Leveraging Artificial Intelligence and Participatory Modeling to Support Paradigm Shifts in Public Health: An Application to Obesity and Evidence-Based Policymaking
Abstract
1. Introduction
- We create a concept map of obesity with 19 experts to combine different views on obesity, physical well-being, and mental well-being. With 98 nodes and 174 edges, it is currently the most comprehensive expert-driven model of obesity that conciliates medical perspectives with those emphasizing well-being.
- We analyze knowledge on obesity using tools from network science and natural language processing and examine the implications for public policymaking.
2. Background: A Primer on Participatory Modeling for Causal Mapping
3. Knowledge Representation by Participatory Systems Mapping
3.1. Key Steps of Our Methodology
3.2. A Starting Point: The PHSA Report on Obesity and Well-Being
3.3. Extending the Map via Semi-Structured Interviews
4. Analyzing Knowledge on Obesity and Well-Being
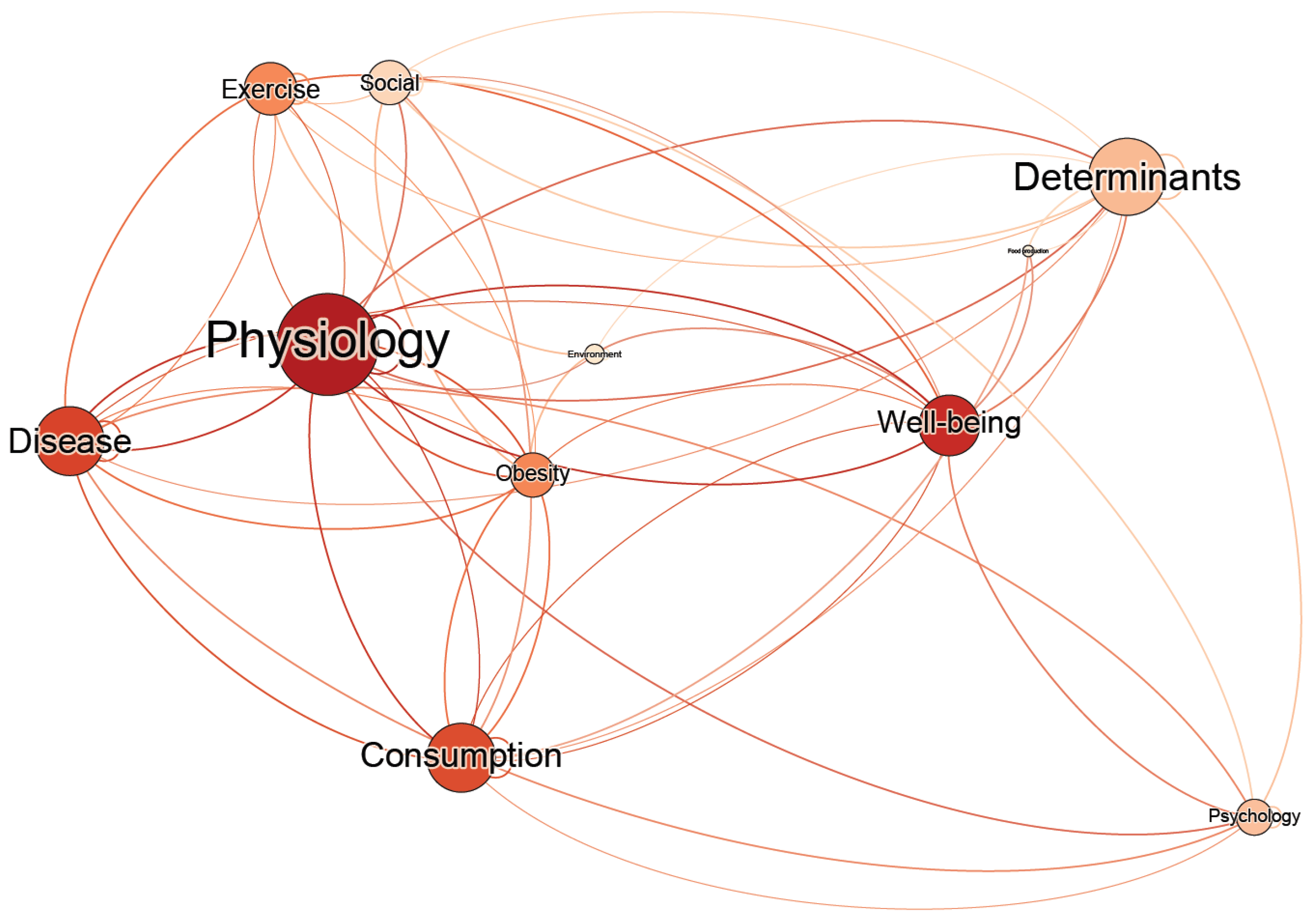
4.1. Overview of the Map
4.2. Network Analysis of the Conceptual Map
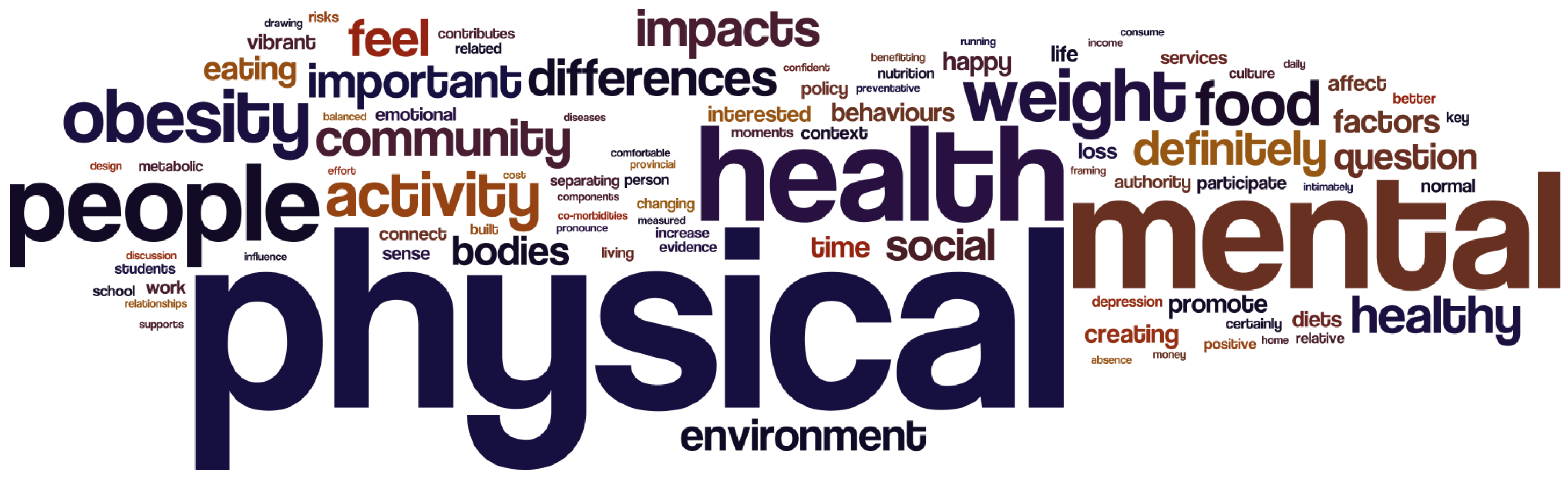
4.3. Natural Language Processing for the Interviews
5. Discussion and Future Work
5.1. Structuring the Relations between Physical and Well-Being in the Context of Obesity
“You don’t have two separate drawers, one is mental health, one is physical health. They’re really part of the same thing.”
“That would entail having access to the resources that are required for everyday living. Access to shelter, to clothing, to reasonable foodstuffs, to entertainment, meet entertainment needs so that people could participate meaningfully in the society or the culture in which they find themselves”.
5.2. Limitations and Suggestions for Future Studies
5.3. Implications of the Map for AI Solutions Focused on Well-Being
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Key Informants
| Name | Position | Fields of Expertise |
|---|---|---|
| Geoff Ball, Ph.D. | Professor and associate chair of research, Department of Pediatrics, Faculty of Medicine & Dentistry, University of Alberta, Canada | Optimize obesity management and prevention for children and families, including via clinical trials or qualitative research |
| Katherine Cianflone, Ph.D. | Professor Emeritus, former Canada Research Chair on Adipose Tissue (Tier 1), Universite Laval, Canada | Adipose tissue metabolism, factors controlling fat, molecular basis of obesity |
| Jean-Pierre Chanoine, Ph.D., MD | Clinical Professor, Pediatric Endocrinologist, Department of Pediatrics, BC Children’s Hospital, Canada. Secretary General of Global Pediatric Endocrinology and Diabetes (GPED) | Pediatric endocrinology, capacity building, access to medicine |
| Jean-Philippe Chaput, Ph.D. | Professor, Department of Pediatrics, University of Ottawa. Research Scientist, Healthy Active Living and Obesity Research Group CHEO Research Institute | Prevention and Treatment of Obesity in Children, Sleep health, Screen time, Physical activity |
| Jean-Pierre Després, Ph.D. | Professor, Department of Kinesiology, Faculty of Medicine, Universite Laval, Canada Scientific. Director, International Chair on Cardiometabolic Risk, Universite Laval. Innovation and Science Director, Alliance Santé Québec | Adipose tissue distribution, visceral obesity, type 2 diabetes, lipids, lipoproteins, cardiovascular disease, and their prevention through physical activity and healthy living |
| Jim Frankish, Ph.D. | Clinical Psychologist & Endowed Professor, School of Population and Public Health, UBC (passed away) | Nutrition education, health literacy, community capacity, healthy communities, and health promotion in primary care |
| Danijela Gasevic, MD, Ph.D. | Associate Professor and Head of Professional Education, School of Public Health and Preventive Medicine, Monash University, Australia | Chronic disease prevention, particularly regarding the effect of physical inactivity and sedentary behavior on health |
| Carolyn Gotay, Ph.D. | Professor Emeritus, founding Canadian Cancer Society Chair in Cancer Primary, School of Population and Public Health, UBC, Canada | Interventions to reduce modifiable cancer risk factors, quality of life in cancer patients and survivors |
| Michael Hayes, Ph.D. | Professor Emeritus and former Director, School of Public Health and Social Policy, University of Victoria, Canada | Health inequities, disability, public policy, obesity, health literacy, population health promotion |
| Terry Huang, Ph.D. | Distinguished Professor and Chair, Department of Health Policy and Management, City University of New York, USA | Chronic disease prevention, design and health, built environment, public-private partnerships, cross-cultural health |
| David Lau, Ph.D., MD | Professor Emeritus, Department of Biochemistry & Molecular Biology, University of Calgary, Canada. Former Chair of Diabetes & Endocrine Research Group and Director of the Julia McFarlane Diabetes Research Centre | Fat cell biology in health and obesity, development of insulin resistance in obesity and diabetes, and cellular mechanisms of diabetic vascular complications |
| Scott Lear, Ph.D. | Professor, Pfizer/Heart & Stroke Foundation Chair in Cardiovascular Prevention Research, Faculty of Health Sciences, Simon Fraser University, Canada | Cardiovascular disease prevention, population health, ethnic disparities |
| Gary Lewis, Ph.D., MD | Professor, Department of Medicine and Department of Physiology, University of Toronto. Director, Division of Endocrinology and Metabolism, University of Toronto | Whole body, integrative, physiological studies in humans |
| Pablo Monsivais, Ph.D. | Associate Professor, Department of Nutrition and Exercise Physiology, Elson S. Floyd College of Medicine, Washington State University, USA | Public Health, Epidemiology, Social Inequalities, Food and Nutrition |
| Kim Raine, Ph.D. | Distinguished Professor, School of Public Health, University of Alberta, Canada | How social conditions and people’s behaviors (particularly food and eating behaviors) interact to transmit obesity and chronic diseases through social means |
| Arya Sharma, Ph.D., MD | Professor of medicine, chair in obesity research and management, University of Alberta. Founder and Scientific Director, Canadian Obesity Network | Evidence-based prevention and management of obesity and related cardiovascular disorders |
| John Spence, Ph.D. | Professor, Faculty of Kinesiology, Sport, & Recreation, University of Alberta, Canada | Benefits and determinants of physical activity and how physical inactivity and sedentary behavior are related to health |
| Tom Warshawski, MD | Associate Clinical Professor of Pediatrics, UBC. Chair of the Childhood Obesity Foundation. Former head of pediatrics, Kelowna General Hospital, Canada | Promoting Healthy Active Living in children and youth |
| James Woodcock, Ph.D. | Professor of Transport and Health Modelling, University of Cambridge, UK | Health impacts of changes to how we travel and how such changes might occur |
Appendix B. Components of the Map
Appendix B.1. Physical Factors
Appendix B.2. Comorbidities of Obesity
“I would die before I reached a BMI of 50 because I do not have the physiology, the genetics which would allow me to put on a lot of subcutaneous fat. Because to become massively obese you must have subcutaneous adipose tissue that has tremendous ability to expand. And you’re able to deal with chronic energy imbalance. Some of us just can’t. We develop diabetes, we have cardiovascular events.”
