Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks
Abstract
1. Introduction
- -
- To design the ANNs to predict the output values of the process;
- -
- To determine the accuracy of the ANNs;
- -
- Process optimization.
2. Material and Methods
2.1. Experiment
2.2. Parameter Measurement Procedure
2.3. Experimental Data Preparation
2.4. Research Tools
3. Results
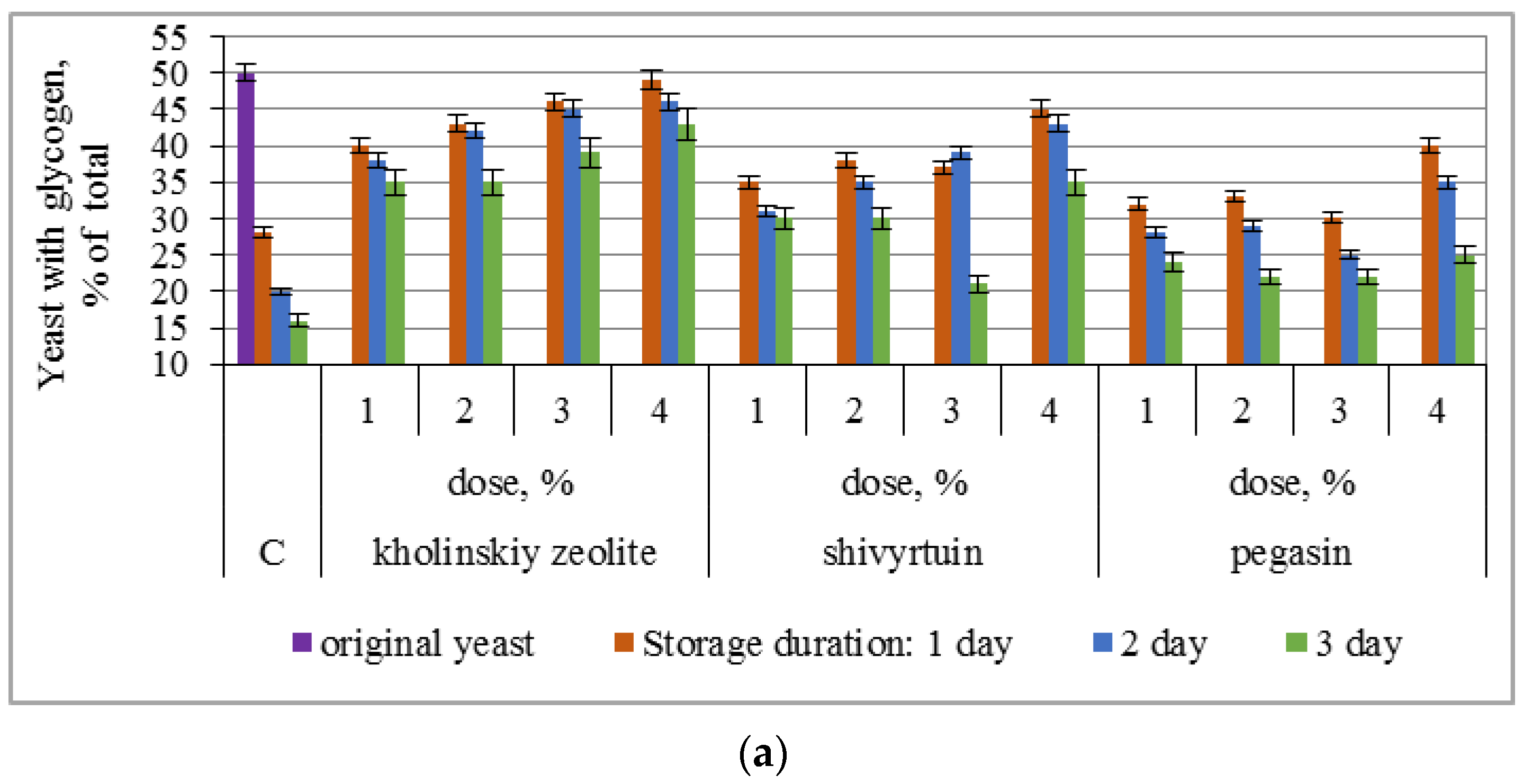
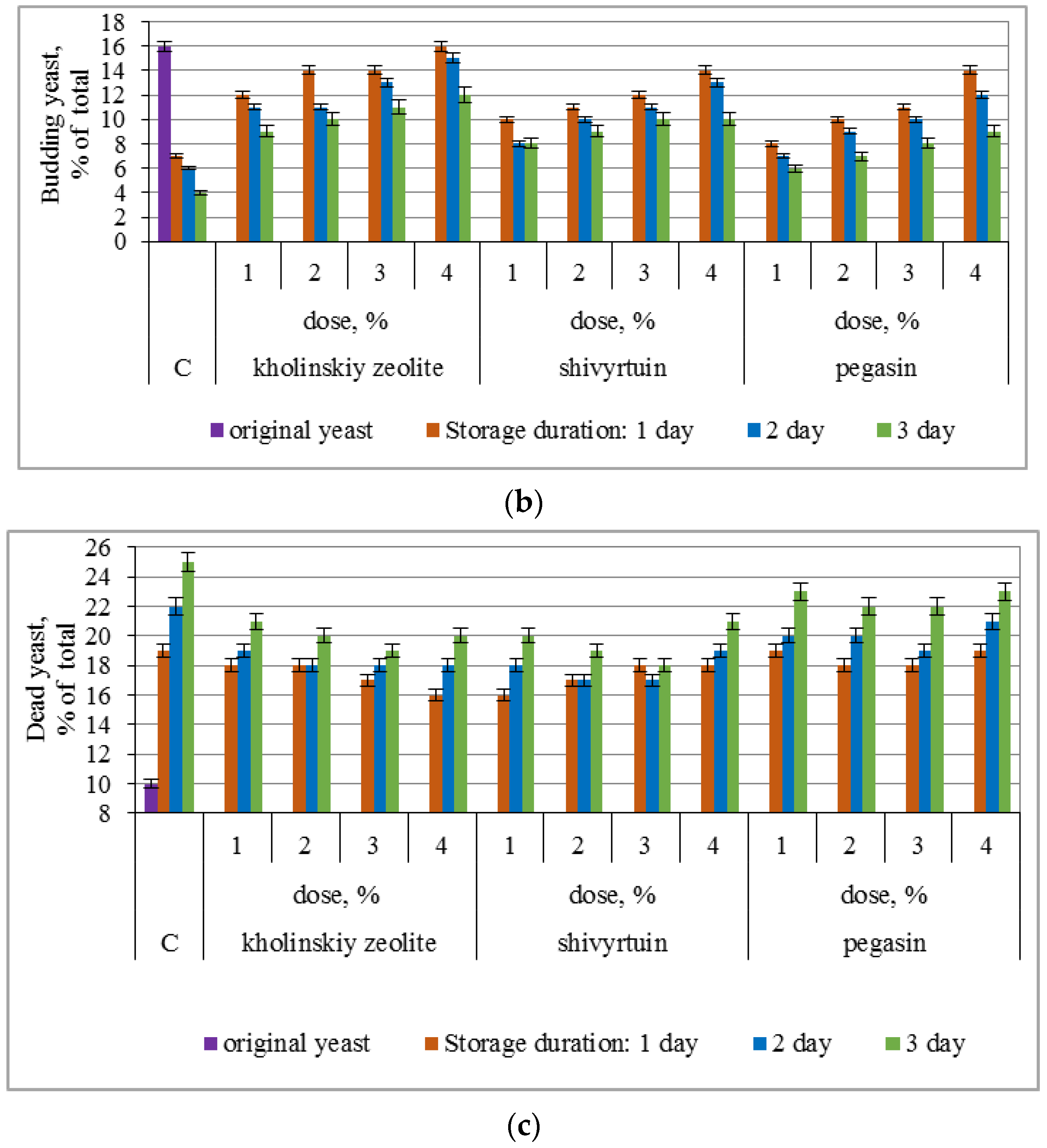
