17, 25. [Google Scholar] [CrossRef]
- Khatoon, A. A blockchain-based smart contract system for healthcare management. Electronics 2020, 9, 94. [Google Scholar] [CrossRef]
- Jurvetson, S. How a quantum computer could break 2048-bit RSA encryption in 8 hours. MIT Technol. Rev. 2019, 30, 9. [Google Scholar]
- Denker, K.; Javaid, A.Y. Quantum computing as a threat to modern cryptography techniques. In Proceedings of the International Conference on Foundations of Computer Science (FCS), Las Vegas, NV, USA, 29 July–1 August 2019; pp. 3–8. [Google Scholar]
- Aggarwal, D.; Brennen, G.K.; Lee, T.; Santha, M.; Tomamichel, M. Quantum attacks on Bitcoin, and how to protect against them. arXiv 2017, arXiv:1710.10377. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, A.; Panigrahi, P.K. Quantum blockchain using weighted hypergraph states. Phys. Rev. Res. 2020, 2, 013322. [Google Scholar] [CrossRef]
- Kiktenko, E.O.; Pozhar, N.O.; Anufriev, M.N.; Trushechkin, A.S.; Yunusov, R.R.; Kurochkin, Y.V.; Lvovsky, A.; Fedorov, A.K. Quantum-secured blockchain. Quantum Sci. Technol. 2018, 3, 035004. [Google Scholar] [CrossRef]
- Alabdulatif, A. Blockchain-Based Privacy-Preserving Authentication and Access Control Model for E-Health Users. Information 2025, 16, 219. [Google Scholar] [CrossRef]
- Khan, A.; Litchfield, A.; Alabdulatif, A.; Khan, F. BlockPres IPFS: Performance evaluation of blockchain based secure patients prescription record storage using IPFS for smart prescription management system. Clust. Comput. 2025, 28, 255. [Google Scholar] [CrossRef]
| Metric | Lab Conditions | Healthcare Reality | Perf. Impact |
|---|---|---|---|
| Throughput (TPS) | 1000+ | 11–20 | −97% to −99% |
| Latency | Sub-second | 2–4 s | +200–400% |
| Data Complexity | Simple key–value | Complex HL7/DICOM | High |
| Consensus Participants | 3–7 nodes | 10–50 institutions | High |
| Compliance Overhead | None | Significant | High |
| AI Model | Description | Accuracy | Computational Cost | Scalability | Best Use Case |
|---|---|---|---|---|---|
| Random Forest (RF) | Ensemble learning method using decision trees | 85–90% | Medium | High | Insurance fraud detection |
| Neural Network (NN) | Multi-layered deep learning model | 92–96% | High | Medium | Transaction anomaly detection |
| BERT Transformer | NLP-based model for fraud detection via transaction logs | 93–98% | Very High | High | Smart-contract security monitoring |
| Support Vector Machine (SVM) | Classification-based algorithm with kernel functions | 80–88% | Medium | Medium | Behavioral fraud analysis |
| Dimension | Categories | Coding Criteria |
|---|---|---|
| D1—Mining | PoW; PoS; Hybrid (PoS-BFT); None/Off-chain emphasis | Identify whether the study relies on on-chain mining incentives or consensus-independent execution; infer from platform defaults if not stated. |
| D2—Consensus Family | PoW/PoS Nakamoto-style; PBFT/RAFT/PoA (BFT-like); DAG/Other | Use the explicitly declared protocol; if absent, map via platform (e.g., Hyperledger Fabric→PBFT-like). Code unclear if insufficient evidence. |