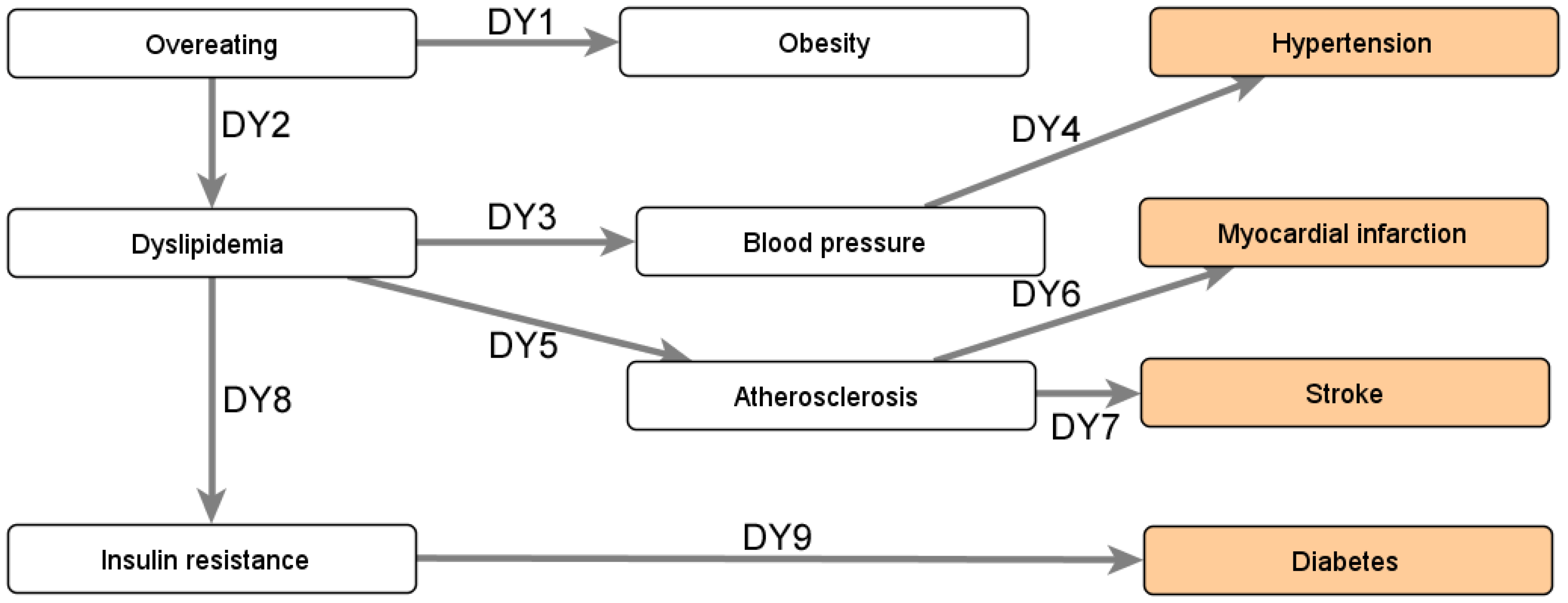
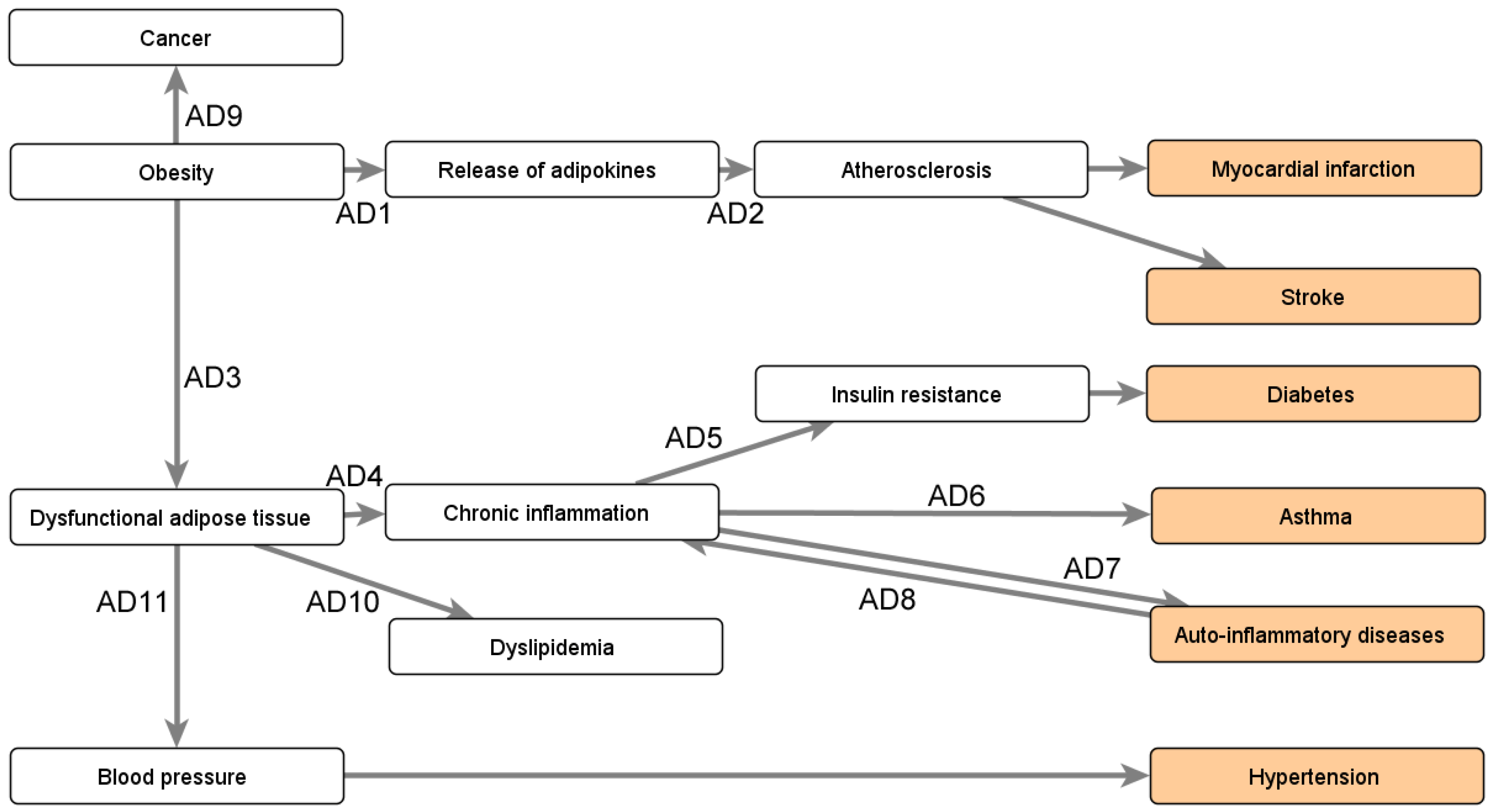
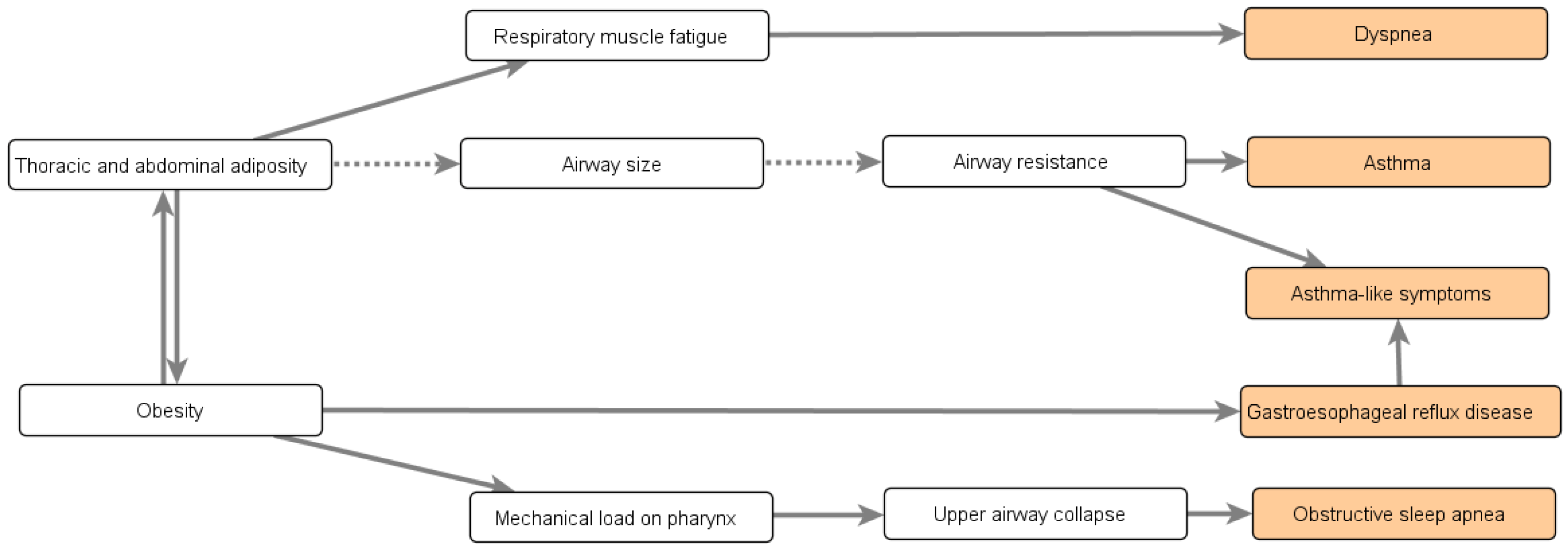
Appendix B.3. Impact of Obesity’s Comorbidities
- Beta-blockers (PH1), which reduce the heart rate, treating conditions such as hypertension (PH2), angina (PH3), or congestive heart failure (PH4);
- Medications prescribed for mental health problems (PH5), as they may affect concentration (PH6–7), coordination (PH8–9), or balance (PH10–11); and
- Medications causing tiredness (PH12–13), which are prescribed for a wide variety of issues ranging from depression to insomnia.