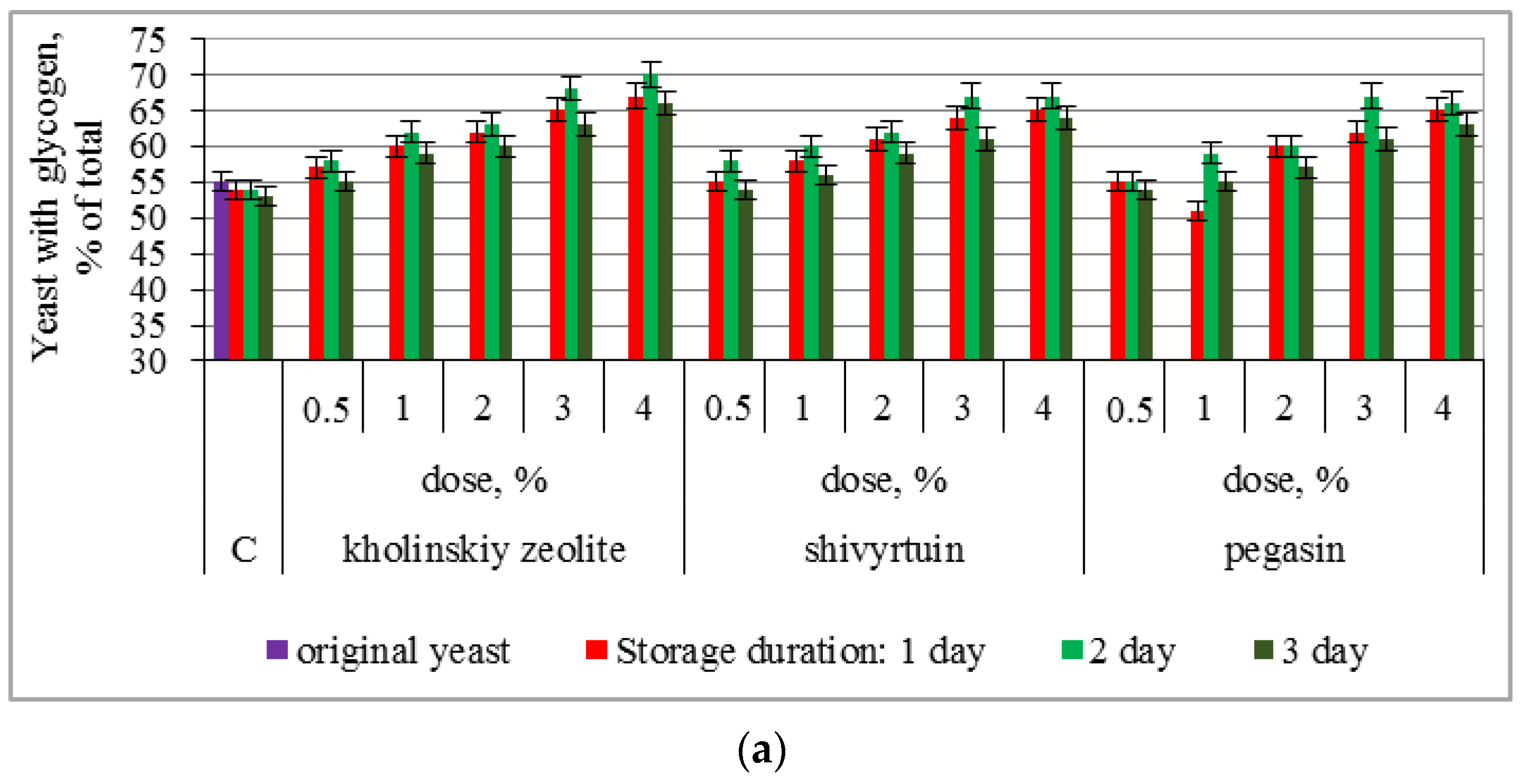
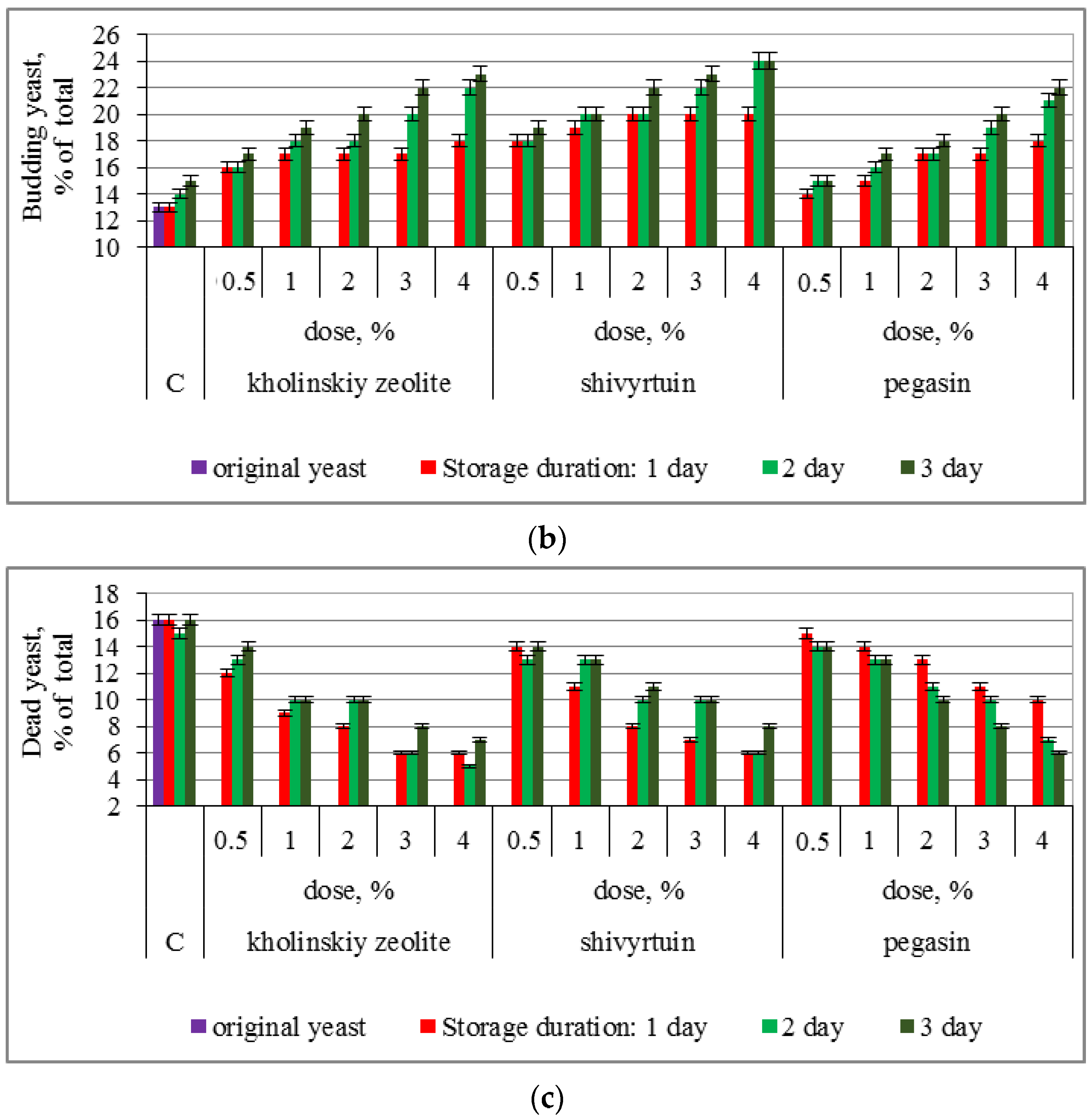
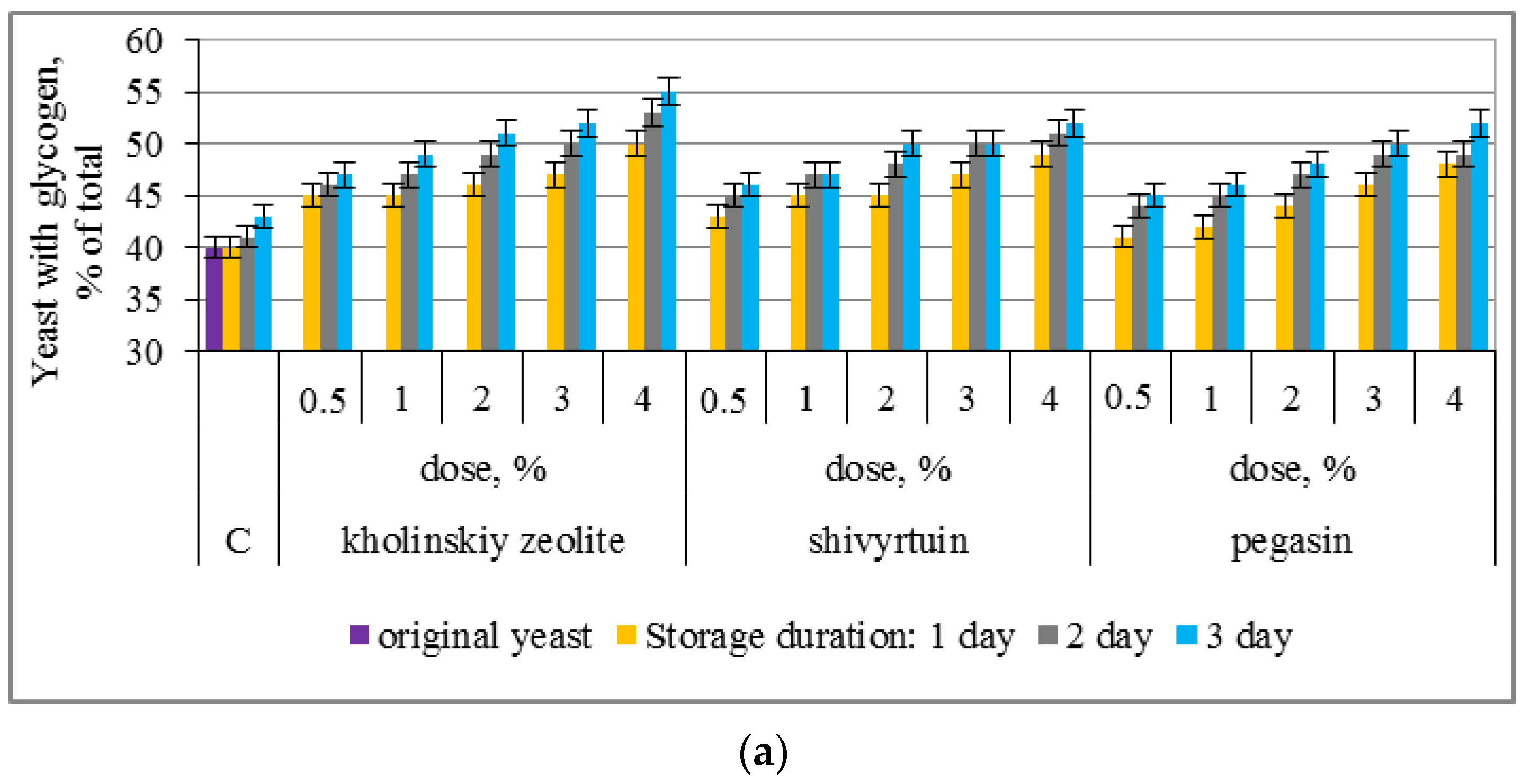
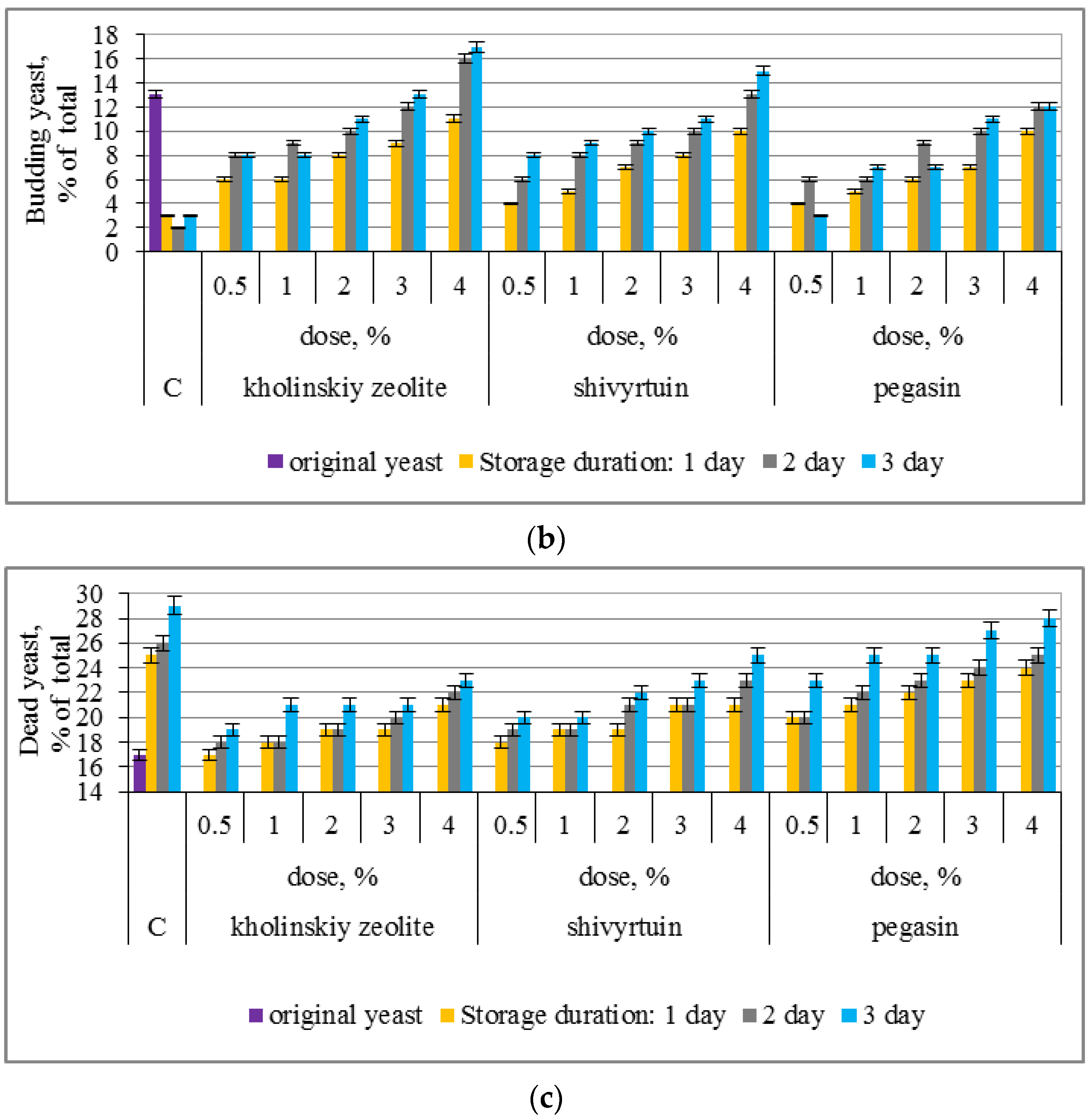
3.1. Experimental Results
3.2. Designing the ANN
- -
- The number of hidden layers;
- -
- The number of neurons in the hidden layers;
- -
- The activation function;
- -
- The loss function;
- -
- The step;
- -
- The optimizer;
- -
- Regularization;
- -
- The size and number of batches;
- -
- The number of epochs.