| D3—Ledger/Network Model | Public; Private-permissioned; Consortium | Classify by governance/identity: open vs. permissioned vs. consortium-operated; check identity management and node control. |
| D4—Smart-Contract Primary Function | Access control; Consent management; Incentive/payment; Provenance/audit; Key management; Orchestration/business logic | Inspect stated purpose, ABI/events, and evaluation focus. If multiple, choose the primary function driving outcomes. |
| D5—Standards and Compliance Linkage | FHIR/HL7; ISO/IEC 27799; HIPAA/GDPR mapping; None | Code explicit standard/regulatory references (article/section). If no concrete mapping, mark ‘None’. |
| ID | Ref. | Author(s) | Year | Publisher | Type |
|---|---|---|---|---|---|
| A20 | [42] | Hang et al. | 2019 | MDPI | Journal |
| A04 | [43] | Jamil et al. | 2020 | MDPI | Journal |
| A19 | [44] | Dhillon | 2020 | Frontiers Media | Journal |
| A21 | [45] | Malamas et al. | 2020 | IEEE | Conference |
| A26 | [46] | Gong and Zhao | 2020 | Springer Nature | Journal |
| A11 | [47] | Ali et al. | 2021 | MDPI | Journal |
| A15 | [38] | Jabarulla and Lee | 2021 | MDPI | Journal |
| A17 | [39] | Iqbal et al. | 2021 | IEEE | Conference |
| A02 | [40] | Mohsan et al. | 2021 | MDPI | Journal |
| A03 | [41] | Ali et al. | 2022 | MDPI | Journal |
| A06 | [48] | Chondrogiannis et al. | 2022 | Elsevier | Journal |
| A07 | [49] | Su et al. | 2022 | Elsevier | Journal |
| A10 | [50] | Sutanto et al. | 2022 | MDPI | Journal |
| A12 | [5] | Zhang et al. | 2022 | IEEE | Journal |
| A13 | [51] | Careline and Godhavari | 2020 | SAI | Journal |
| A16 | [52] | Salonikias et al. | 2022 | MDPI | Journal |
| A25 | [53] | De Olivera et al. | 2022 | IEEE | Conference |
| A27 | [54] | Bhandawat et al. | 2022 | Elsevier | Journal |
| A08 | [55] | Haritha and Anitha | 2023 | IEEE | Conference |
| A18 | [56] | Thantharate and Thantharate | 2023 | MDPI | Journal |
| A22 | [57] | Abdelgalil and Mejri | 2023 | MDPI | Journal |
| A23 | [58] | Chandini and Basarkod | 2023 | Springer Nature | Journal |
| A24 | [59] | Karmakar et al. | 2023 | Elsevier | Journal |
| A43 | [60] | Selvarajan et al. | 2023 | Springer Nature | Journal |
| A44 | [61] | Liu et al. | 2023 | Elsevier | Journal |
| A45 | [11] | Prajapat et al. | 2024 | IEEE | Journal |
| A46 | [62] | Balasubramaniam et al. | 2024 | MDPI | Journal |
| A47 | [63] | Venkatesh et al. | 2024 | IEEE | Conference |
| A01 | [64] | Pu et al. | 2024 | Frontiers Media | Journal |
| A05 | [65] | Kaur et al. | 2024 | Springer Nature | Journal |
| A09 | [66] | Wang et al. | 2024 | Elsevier | Journal |
| A14 | [67] | Li et al. | 2024 | Elsevier | Journal |
| A28 | [68] | Bobrova et al. | 2024 | MDPI | Journal |
| A29 | [69] | Igboanusi et al. | 2024 | Springer Nature | Journal |
| A30 | [70] | Kaafarani et al. | 2024 | JMIR | Journal |
| A31 | [71] | Liang et al. | 2024 | JMIR | Journal |
| A32 | [72] | Mahdi et al. | 2024 | Springer Nature | Journal |
| A33 | [73] | Takahashi et al. | 2024 | Springer Nature | Journal |
| A34 | [74] | Wang et al. | 2024 | JMIR | Journal |
| A36 | [75] | Yang and Li | 2024 | Springer Nature | Journal |
| A37 | [76] | Duc et al. | 2024 | SAI | Journal |
| A38 | [77] | Guerra et al. | 2024 | Taylor & Francis | Journal |