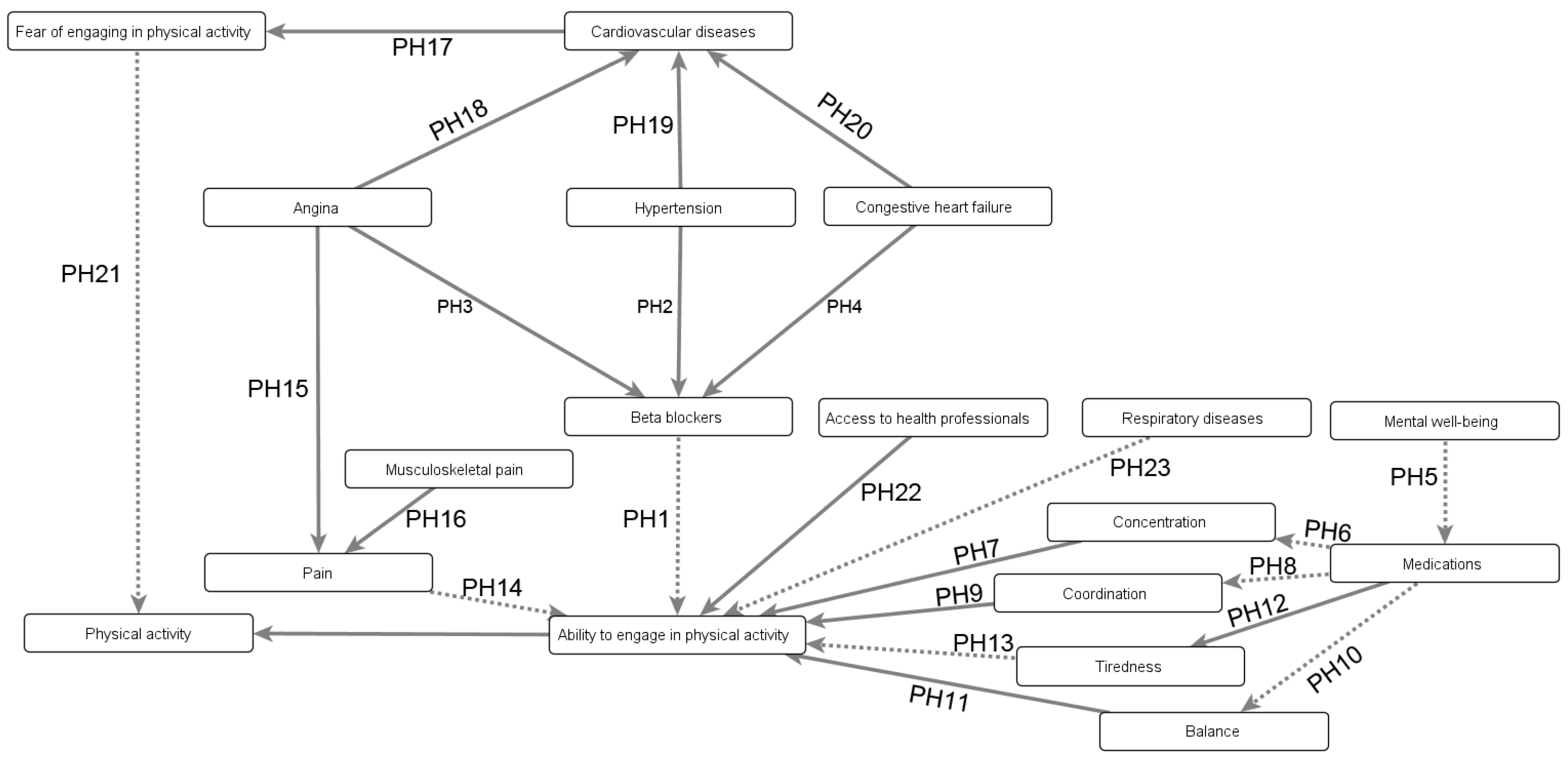
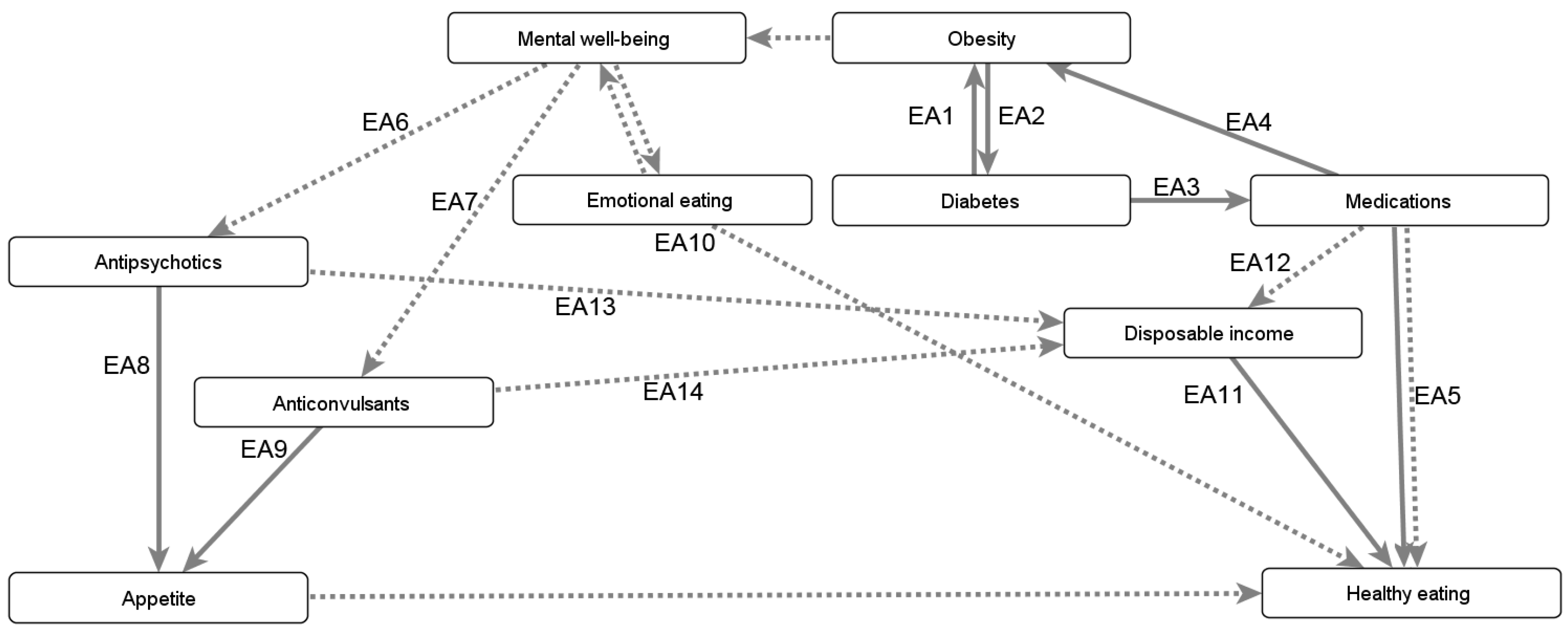
Appendix B.3.1. Mitigating the Negative Consequences of Obesity’s Comorbidities

Appendix B.3.2. Sleep Duration

Appendix B.4. Environmental Factors
Appendix B.4.1. Influence of the Built and Social Environments on Eating Behaviours
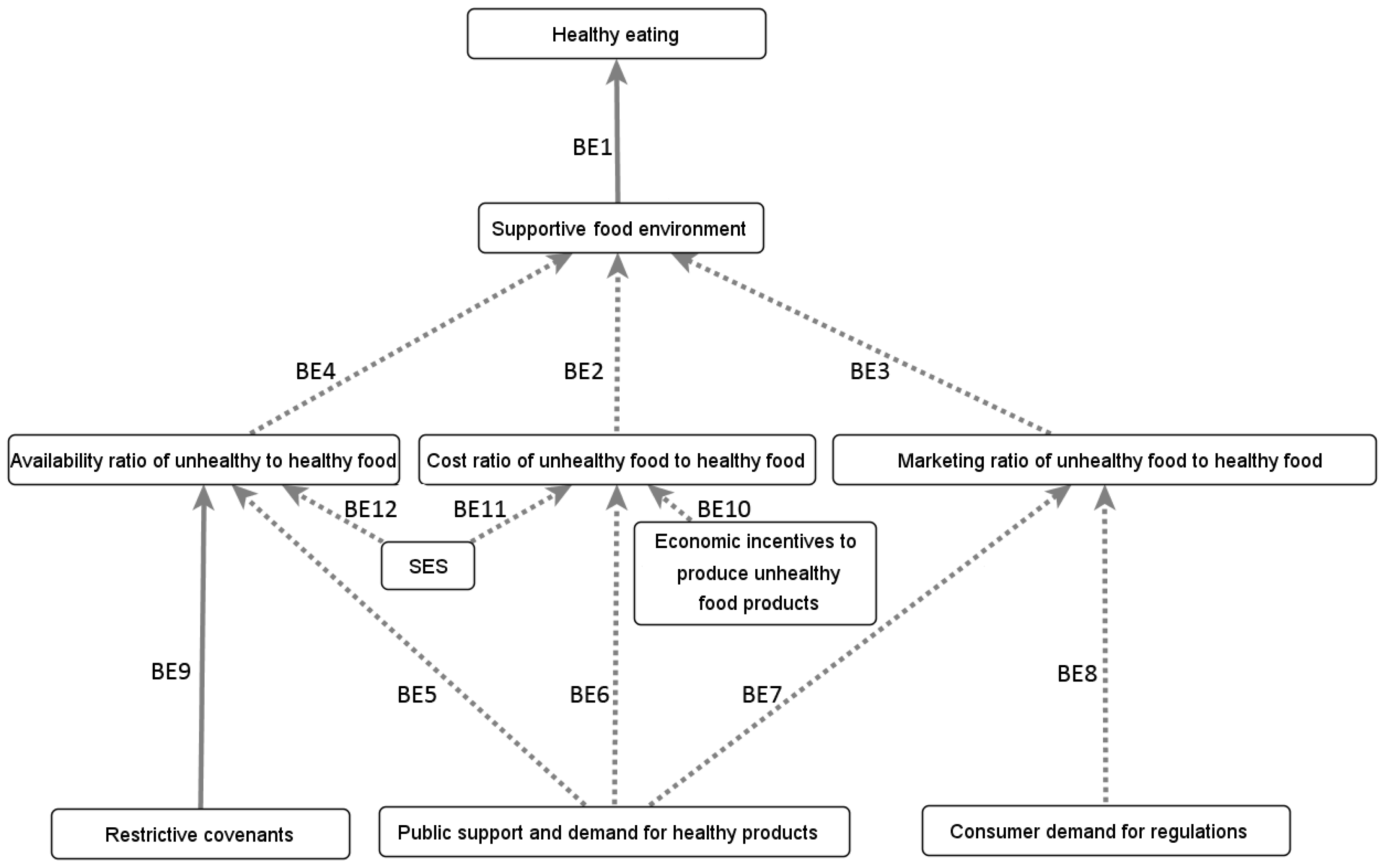
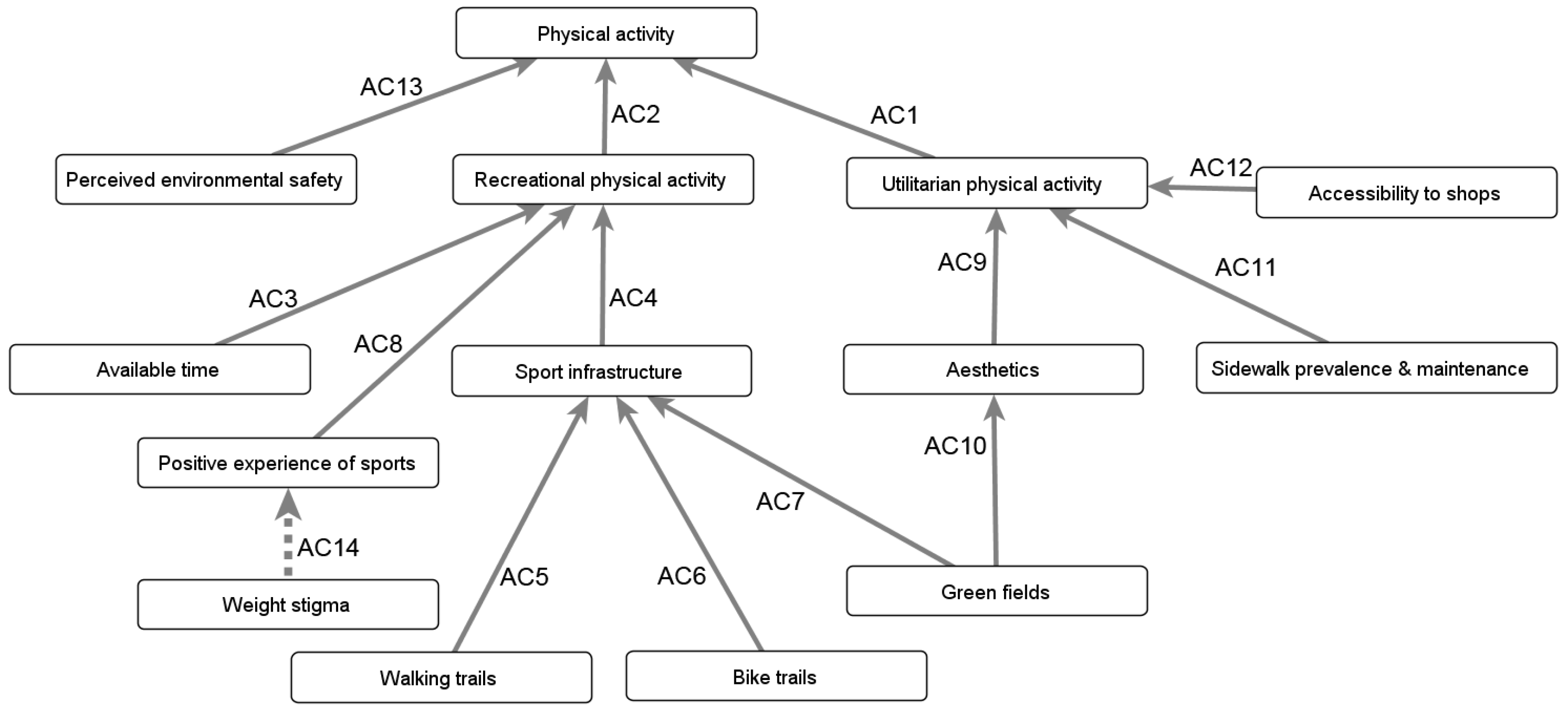
Appendix B.4.2. Influence of the Built and Social Environments on Physical Well-Being
“As soon as we build it and if we think it’s really important, we should promote it. [...] But before it is built, and that’s the biggest mistake now, things are often built without consulting with the community. [...] What are the needs of the community? Because gyms may be built in communities where people are of low-income and cannot afford to go to the gym, so what is the use of it? [...] Maybe they would prefer [...] children’s playground or playing fields where kids could play.”
Appendix B.5. Factors Not Included
References
- Statistics Canada. Overweight and Obese Adults, 2018; Statistics Canada: Ottawa, ON, Canada, 2019.
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kotz, C.M.; Kahan, S.; Kelly, A.S.; Heymsfield, S.B. Obesity as a disease: The obesity society 2018 position statement. Obesity 2019, 27, 7–9. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Perriard-Abdoh, S.; Chadwick, P.; Chater, A.M.; Chisolm, A.; Doyle, J.; Gillison, F.B.; Greaves, C.; Liardet, J.; Llewellyn, C.; McKenna, I.; et al. Psychological Perspectives on Obesity: Addressing Policy, Practice and Research Priorities; University College London: London, UK, 2019. [Google Scholar]
- Vallis, M.; Macklin, D. When behaviour meets biology: If obesity is a chronic medical disease what is obesity management? Clin. Obes. 2021, 11, e12443. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight loss strategies for treatment of obesity: Lifestyle management and pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef]
