Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate on Markers of Inflammation and Fibrosis in a Rat Model of Pulmonary Silicosis
Abstract
1. Introduction
2. Results
2.1. Changes in the Counts of Leukocytes in the Blood and Bronchoalveolar Lavage Fluid (BALF)
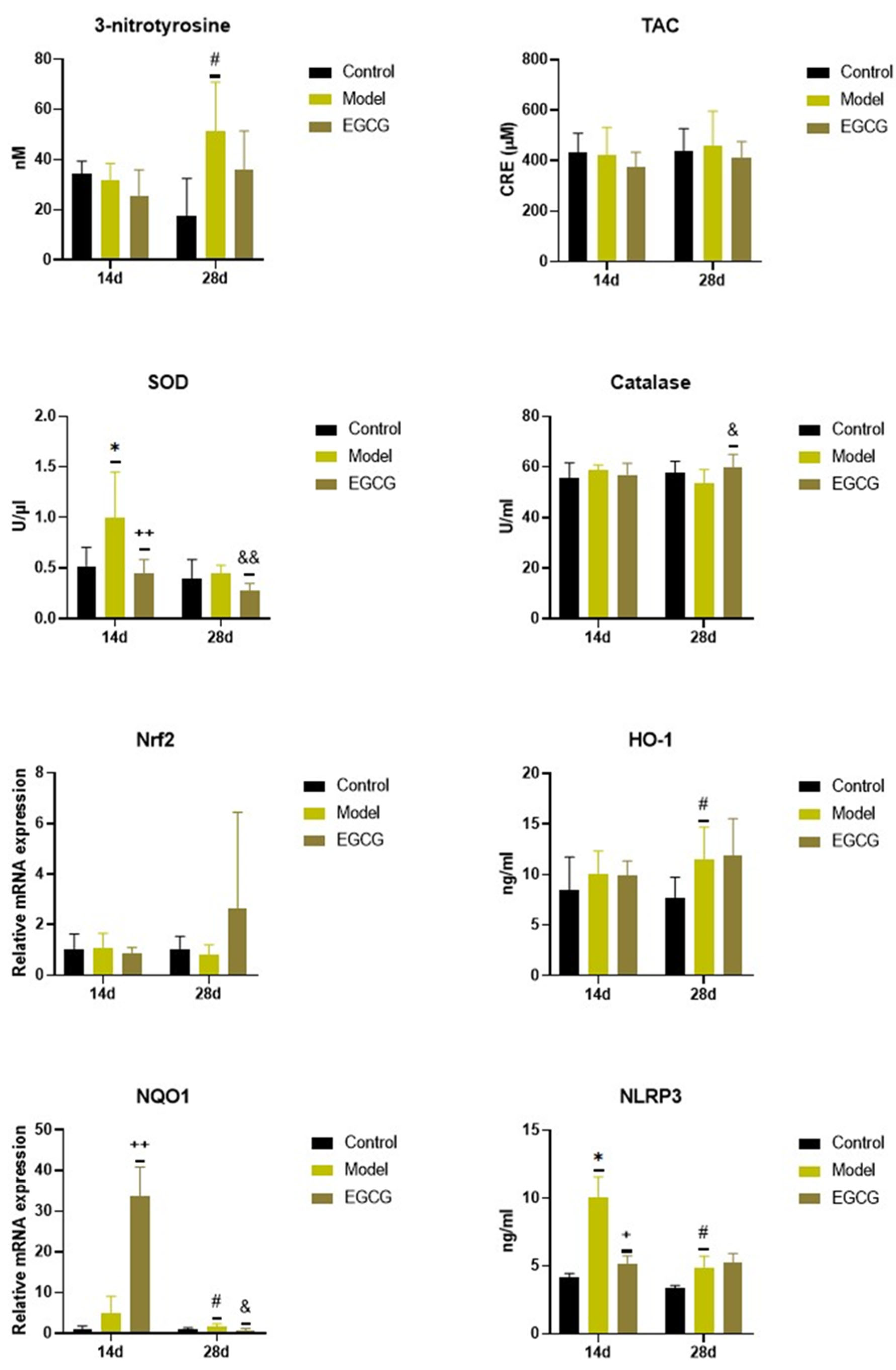