4. Discussion
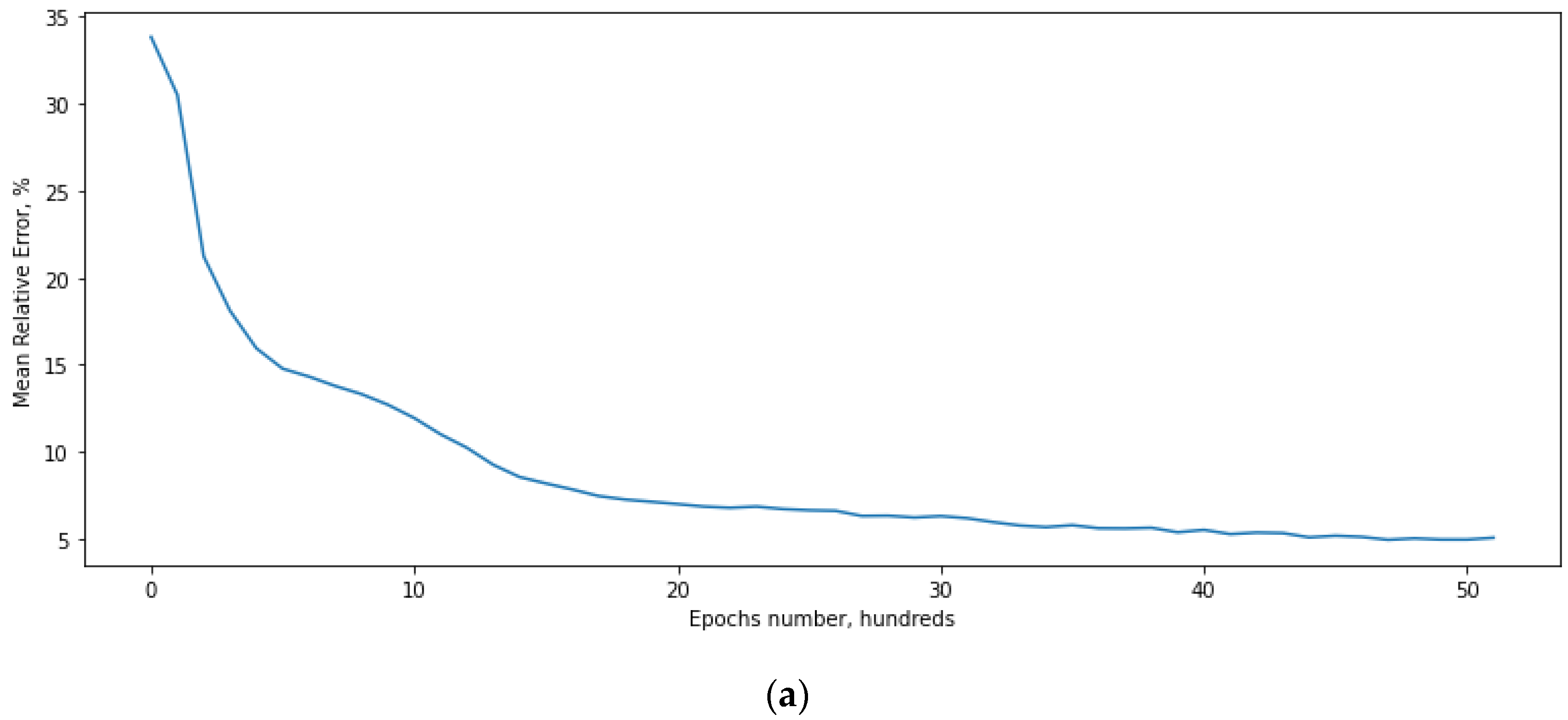
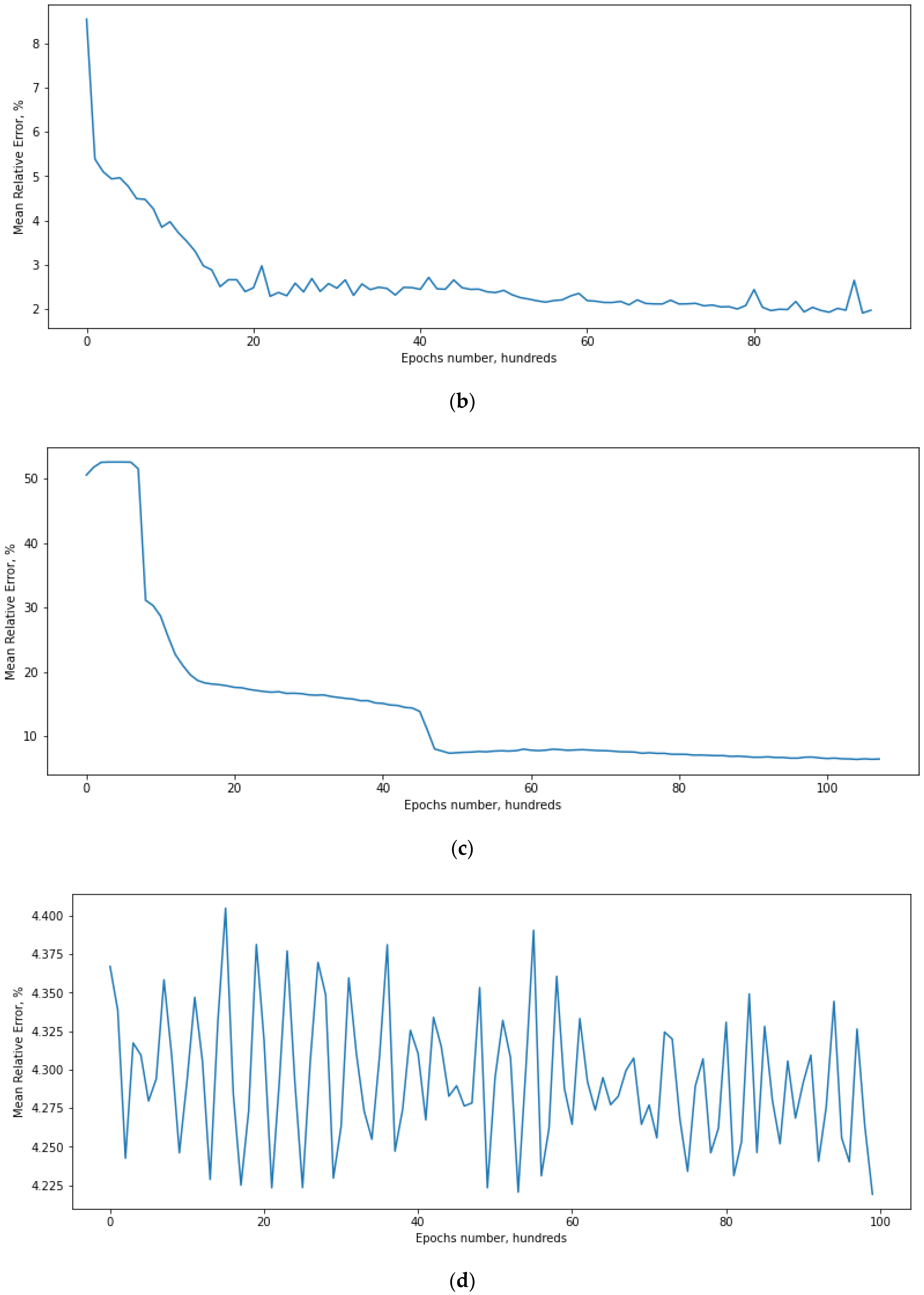
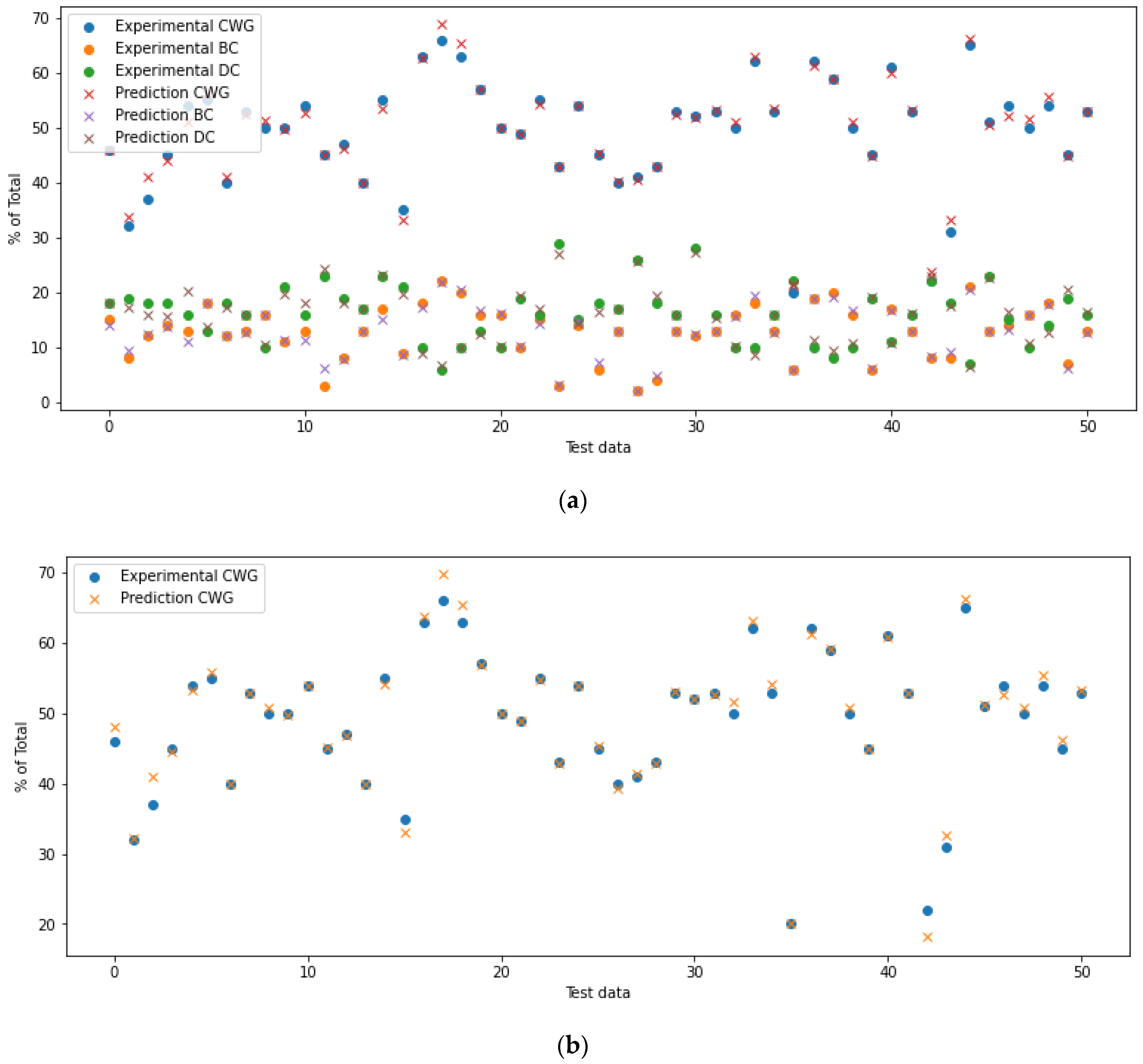
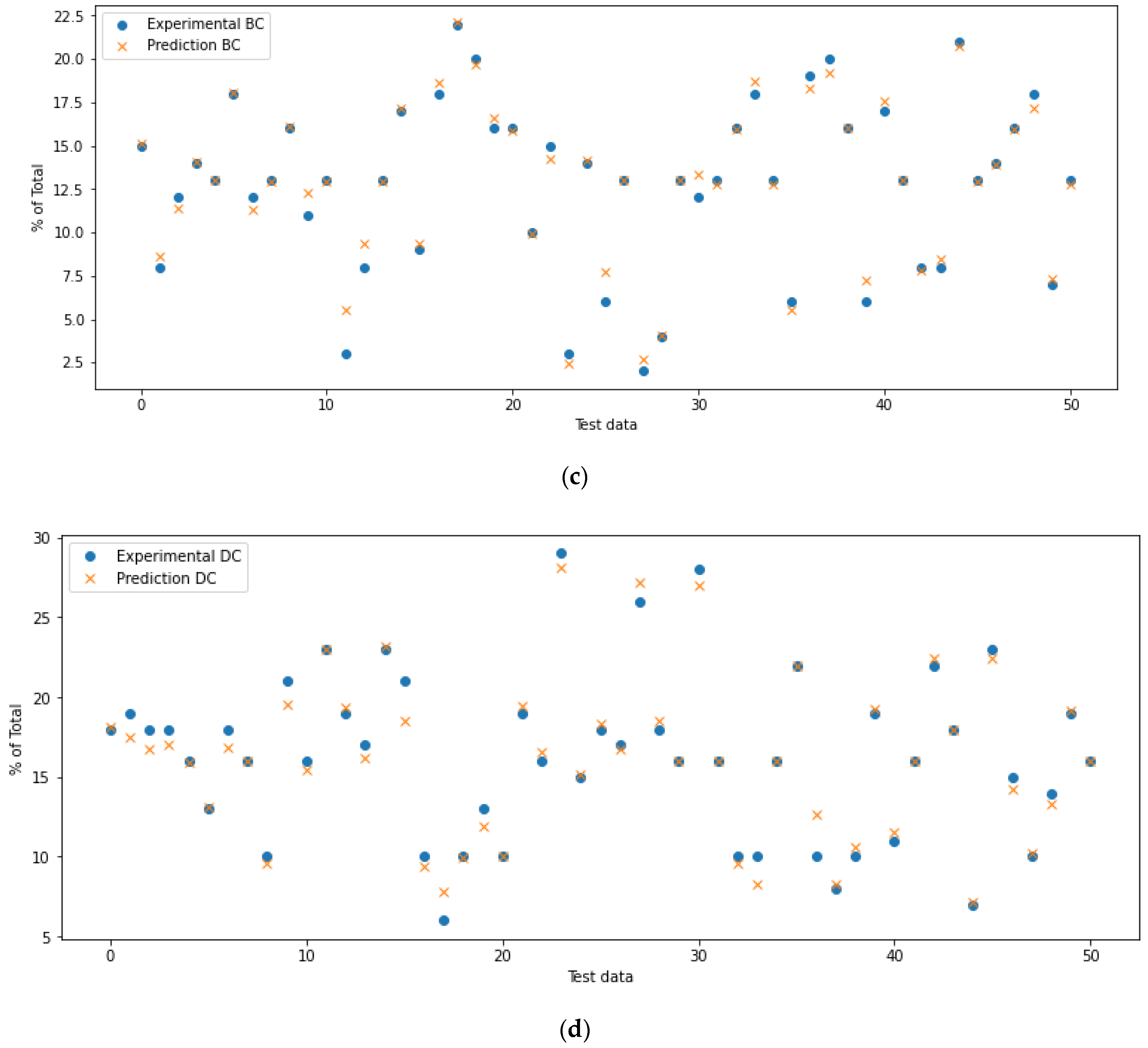
4.1. Defining the ANN Accuracy
4.2. Process Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Narziβ, L. Abriss der Bierbrauerei. Weinheim; Wiley-VCH Verlag GmbH & Co. KGaA: Wienheim, Germany, 2005. [Google Scholar]
- Back, W. Ausgewählte Kapitel der Brauereitechnologie; Fachverlag Hans Karl: Nuremberg, Germany, 2008. [Google Scholar]
- Annemuller, G.; Manger, H.-J.; Lietz, P. The Yeast in the Brewery; VLB Berlin: Berlin, Germany, 2011. [Google Scholar]
- Permyakova, L.V. Classification of preparations to promote yeast vital activity. Food Process. Tech. Technol. 2016, 42, 46–55. [Google Scholar]
- Meledina, T.V. Raw Materials and Auxiliary Materials in Brewing: Reference Book; Professiya Publication: St. Peterburg, Russia, 2003. [Google Scholar]
- Abramov, S.A.; Vlasova, O.K. Nitrogen exchange of yeasts of the genus Saccharomyces on siliceous media. Wine-Mak. Vitic. 2007, 4, 16–17. [Google Scholar]
- Khalilova, E.A.; Islammagomedova, E.A.; Kotenko, S.T. Some features of amino acid metabolism in the metabolism of yeast Saccharomyces cerevisiae Y-503 on nutrient medium with geothermal water of phenolic class. Prod. Alcohol Alcohol. Beverages 2011, 2, 9–12. [Google Scholar]
- Polyakov, I.V.; Lavrova, V.L.; Shishkov, Y.I. The effect of the chemical composition of the yeast Saccharomyces cerevisiae on their physiological and biochemical activity. Storage Process. Farm Prod. 2007, 7, 54–56. [Google Scholar]
- Shiyan, P.; Mudrak, T.; Kyrylenko, R.; Kovalchuk, S. Effect of nitrogen and mineral composition of the high-concentrated wort made from starch-containing raw materials on the cultivation of yeast. East. –Eur. J. Enterp. Technol. 2017, 6, 72–77. [Google Scholar] [CrossRef]