- Bombak, A. Obesity, health at every size, and public health policy. Am. J. Public Health 2014, 104, e60–e67. [Google Scholar] [CrossRef]
- Gibson, G. Health (ism) at every size: The duties of the “good fatty”. Fat Stud. 2022, 11, 22–35. [Google Scholar] [CrossRef]
- Zafir, S.; Jovanovski, N. The weight of words: Discursive constructions of health in weight-neutral peer-reviewed journal articles. Body Image 2022, 40, 358–369. [Google Scholar] [CrossRef]
- Jovanovski, N.; Jaeger, T. Unpacking the ‘anti-diet movement’: Domination and strategies of resistance in the broad anti-diet community. Soc. Mov. Stud. 2022, 23, 172–189. [Google Scholar] [CrossRef]
- Brown, A.; Flint, S.W.; Batterham, R.L. Pervasiveness, impact and implications of weight stigma. EClinicalMedicine 2022, 47, 101408. [Google Scholar] [CrossRef]
- Flint, S.W. Addressing weight stigma: A timely call. Lancet Public Health 2019, 4, e322. [Google Scholar] [CrossRef]
- Pearl, R.L. Weight bias and stigma: Public health implications and structural solutions. Soc. Issues Policy Rev. 2018, 12, 146–182. [Google Scholar] [CrossRef]
- Hill, B.; Bergmeier, H.; Incollingo Rodriguez, A.C.; Barlow, F.K.; Chung, A.; Ramachandran, D.; Savaglio, M.; Skouteris, H. Weight stigma and obesity-related policies: A systematic review of the state of the literature. Obes. Rev. 2021, 22, e13333. [Google Scholar] [CrossRef]
- Wharton, S.; Lau, D.C.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. Cmaj 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- GermAnn, K.; MacKean, G.; Casselman, L.; Daghofer, D. From Weight to Well-Being: Time for Shift in Paradigms. 2013. Available online: http://www.bccdc.ca/pop-public-health/Documents/W2WBSummaryReport_20130208FINAL1.pdf (accessed on 7 February 2024).