| A39 | [78] | Li et al. | 2024 | Springer Nature | Journal |
| A40 | [79] | Rekik et al. | 2024 | IEEE | Conference |
| A41 | [80] | Saha et al. | 2024 | IEEE | Journal |
| A42 | [81] | Vidhya et al. | 2024 | Wiley | Journal |
| A48 | [82] | Arabnouri & Shafieinejad | 2024 | Springer Nature | Journal |
| A49 | [83] | Bunia et al. | 2024 | IEEE | Conference |
| A50 | [84] | Bieniek et al. | 2024 | Wiley | Journal |
| A51 | [85] | Alharbi et al. | 2024 | MDPI | Journal |
| A52 | [86] | Kumar & Ali | 2024 | Elsevier | Journal |
| A53 | [87] | Rohini et al. | 2024 | PKP | Journal |
| A54 | [88] | Li et al. | 2024 | Elsevier | Journal |
| A56 | [89] | Zhu et al. | 2024 | MDPI | Journal |
| A58 | [90] | Kar et al. | 2024 | IEEE | Journal |
| A59 | [91] | Zhang et al. | 2024 | Elsevier | Journal |
| A62 | [92] | Abid et al. | 2024 | OUP | Journal |
| A64 | [93] | Ahmed et al. | 2024 | Univ. of New Mexico | Journal |
| A65 | [94] | Ahmed et al. | 2024 | MDPI | Journal |
| A67 | [95] | Akhyani et al. | 2024 | IEEE | Conference |
| A69 | [96] | Ansar et al. | 2024 | ETP | Journal |
| A70 | [97] | Badidi et al. | 2024 | IEEE | Conference |
| A71 | [98] | Basudan | 2024 | Taylor & Francis | Journal |
| A73 | [99] | Chegenizadeh & Tessone | 2024 | IEEE | Conference |
| A74 | [100] | Devgun et al. | 2024 | IEEE | Conference |
| A77 | [101] | Kumari et al. | 2024 | Elsevier | Journal |
| A78 | [102] | Sun et al. | 2024 | IEEE | Journal |
| A79 | [103] | Rani et al. | 2024 | Springer Nature | Journal |
| A80 | [104] | Riahi et al. | 2024 | IEEE | Journal |
| A55 | [105] | Cihan et al. | 2025 | Wiley | Journal |
| A57 | [106] | Aakanksha & Sundaram, | 2025 | HICSS | Conference |
| A60 | [107] | Ding et al. | 2025 | IEEE | Journal |
| A61 | [108] | Abdunabi et al. | 2025 | SAGE | Journal |
| A63 | [109] | Ahanger et al. | 2025 | Elsevier | Journal |
| A66 | [110] | Ahmed et al. | 2025 | Elsevier | Journal |
| A68 | [111] | Ali et al. | 2025 | ACM | Journal |
| A35 | [112] | Mishra and Mehra | 2025 | Springer Nature | Journal |
| A72 | [113] | Chaudhry et al. | 2025 | Elsevier | Journal |
| A75 | [114] | Guo et al. | 2025 | MDPI | Journal |
| A76 | [115] | Gupta & Lakhwani | 2025 | Springer Nature | Journal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurt, K.K.; Timurtaş, M.; Pınar, S.; Ozaydin, F.; Türkeli, S. Smart Contracts, Blockchain, and Health Policies: Past, Present, and Future. Information 2025, 16, 853. https://doi.org/10.3390/info16100853
Kurt KK, Timurtaş M, Pınar S, Ozaydin F, Türkeli S. Smart Contracts, Blockchain, and Health Policies: Past, Present, and Future. Information. 2025; 16(10):853. https://doi.org/10.3390/info16100853
Chicago/Turabian StyleKurt, Kenan Kaan, Meral Timurtaş, Sevcan Pınar, Fatih Ozaydin, and Serkan Türkeli. 2025. "Smart Contracts, Blockchain, and Health Policies: Past, Present, and Future" Information 16, no. 10: 853. https://doi.org/10.3390/info16100853
APA StyleKurt, K. K., Timurtaş, M., Pınar, S., Ozaydin, F., & Türkeli, S. (2025). Smart Contracts, Blockchain, and Health Policies: Past, Present, and Future. Information, 16(10), 853. https://doi.org/10.3390/info16100853