- Petranovskii, V.; Chaves-Rivas, F.; Espinoza, M.H.; Pestryakov, A.; Kolobova, E. Potential uses of natural zeolites for the development of new materials: Short review. MATEC Web Conf. 2016, 85, 01014. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López-Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for food applications: A review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Savchenkov, M.F. Zeolites of Russia. XXI century. Technosphere Saf. 2017, 2, 38–44. [Google Scholar]
- Pavlenko, Y. Research and production cluster as a strategy for the study and integrated use of zeolites in East Transbaikalia. Bull. Trans-Baikal State Univ. 2020, 26, 23–33. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chai, Q.Q.; Lin, N.; Li, X.H.; Wang, W. 1D convolutional neural network for the discrimination of aristolochic acids and their analogues based on near-infrared spectroscopy. Anal. Methods 2019, 11, 5118–5125. [Google Scholar] [CrossRef]
- Ikonic, B.; Bera, O.; Pavlicevic, J.; Kojic, P.; Jokic, A.; Ikonic, P.; Saranovic, Z. Artificial neural network modeling and optimization of wheat starch suspension microfiltration using twisted tape as a turbulence promoter. J. Food Process. Preserv. 2019, 43, 14219. [Google Scholar] [CrossRef]
- Codina, G.G.; Dabija, A.; Oroian, M. Prediction of pasting properties of dough from mixolab measurements using artificial neuronal networks. Foods 2019, 8, 447. [Google Scholar] [CrossRef]
- Sadeghi, E.; Asl, A.H.; Movagharnejad, K. Mathematical modelling of infrared-dried kiwifruit slices under natural and forced convection. Food Sci. Nutr. 2019, 7, 3589–3606. [Google Scholar] [CrossRef]
- Sadeghi, E.; Movagharnejad, K.; Asl, A.H. Mathematical modeling of infrared radiation thin-layer drying of pumpkin samples under natural and forced convection. J. Food Process. Preserv. 2019, 43, 14229. [Google Scholar] [CrossRef]
- Stangierski, J.; Weiss, D.; Kaczmarek, A. Multiple regression models and artificial neural network (ANN) as prediction tools of changes in overall quality during the storage of spreadable processed Gouda cheese. Eur. Food Res. Technol. 2019, 245, 2539–2547. [Google Scholar] [CrossRef]