- Bays, H.E.; Fitch, A.; Cuda, S.; Gonsahn-Bollie, S.; Rickey, E.; Hablutzel, J.; Coy, R.; Censani, M. Artificial intelligence and obesity management: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023. Obes. Pillars 2023, 6, 100065. [Google Scholar] [CrossRef]
- Colmenarejo, G. Machine learning models to predict childhood and adolescent obesity: A review. Nutrients 2020, 12, 2466. [Google Scholar] [CrossRef]
- DeGregory, K.; Kuiper, P.; DeSilvio, T.; Pleuss, J.; Miller, R.; Roginski, J.; Fisher, C.; Harness, D.; Viswanath, S.; Heymsfield, S.; et al. A review of machine learning in obesity. Obes. Rev. 2018, 19, 668–685. [Google Scholar] [CrossRef]
- Siddiqui, H.; Rattani, A.; Woods, N.K.; Cure, L.; Lewis, R.; Twomey, J.; Smith-Campbell, B.; Hill, T. A survey on machine and deep learning models for childhood and adolescent obesity. IEEE Access 2021, 9, 157337–157360. [Google Scholar] [CrossRef]
- Ferreras, A.; Sumalla-Cano, S.; Martínez-Licort, R.; Elío, I.; Tutusaus, K.; Prola, T.; Vidal-Mazón, J.L.; Sahelices, B.; de la Torre Díez, I. Systematic Review of Machine Learning applied to the Prediction of Obesity and Overweight. J. Med Syst. 2023, 47, 8. [Google Scholar] [CrossRef]
- Giabbanelli, P.; Flarsheim, R.; Vesuvala, C.; Drasic, L. Developing technology to support policymakers in taking a systems science approach to obesity and well-being. Obes. Rev. 2016, 17, 194–195. [Google Scholar]
- Barlas, T.; Altinova, A.E.; Akturk, M.; Toruner, F.B. Credibility of ChatGPT in the assessment of obesity in type 2 diabetes according to the guidelines. Int. J. Obes. 2023, 48, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S. Exploring the Potential of Chat GPT in Personalized Obesity Treatment. Ann. Biomed. Eng. 2023, 51, 1887–1888. [Google Scholar] [CrossRef]
- Ali, H. The potential of GPT-4 as a personalized virtual assistant for bariatric surgery patients. Obes. Surg. 2023, 33, 1605. [Google Scholar] [CrossRef]
- Crutzen, R. Hardwired... to self-destruct? Using technology to improve behavior change science. Health Psychol. Bull. 2021, 5, 70–80. [Google Scholar] [CrossRef]
- Belghali, M.; Statsenko, Y.; Al-Za’abi, A. Improving serious games to tackle childhood obesity. Front. Psychol. 2021, 12, 657289. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Crutzen, R. Supporting self-management of obesity using a novel game architecture. Health Inform. J. 2015, 21, 223–236. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Tison, B.; Keith, J. The application of modeling and simulation to public health: Assessing the quality of agent-based models for obesity. Simul. Model. Pract. Theory 2021, 108, 102268. [Google Scholar] [CrossRef]
- Xue, H.; Slivka, L.; Igusa, T.; Huang, T.; Wang, Y. Applications of systems modelling in obesity research. Obes. Rev. 2018, 19, 1293–1308. [Google Scholar] [CrossRef]
- Sukhwal, P.C.; Kankanhalli, A. Agent-based Modeling in Digital Governance Research: A Review and Future Research Directions. Sci. Found. Digit. Gov. Transform. 2022, 38, 303–331. [Google Scholar]
- McPherson, K.; Marsh, T.; Brown, M. Foresight report on obesity. Lancet 2007, 370, 1755. [Google Scholar] [CrossRef]
- Allender, S.; Owen, B.; Kuhlberg, J.; Lowe, J.; Nagorcka-Smith, P.; Whelan, J.; Bell, C. A community based systems diagram of obesity causes. PLoS ONE 2015, 10, e0129683. [Google Scholar] [CrossRef]
- McGlashan, J.; Johnstone, M.; Creighton, D.; de la Haye, K.; Allender, S. Quantifying a systems map: Network analysis of a childhood obesity causal loop diagram. PLoS ONE 2016, 11, e0165459. [Google Scholar] [CrossRef]
- Pronk, N.P.; Eneli, I.; Economos, C.D.; Bradley, D.; Fassbender, J.; Calancie, L.; Patawaran, W.; Hovmand, P.S. Using Systems Science for Strategic Planning of Obesity Prevention and Treatment: The Roundtable on Obesity Solutions Experience. Curr. Probl. Cardiol. 2022, 48, 101240. [Google Scholar] [CrossRef]
- Hennessy, E.; Economos, C.D.; Hammond, R.A. Integrating complex systems methods to advance obesity prevention intervention research. Health Educ. Behav. 2020, 47, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Wutich, A.; Quimby, B.; Porter, S.; Zheng, M.; Hossain, M.J.; Brewis, A. Participatory approaches in water research: A review. Wiley Interdiscip. Rev. Water 2022, 9, e1577. [Google Scholar] [CrossRef]
- Quimby, B.; Beresford, M. Participatory modeling: A methodology for engaging stakeholder knowledge and participation in social science research. Field Methods 2022, 35, 73–82. [Google Scholar] [CrossRef]
- Voinov, A.; Jenni, K.; Gray, S.; Kolagani, N.; Glynn, P.D.; Bommel, P.; Prell, C.; Zellner, M.; Paolisso, M.; Jordan, R.; et al. Tools and methods in participatory modeling: Selecting the right tool for the job. Environ. Model. Softw. 2018, 109, 232–255. [Google Scholar] [CrossRef]
- Hudson-Doyle, E.E.; Harrison, S.E.; Hill, S.R.; Williams, M.; Paton, D.; Bostrom, A. Eliciting mental models of science and risk for disaster communication: A scoping review of methodologies. Int. J. Disaster Risk Reduct. 2022, 77, 103084. [Google Scholar] [CrossRef]
- de Pinho, H. Mapping Complex Systems of Population Health. In Systems Science and Population Health; El-Sayed, A.M., Galea, S., Eds.; Oxford University Press: Oxford, UK, 2017; pp. 60–76. [Google Scholar]
- Barbrook-Johnson, P.; Penn, A.S. Systems Mapping: How to Build and Use Causal Models of Systems; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Stankov, I.; Henson, R.M.; Headen, I.; Purtle, J.; Langellier, B.A. Use of qualitative systems mapping and causal loop diagrams to understand food environments, diet and obesity: A scoping review protocol. BMJ Open 2023, 13, e066875. [Google Scholar] [CrossRef] [PubMed]
- Klepp, K.I.; Helleve, A.; Brinsden, H.; Bröer, C.; Budin-Ljøsne, I.; Harbron, J.; Knai, C.; Lien, N.; Luszczynska, A.; Nesrallah, S.; et al. Overweight and obesity prevention for and with adolescents: The “Confronting obesity: Co-creating policy with youth”(CO-CREATE) project. Obes. Rev. 2023, 24, e13540. [Google Scholar] [CrossRef]
- Felmingham, T.; Bolton, K.A.; Fraser, P.; Allender, S.; Brown, A.D. Measuring Shifts in Mental Models in the Prevention of Childhood Obesity in Rural Australia. Health Educ. Behav. 2023, 50, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Barbrook-Johnson, P.; Penn, A. Participatory systems mapping for complex energy policy evaluation. Evaluation 2021, 27, 57–79. [Google Scholar] [CrossRef]
- Király, G.; Miskolczi, P. Dynamics of participation: System dynamics and participation—An empirical review. Syst. Res. Behav. Sci. 2019, 36, 199–210. [Google Scholar] [CrossRef]
- Fagerholm, N.; Raymond, C.M.; Olafsson, A.S.; Brown, G.; Rinne, T.; Hasanzadeh, K.; Broberg, A.; Kyttä, M. A methodological framework for analysis of participatory mapping data in research, planning, and management. Int. J. Geogr. Inf. Sci. 2021, 35, 1848–1875. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Rice, K.L.; Galgoczy, M.C.; Nataraj, N.; Brown, M.M.; Harper, C.R.; Nguyen, M.D.; Foy, R. Pathways to suicide or collections of vicious cycles? Understanding the complexity of suicide through causal mapping. Soc. Netw. Anal. Min. 2022, 12, 60. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Torsney-Weir, T.; Mago, V.K. A fuzzy cognitive map of the psychosocial determinants of obesity. Appl. Soft Comput. 2012, 12, 3711–3724. [Google Scholar] [CrossRef]
- Moon, K.; Browne, N.K. Developing shared qualitative models for complex systems. Conserv. Biol. 2021, 35, 1039–1050. [Google Scholar] [CrossRef]
- Edwards, G.I.; Kok, K. Building a Fuzzy Cognitive Map from stakeholder knowledge: An Episodic, asynchronous approach. Curr. Res. Environ. Sustain. 2021, 3, 100053. [Google Scholar] [CrossRef]
- Gray, S.; Voinov, A.; Paolisso, M.; Jordan, R.; BenDor, T.; Bommel, P.; Glynn, P.; Hedelin, B.; Hubacek, K.; Introne, J.; et al. Purpose, processes, partnerships, and products: Four Ps to advance participatory socio-environmental modeling. Ecol. Appl. 2018, 28, 46–61. [Google Scholar] [CrossRef]
- Firmansyah, H.S.; Supangkat, S.H.; Arman, A.A.; Giabbanelli, P.J. Identifying the components and interrelationships of smart cities in Indonesia: Supporting policymaking via fuzzy cognitive systems. IEEE Access 2019, 7, 46136–46151. [Google Scholar] [CrossRef]
- Wallis, S.E. Understanding and improving the usefulness of conceptual systems: An Integrative Propositional Analysis-based perspective on levels of structure and emergence. Syst. Res. Behav. Sci. 2021, 38, 426–447. [Google Scholar] [CrossRef]
- Kiekens, A.; Dierckx de Casterlé, B.; Vandamme, A.M. Qualitative systems mapping for complex public health problems: A practical guide. PLoS ONE 2022, 17, e0264463. [Google Scholar] [CrossRef] [PubMed]
- Hedelin, B.; Gray, S.; Woehlke, S.; BenDor, T.K.; Singer, A.; Jordan, R.; Zellner, M.; Giabbanelli, P.; Glynn, P.; Jenni, K.; et al. What’s left before participatory modeling can fully support real-world environmental planning processes: A case study review. Environ. Model. Softw. 2021, 143, 105073. [Google Scholar] [CrossRef]
- Sterling, E.J.; Zellner, M.; Jenni, K.E.; Leong, K.; Glynn, P.D.; BenDor, T.K.; Bommel, P.; Hubacek, K.; Jetter, A.J.; Jordan, R.; et al. Try, try again: Lessons learned from success and failure in participatory modeling. Elem. Sci. Anthr. 2019, 7, 9. [Google Scholar] [CrossRef]
- Reddy, T.; Giabbanelli, P.J.; Mago, V.K. The artificial facilitator: Guiding participants in developing causal maps using voice-activated technologies. In Proceedings of the International Conference on Human-Computer Interaction, Orlando, FL, USA, 26–31 July 2019; pp. 111–129. [Google Scholar]
- Freebairn, L.; Atkinson, J.A.; Kelly, P.M.; McDonnell, G.; Rychetnik, L. Decision makers’ experience of participatory dynamic simulation modelling: Methods for public health policy. BMC Med Inform. Decis. Mak. 2018, 18, 131. [Google Scholar] [CrossRef]
- Freund, A.J.; Giabbanelli, P.J. Automatically Combining Conceptual Models Using Semantic and Structural Information. In Proceedings of the 2021 Annual Modeling and Simulation Conference (ANNSIM), Fairfax, VA, USA, 19–22 July 2021; pp. 1–12. [Google Scholar]
- Gray, S.; Hilsberg, J.; McFall, A.; Arlinghaus, R. The structure and function of angler mental models about fish population ecology: The influence of specialization and target species. J. Outdoor Recreat. Tour. 2015, 12, 1–13. [Google Scholar] [CrossRef]