2.2. Changes in the Oxidant/Antioxidant Markers
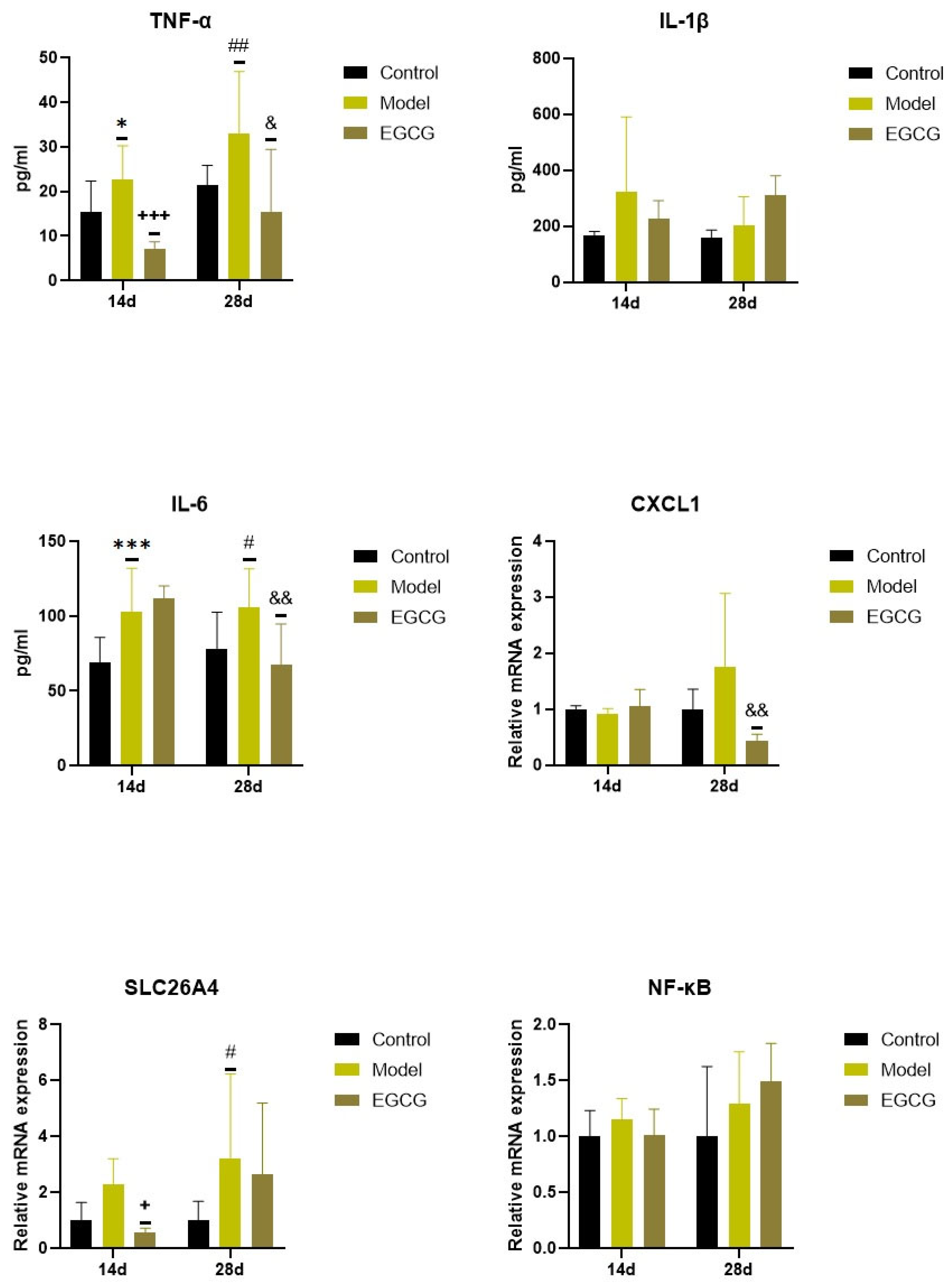
2.3. Changes in the Inflammatory Markers