- Ali, A.; Qadri, S.; Mashwani, W.K.; Belhaouari, S.B.; Naeem, S.; Rafique, S.; Anam, S. Machine learning approach for the classification of corn seed using hybrid features. Int. J. Food Prop. 2020, 23, 1097–1111. [Google Scholar] [CrossRef]
- An, T.; Yu, H.; Yang, C.S.; Liang, G.Z.; Chen, J.Y.; Hu, Z.H.; Dong, C.W. Black tea withering moisture detection method based on convolution neural network confidence. J. Food Process Eng. 2020, 43, 13428. [Google Scholar] [CrossRef]
- Chen, J.D.; Zhang, D.F.; Nanehkaran, Y.A.; Li, D.L. Detection of rice plant diseases based on deep transfer learning. J. Sci. Food Agric. 2020, 100, 3246–3256. [Google Scholar] [CrossRef]
- Ekiz, B.; Baygul, O.; Yalcintan, H.; Ozcan, M. Comparison of the decision tree, artificial neural network and multiple regression methods for prediction of carcass tissues composition of goat kids. Meat Sci. 2020, 161, 108011. [Google Scholar] [CrossRef]
- Lu, A.N.; Wei, X.X.; Cai, R.K.; Xiao, S.J.; Yuan, H.N.; Gong, J.Y.; Xiao, G.N.; Chu, B. Modeling the effect of vibration on the quality of stirred yogurt during transportation. Food Sci. Biotechnol. 2020, 29, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Kaur, B.P.; Nema, P.K.; Babar, O.A.; Kumar, D. Using a combined neural network—Genetic algorithm approach for predicting the complex rheological characteristics of microfluidized sugarcane juice. Lwt-Food Sci. Technol. 2020, 123, 109058. [Google Scholar] [CrossRef]
- Torshizi, M.V.; Asghari, A.; Tabarsa, F.; Danesh, P.; Akbarzadeh, A. Classification by artificial neural network for mushroom color changing under effect UV-A irradiation. Carpathian J. Food Sci. Technol. 2020, 12, 157–167. [Google Scholar] [CrossRef]
- Vakula, A.; Pavlic, B.; Pezo, L.; Horecki, A.T.; Danicic, T.; Raicevic, L.; Sumic, Z. Vacuum drying of sweet cherry: Artificial neural networks approach in process optimization. J. Food Process. Preserv. 2020, 44, 14863. [Google Scholar] [CrossRef]
- Vasighi-Shojae, H.; Gholami-Parashkouhi, M.; Mohammadzamani, D.; Soheili, A. Predicting mechanical properties of golden delicious apple using ultrasound technique and artificial neural network. Food Anal. Methods 2020, 13, 699–705. [Google Scholar] [CrossRef]