- Freebairn, L.; Occhipinti, J.A.; Song, Y.J.C.; Skinner, A.; Lawson, K.; Lee, G.Y.; Hockey, S.J.; Huntley, S.; Hickie, I.B. Participatory Methods for Systems Modeling of Youth Mental Health: Implementation Protocol. JMIR Res. Protoc. 2022, 11, e32988. [Google Scholar] [CrossRef]
- Mkhitaryan, S.; Giabbanelli, P.J.; de Vries, N.K.; Crutzen, R. Dealing with complexity: How to use a hybrid approach to incorporate complexity in health behavior interventions. Intell.-Based Med. 2020, 3, 100008. [Google Scholar] [CrossRef]
- Sohns, A.; Ford, J.D.; Adamowski, J.; Robinson, B.E. Participatory modeling of water vulnerability in remote Alaskan households using causal loop diagrams. Environ. Manag. 2021, 67, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Kwon, J.; Swinburn, B.; Sacks, G. Understanding the dynamics of obesity prevention policy decision-making using a systems perspective: A case study of Healthy Together Victoria. PLoS ONE 2021, 16, e0245535. [Google Scholar] [CrossRef] [PubMed]
- Will, M.; Dressler, G.; Kreuer, D.; Thulke, H.H.; Grêt-Regamey, A.; Müller, B. How to make socio-environmental modelling more useful to support policy and management? People Nat. 2021, 3, 560–572. [Google Scholar] [CrossRef]
- de Jong, C.E.; Kok, K. Ambiguity in social ecological system understanding: Advancing modelling of stakeholder perceptions of climate change adaptation in Kenya. Environ. Model. Softw. 2021, 141, 105054. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Galgoczy, M.C.; Nguyen, D.M.; Foy, R.; Rice, K.L.; Nataraj, N.; Brown, M.M.; Harper, C.R. Mapping the complexity of suicide by combining participatory modeling and network science. In Proceedings of the 2021 IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining, Virtual Event, 8–11 November 2021; pp. 339–342. [Google Scholar]
- Uusitalo, L.; Jernberg, S.; Korn, P.; Puntila-Dodd, R.; Skyttä, A.; Vikström, S. Fuzzy cognitive mapping of Baltic Archipelago Sea food webs reveals no cliqued views of the system structure between stakeholder groups. Socio-Environ. Syst. Model. 2020, 2, 16343. [Google Scholar] [CrossRef]
- Eakin, H.; Siqueiros-García, J.M.; Hernández-Aguilar, B.; Shelton, R.; Bojórquez-Tapia, L.A. Mental models, meta-narratives, and solution pathways associated with socio-hydrological risk and response in Mexico City. Front. Sustain. Cities 2019, 1, 4. [Google Scholar] [CrossRef]
- Swierad, E.; Huang, T.T.K.; Ballard, E.; Flórez, K.; Li, S. Developing a socioculturally nuanced systems model of childhood Obesity in manhattan’s Chinese American community via Group Model building. J. Obes. 2020, 2020, 4819143. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, G.; Savona, N.; Aguiar, A.; Alaba, O.; Booley, S.; Malczyk, S.; Nwosu, E.; Knai, C.; Rutter, H.; Klepp, K.I.; et al. Adolescents’ Perspectives on the Drivers of Obesity Using a Group Model Building Approach: A South African Perspective. Int. J. Environ. Res. Public Health 2022, 19, 2160. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, J.; Hayward, J.; Brown, A.; Owen, B.; Millar, L.; Johnstone, M.; Creighton, D.; Allender, S. Comparing complex perspectives on obesity drivers: Action-driven communities and evidence-oriented experts. Obes. Sci. Pract. 2018, 4, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, D.J.; Moreira, L.B.; Kerkhoff, A.; Fuchs, S.C.; Fuchs, F.D. Health-related quality of life and hypertension: A systematic review and meta-analysis of observational studies. J. Hypertens. 2011, 29, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Knapp, E.A.; Bilal, U.; Burke, B.T.; Dougherty, G.B.; Glass, T.A. A network approach to understanding obesogenic environments for children in Pennsylvania. Connections 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Davis, C.W.; Jetter, A.J.; Giabbanelli, P.J. Automatically Generating Scenarios from a Text Corpus: A Case Study on Electric Vehicles. Sustainability 2022, 14, 7938. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Macewan, G. Repository for this article on the Open Science Framework. Available online: https://osf.io/7ztwu/ (accessed on 7 February 2024).
- Verigin, T.; Giabbanelli, P.J.; Davidsen, P.I. Supporting a systems approach to healthy weight interventions in british columbia by modeling weight and well-being. In Proceedings of the 49th Annual Simulation Symposium, Pasadena, CA, USA, 3–6 April 2016; pp. 1–10. [Google Scholar]
- Adams, S.; Rhodes, T.; Lancaster, K. New directions for participatory modelling in health: Redistributing expertise in relation to localised matters of concern. Global Public Health 2021, 17, 1827–1841. [Google Scholar] [CrossRef]
- Barbrook-Johnson, P.; Carrick, J. Combining complexity-framed research methods for social research. Int. J. Soc. Res. Methodol. 2021, 25, 835–848. [Google Scholar] [CrossRef]
- Radonic, L. When catching the rain: A cultural model approach to green infrastructure in water governance. Hum. Organ. 2018, 77, 172–184. [Google Scholar] [CrossRef]
- LaMere, K.; Mäntyniemi, S.; Vanhatalo, J.; Haapasaari, P. Making the most of mental models: Advancing the methodology for mental model elicitation and documentation with expert stakeholders. Environ. Model. Softw. 2020, 124, 104589. [Google Scholar] [CrossRef]
- Kropf, B.; Schmid, E.; Mitter, H. Multi-step cognitive mapping of perceived nexus relationships in the Seewinkel region in Austria. Environ. Sci. Policy 2021, 124, 604–615. [Google Scholar] [CrossRef]
- Finegood, D.T.; Merth, T.D.; Rutter, H. Implications of the foresight obesity system map for solutions to childhood obesity. Obesity 2010, 18, S13. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, J.; de la Haye, K.; Wang, P.; Allender, S. Collaboration in complex systems: Multilevel network analysis for community-based obesity prevention interventions. Sci. Rep. 2019, 9, 12599. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.G.; Findlay, C.S.; Haas, G.; LaFrance, B.; Laughing, W.; Pembleton, S. Integrating conventional science and aboriginal perspectives on diabetes using fuzzy cognitive maps. Soc. Sci. Med. 2007, 64, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Giabbanelli, P.J. Analyzing the complexity of behavioural factors influencing weight in adults. In Advanced Data Analytics in Health; Springer: Berlin/Heidelberg, Germany, 2018; pp. 163–181. [Google Scholar]
- McAlister, M.M.; Zhang, Q.; Annis, J.; Schweitzer, R.W.; Guidotti, S.; Mihelcic, J.R. Systems thinking for effective interventions in global environmental health. Environ. Sci. Technol. 2022, 56, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.J.; Giabbanelli, P.J. An Experimental Study on the Scalability of Recent Node Centrality Metrics in Sparse Complex Networks. Front. Big Data 2022, 5, 797584. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.A.; Wilkins, E.; Timmins, K.A.; Bryant, M.; Birkin, M.; Griffiths, C. Can big data solve a big problem? Reporting the obesity data landscape in line with the Foresight obesity system map. Int. J. Obes. 2018, 42, 1963–1976. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.E.; Wattick, R.A.; Olfert, M.D. The application of systems science in nutrition-related behaviors and outcomes implementation research: A scoping review. Curr. Dev. Nutr. 2021, 5, nzab105. [Google Scholar] [CrossRef] [PubMed]
- Galgoczy, M.C.; Phatak, A.; Vinson, D.; Mago, V.K.; Giabbanelli, P.J. (Re) shaping online narratives: When bots promote the message of President Trump during his first impeachment. PeerJ Comput. Sci. 2022, 8, e947. [Google Scholar] [CrossRef] [PubMed]
- Wanniarachchi, V.U.; Mathrani, A.; Susnjak, T.; Scogings, C. Methodological Aspects in Study of Fat Stigma in Social Media Contexts: A Systematic Literature Review. Appl. Sci. 2022, 12, 5045. [Google Scholar] [CrossRef]
- Nguyen, Q.C.; Brunisholz, K.D.; Yu, W.; McCullough, M.; Hanson, H.A.; Litchman, M.L.; Li, F.; Wan, Y.; VanDerslice, J.A.; Wen, M.; et al. Twitter-derived neighborhood characteristics associated with obesity and diabetes. Sci. Rep. 2017, 7, 16425. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.; Giabbanelli, P.J.; Mago, V.K. From social media to expert reports: The impact of source selection on automatically validating complex conceptual models of obesity. In Proceedings of the International Conference on Human-Computer Interaction. Springer, Orlando, FL, USA, 26–31 July 2019; pp. 434–452. [Google Scholar]
- Giabbanelli, P.J.; Adams, J.; Pillutla, V.S. Feasibility and framing of interventions based on public support: Leveraging text analytics for policymakers. In Proceedings of the International Conference on Social Computing and Social Media, Toronto, ON, Canada, 17–22 July 2016; pp. 188–200. [Google Scholar]
- Phatak, A.; Mago, V.K.; Agrawal, A.; Inbasekaran, A.; Giabbanelli, P.J. Narrating Causal Graphs with Large Language Models. In Proceedings of the 57th Hawaii International Conference on System Sciences, Waikiki, HI, USA, 3–6 January 2024; pp. 7530–7540. [Google Scholar]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef]
- Shiri, R.; Karppinen, J.; Leino-Arjas, P.; Solovieva, S.; Viikari-Juntura, E. The association between obesity and low back pain: A meta-analysis. Am. J. Epidemiol. 2010, 171, 135–154. [Google Scholar] [CrossRef]
- Peiris, W.L.; Cicuttini, F.M.; Hussain, S.M.; Estee, M.M.; Romero, L.; Ranger, T.A.; Fairley, J.L.; McLean, E.C.; Urquhart, D.M. Is adiposity associated with back and lower limb pain? A systematic review. PLoS ONE 2021, 16, e0256720. [Google Scholar] [CrossRef]
- Sebastian, J.C. Respiratory physiology and pulmonary complications in obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 157–161. [Google Scholar] [CrossRef]
- Poulain, M.; Doucet, M.; Major, G.C.; Drapeau, V.; Sériès, F.; Boulet, L.P.; Tremblay, A.; Maltais, F. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. Cmaj 2006, 174, 1293–1299. [Google Scholar] [CrossRef]
- Gol, R.M.; Rafraf, M. Association between abdominal obesity and pulmonary function in apparently healthy adults: A systematic review. Obes. Res. Clin. Pract. 2021, 15, 415–424. [Google Scholar]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical problems caused by obesity. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Mitri, J.; Hamdy, O. Diabetes medications and body weight. Expert Opin. Drug Saf. 2009, 8, 573–584. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical activity in cancer prevention and survival: A systematic review. Med. Sci. Sport. Exerc. 2019, 51, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. What Is Atherosclerosis? National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2022.