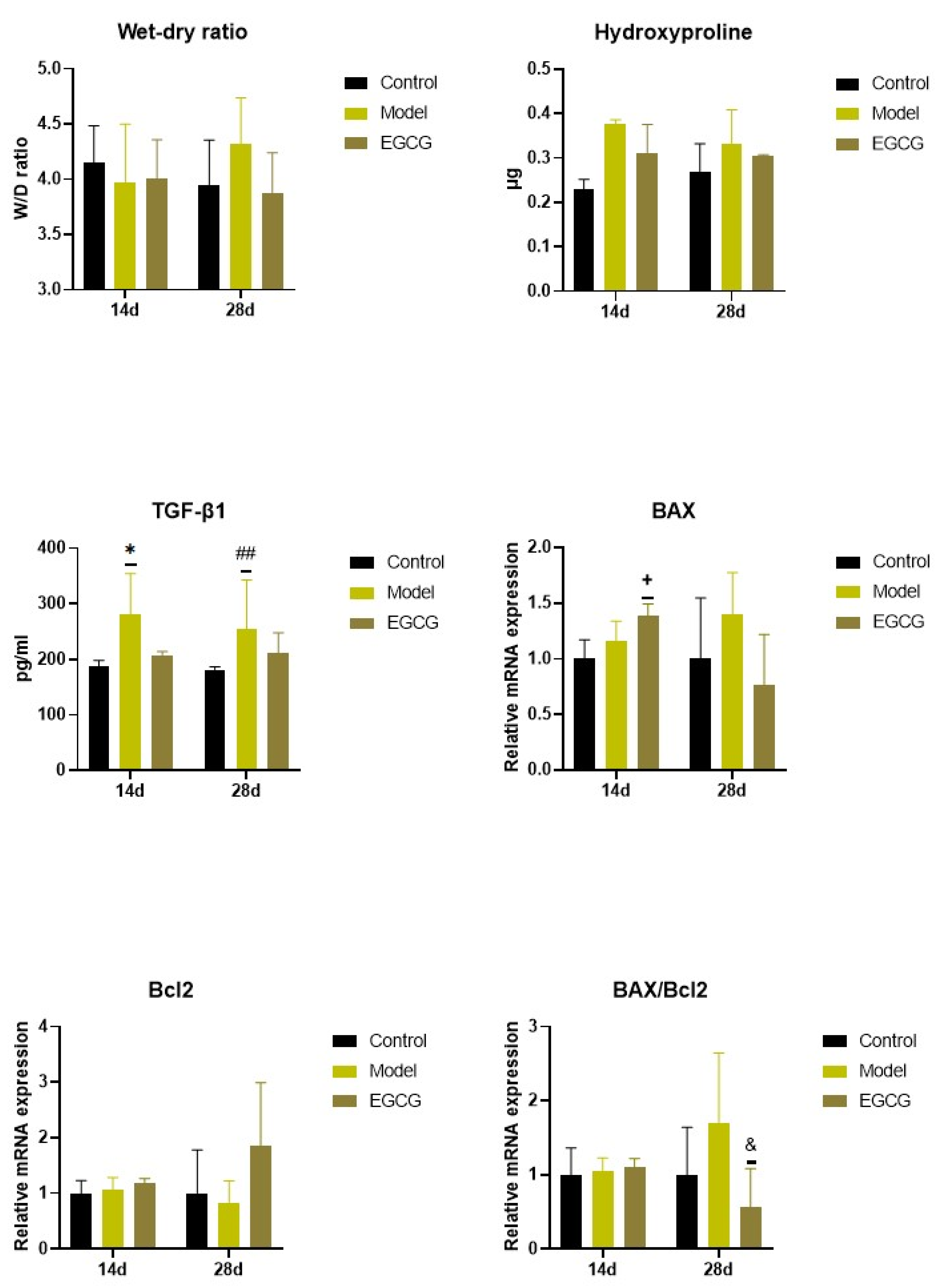
2.4. Changes in the Wet/Dry (W/D) Lung Weight Ratio
2.5. Changes in the Lung Cell Apoptosis
2.6. Changes of Markers of Lung Fibrosis