- Bhargava, A.; Barisal, A. Automatic detection and grading of multiple fruits by machine learning. Food Anal. Methods 2020, 13, 751–761. [Google Scholar] [CrossRef]
- Permyakova, L.V. Research of the influence of the storage environment on the physiological-biochemical and technological indicators of beer yeast. Polzunovskiy Vestn. 2018, 1, 54–58. [Google Scholar] [CrossRef]
- Soyuduru, D.; Ergun, M.; Tosun, A. Application of a statistical technique to investigate calcium, sodium, and magnesium ion effect in yeast fermentation. Appl. Biochem. Biotechnol. 2009, 152, 326–333. [Google Scholar] [CrossRef]
- Trofimova, Y.; Walker, G.; Rapoport, A. Anhydrobiosis in yeast: Influence of calcium and magnesium ions on yeast resistance to dehydration–rehydration. FEMS Microbiol. Lett. 2010, 308, 55–61. [Google Scholar] [CrossRef]
- Nweke, C.O. Effects of metals on dehydrogenase activity and glucose utilization by Saccharomyces cerevisiae. Niger. J. Biochem. Mol. Biol. 2010, 25, 28–35. [Google Scholar]
- Wietstock, P.C.; Kunz, T.; Waterkamp, H.; Methner, F.-J. Uptake and release of Ca, Cu, Fe, Mg, and Zn during beer production. J. Am. Soc. Brew. Chem. 2015, 73, 179–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafrai, A.V.; Permyakova, L.V.; Borodulin, D.M.; Sergeeva, I.Y. Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks. Information 2022, 13, 529. https://doi.org/10.3390/info13110529
Shafrai AV, Permyakova LV, Borodulin DM, Sergeeva IY. Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks. Information. 2022; 13(11):529. https://doi.org/10.3390/info13110529
Chicago/Turabian StyleShafrai, Anton V., Larisa V. Permyakova, Dmitriy M. Borodulin, and Irina Y. Sergeeva. 2022. "Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks" Information 13, no. 11: 529. https://doi.org/10.3390/info13110529
APA StyleShafrai, A. V., Permyakova, L. V., Borodulin, D. M., & Sergeeva, I. Y. (2022). Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks. Information, 13(11), 529. https://doi.org/10.3390/info13110529