- Tornheim, K.; Ruderman, N.B. Intermediary metabolism of carbohydrate, protein, and fat. In Metabolic Basis of Obesity; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–51. [Google Scholar]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; D’Agostino, R., Jr.; Karter, A.J.; Haffner, S.M.; Rewers, M.J.; Saad, M.; Bergman, R.N.; IRAS investigators. Intensity and amount of physical activity in relation to insulin sensitivity: The Insulin Resistance Atherosclerosis Study. JAMA 1998, 279, 669–674. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Milrad, S.F.; Hall, D.L.; Jutagir, D.R.; Lattie, E.G.; Ironson, G.H.; Wohlgemuth, W.; Nunez, M.V.; Garcia, L.; Czaja, S.J.; Perdomo, D.M.; et al. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J. Neuroimmunol. 2017, 303, 43–50. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, A.; Woodcock, J. Understanding bicycling in cities using system dynamics modelling. J. Transp. Health 2017, 7, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Unwin, N.; Garcia, L.; Woodcock, J.; Samuels, T.A.; Guell, C. Systems science for developing policy to improve physical activity, the Caribbean. Bull. World Health Organ. 2021, 99, 722. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Tzeravini, E.; Koliaki, C.; Dalamaga, M.; Kokkinos, A. The role of mitochondrial adaptation and metabolic flexibility in the pathophysiology of obesity and insulin resistance: An updated overview. Curr. Obes. Rep. 2021, 10, 191–213. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Baulies, A.; Mari, M.; García-Rovés, P.M.; Fernandez-Checa, J.C. Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: Cause or consequence? Free Radic. Res. 2013, 47, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.A.; Brown, D.M.; West, D.S. The role of postpartum weight retention in obesity among women: A review of the evidence. Ann. Behav. Med. 2003, 26, 149–159. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Herring, S.J.; Rich-Edwards, J.W.; Oken, E.; Rifas-Shiman, S.L.; Kleinman, K.P.; Gillman, M.W. Association of postpartum depression with weight retention 1 year after childbirth. Obesity 2008, 16, 1296–1301. [Google Scholar] [CrossRef]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; von Kries, R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
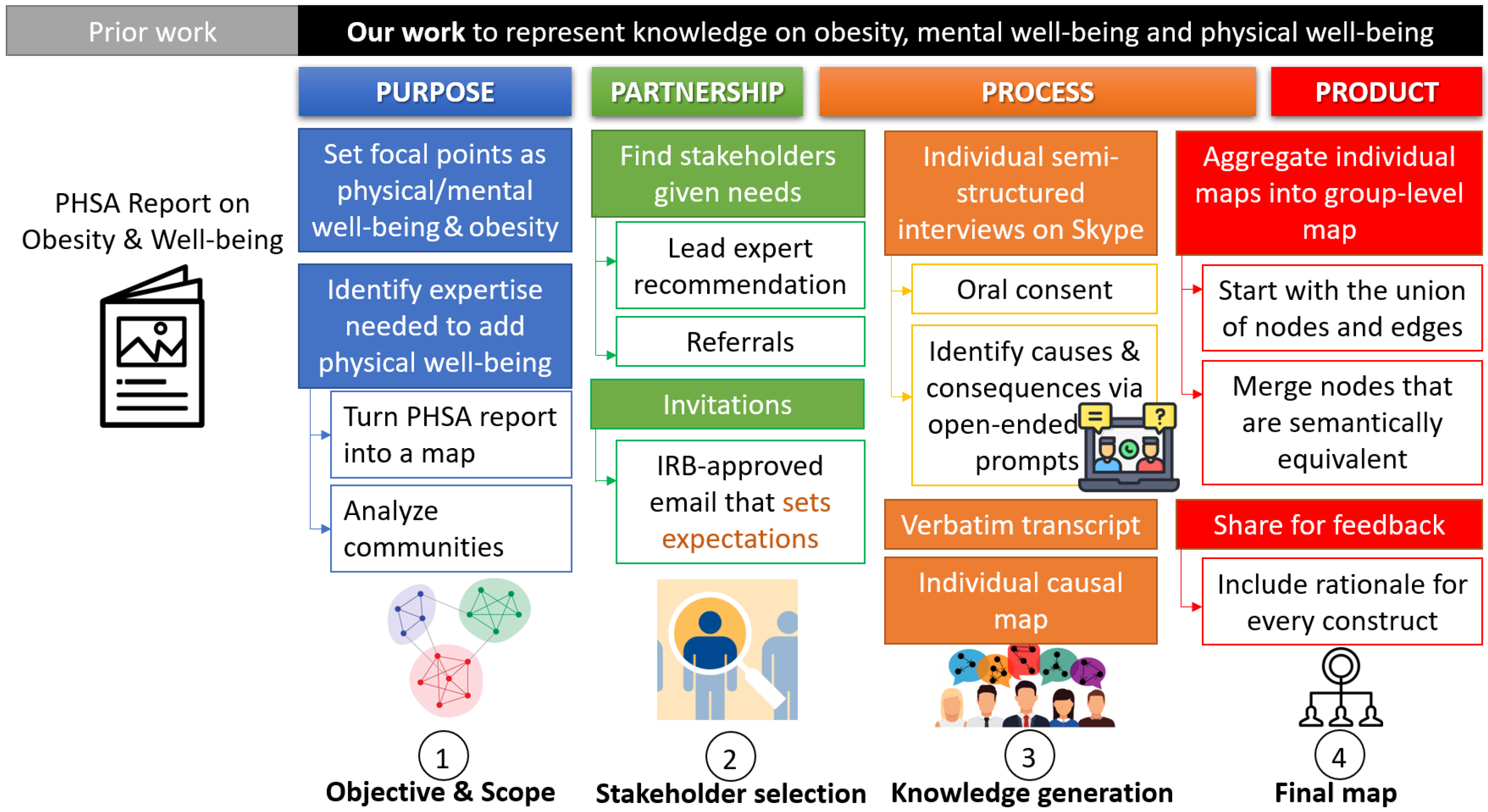
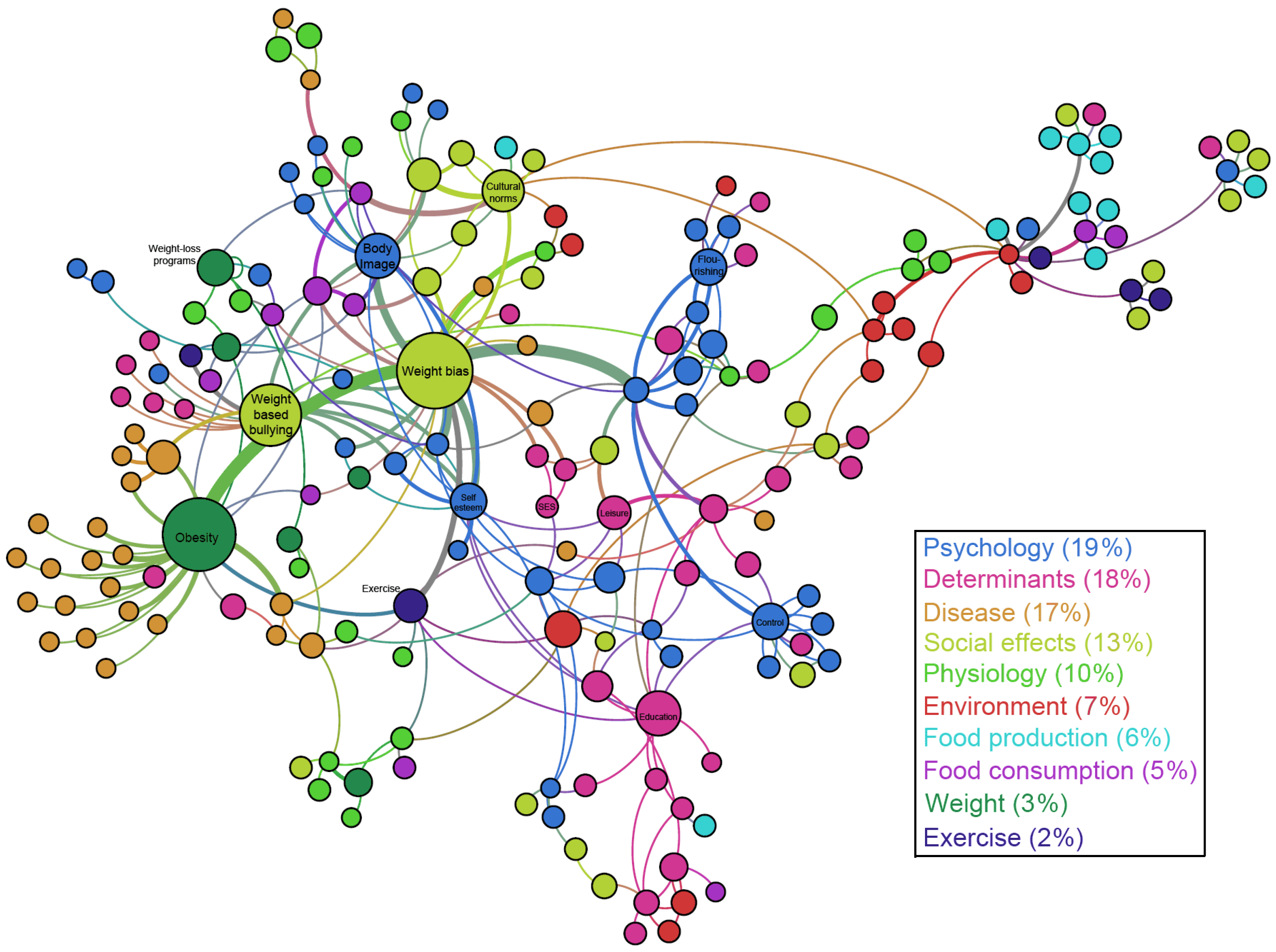
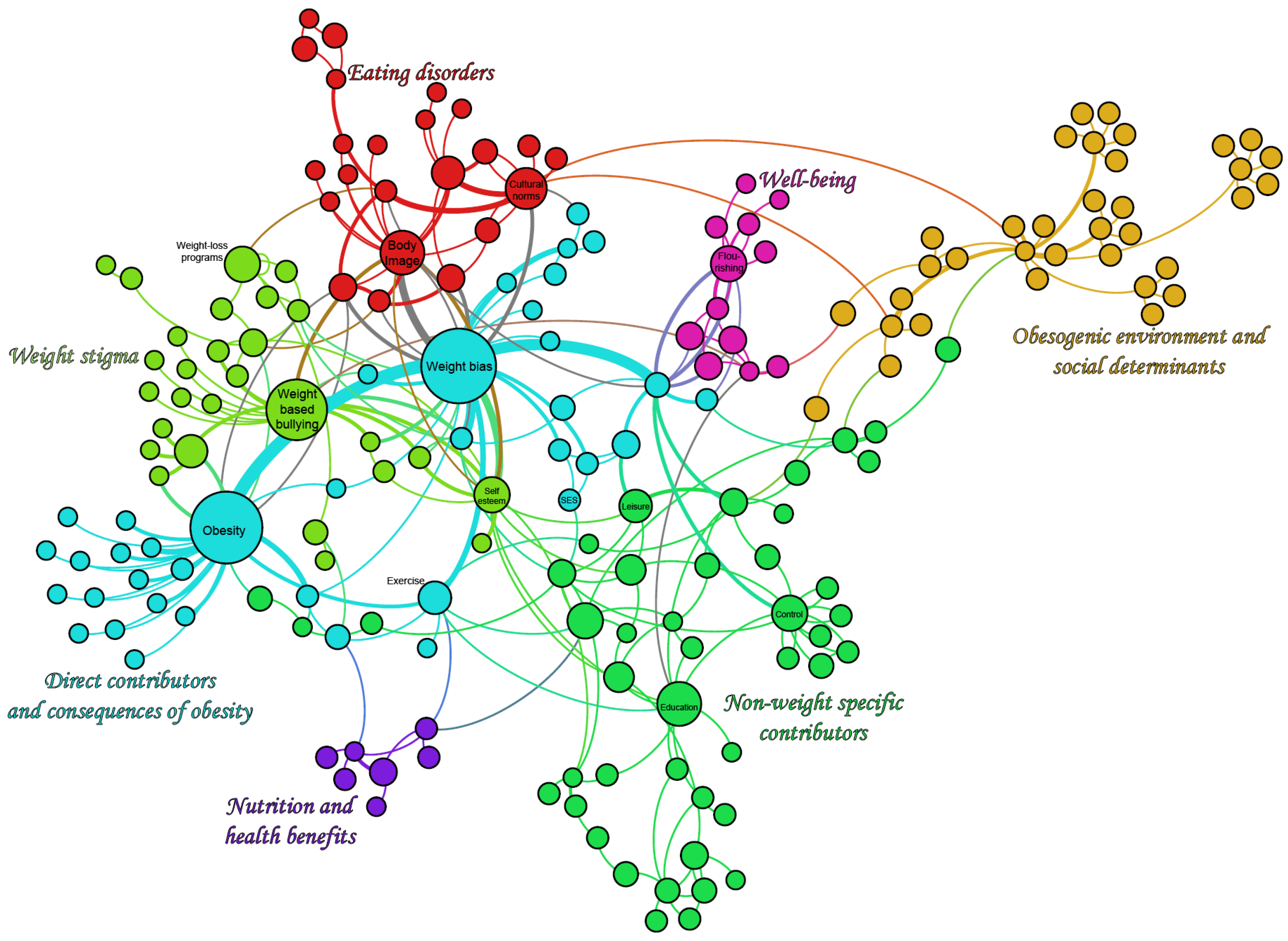
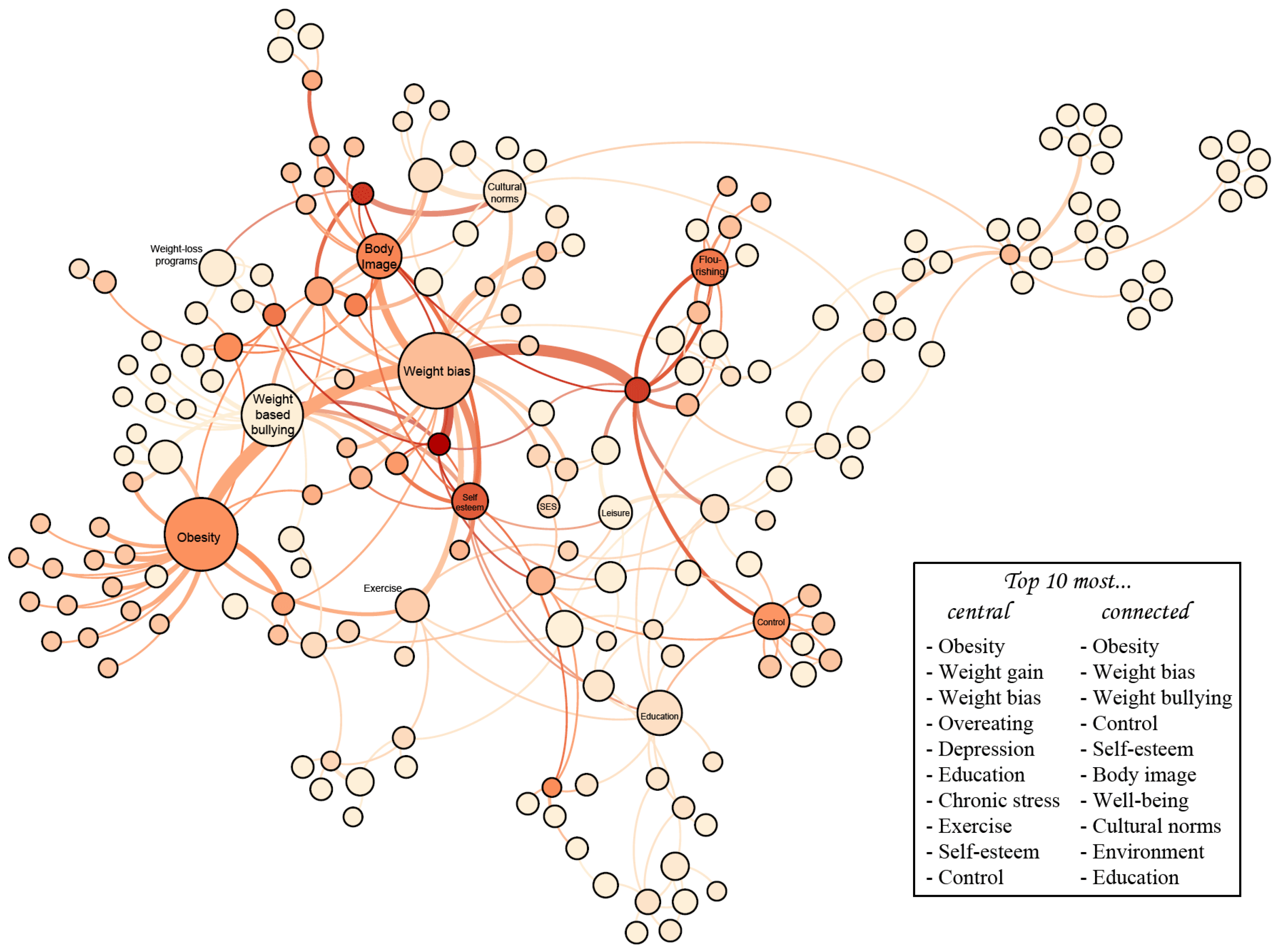

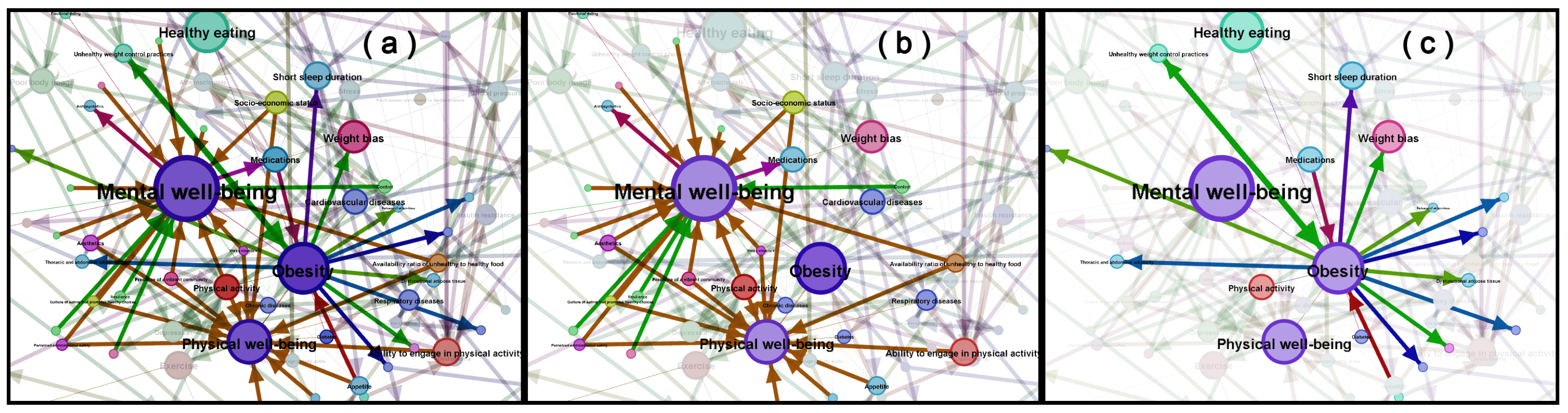

| Strengths of the PHSA Report | Areas of Emphasis in Our Work | Areas Peripheral to Our Work |
|---|---|---|
| Psycho-social pathways (e.g., consequences of weight stigma) | Clinical pathways (e.g., consequences of comorbidities, impact of nutrition) | Food production |