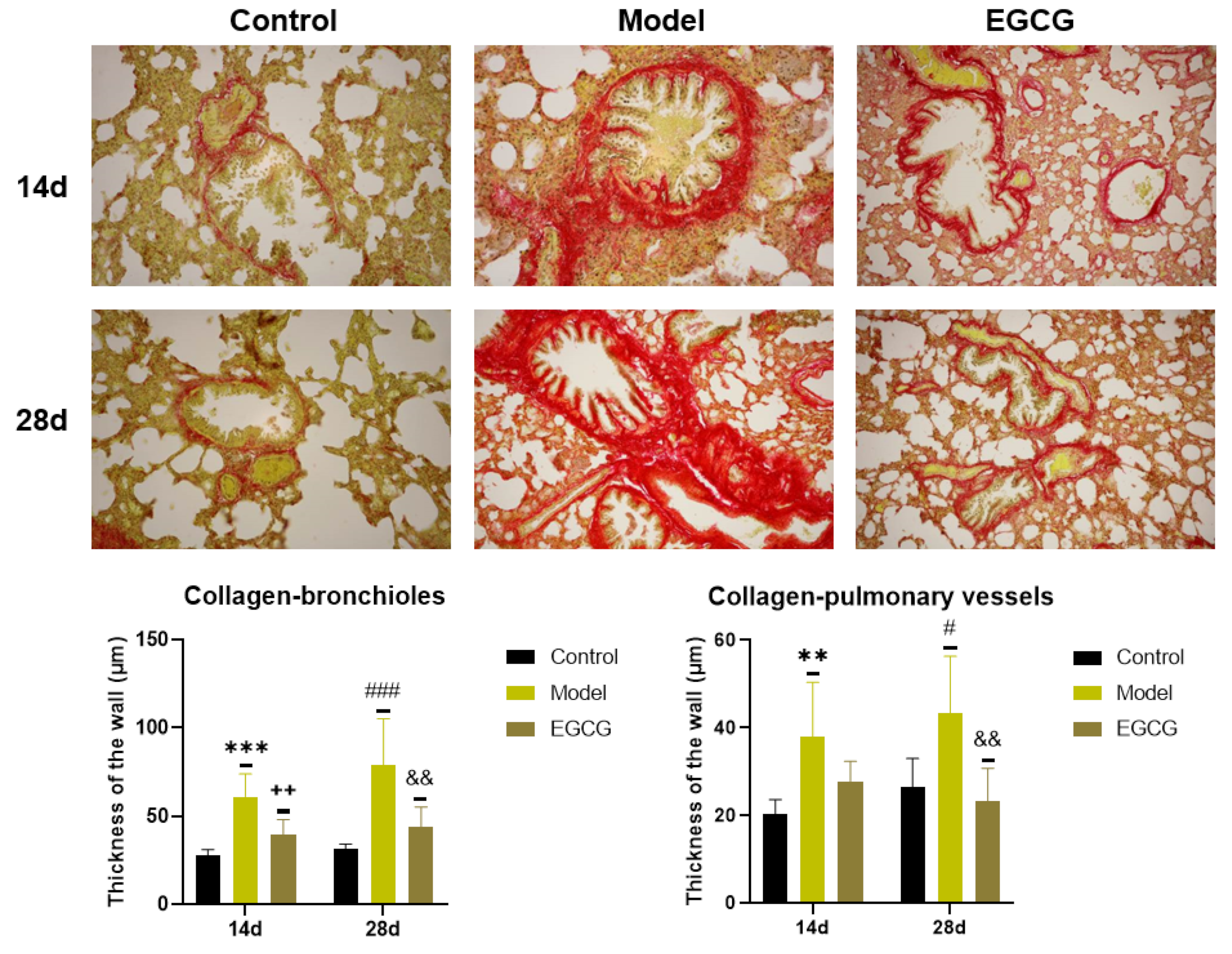
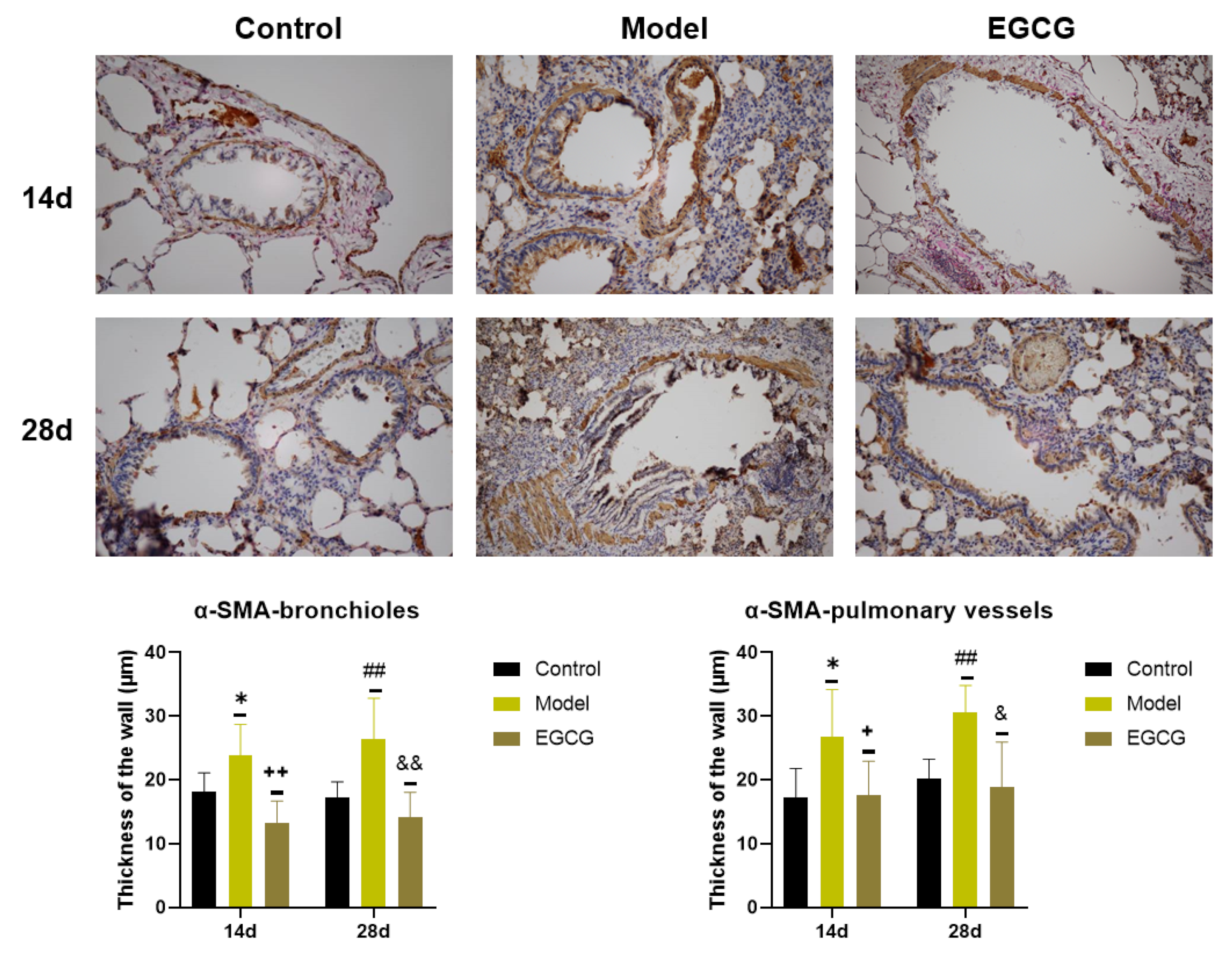
2.7. Immunohistochemical Detection of Collagen and