| Mental well-being | Physical well-being | Food consumption |
| Resources impacted by obesity (e.g., job opportunities) | Resources enabling a high level of physical well-being (e.g., the built environment) | Genetics |
| Obesity | Well-Being | |
|---|---|---|
| Causes | Medications | Perceived environmental safety |
| Overeating | Presence of a vibrant community | |
| Physical activity | Resilience | |
| Diabetes | Ability to engage in physical activity | |
| Consequences | Short sleep duration | Antipsychotics |
| Cancer | Medications | |
| Dysfunctional adipose tissue | ||
| Weight bias |
| Mental Well-Being | Physical Well-Being | Both |
|---|---|---|
| Bias, bullying, culture of eating that promotes healthy choices instead of weight loss, deep relationships, discrimination, feeling able to contribute, feeling comfortable, feeling valued, happiness, physical activity, presence of a vibrant community, psychological stability, self-confidence, stigma | Ability to be physically active, appetite, broken bones, built environment, cardiovascular health, diabetes risk, eating behavior, energy balance, food quality, hormonal systems, metabolic health, nutrition, overweight/obesity, physical activity, sleep | Availability of healthy food options, built environment (pollution level, aesthetics, infrastructure), community ties, the culture of eating healthy food, eating behavior, economy, exercise, family (where kids go, how they arrive there, what they eat, how they spend their time), income, perceptions of neighborhood safety, political climate, public health messaging, school environment, work environment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giabbanelli, P.J.; MacEwan, G. Leveraging Artificial Intelligence and Participatory Modeling to Support Paradigm Shifts in Public Health: An Application to Obesity and Evidence-Based Policymaking. Information 2024, 15, 115. https://doi.org/10.3390/info15020115
Giabbanelli PJ, MacEwan G. Leveraging Artificial Intelligence and Participatory Modeling to Support Paradigm Shifts in Public Health: An Application to Obesity and Evidence-Based Policymaking. Information. 2024; 15(2):115. https://doi.org/10.3390/info15020115
Chicago/Turabian StyleGiabbanelli, Philippe J., and Grace MacEwan. 2024. "Leveraging Artificial Intelligence and Participatory Modeling to Support Paradigm Shifts in Public Health: An Application to Obesity and Evidence-Based Policymaking" Information 15, no. 2: 115. https://doi.org/10.3390/info15020115
APA StyleGiabbanelli, P. J., & MacEwan, G. (2024). Leveraging Artificial Intelligence and Participatory Modeling to Support Paradigm Shifts in Public Health: An Application to Obesity and Evidence-Based Policymaking. Information, 15(2), 115. https://doi.org/10.3390/info15020115