α-Smooth Muscle Actin (α-SMA) in the Lung
3. Discussion
4. Materials and Methods
4.1. Silica
4.2. Animals
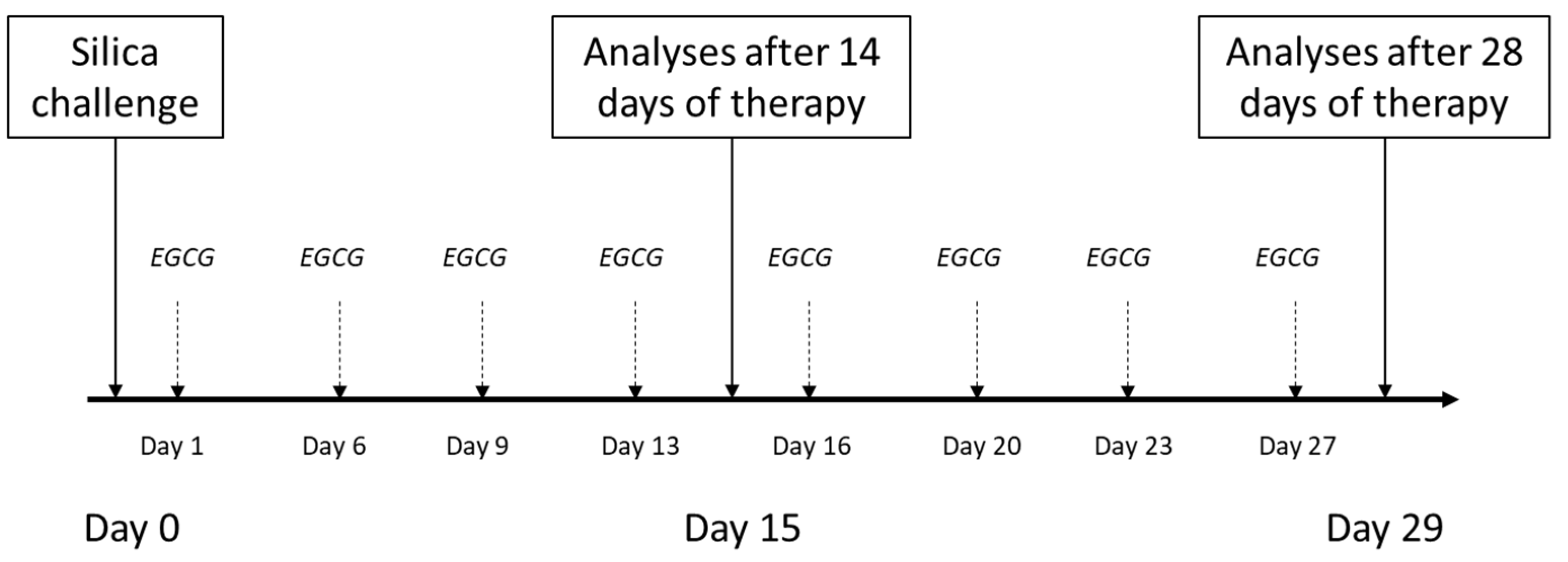
4.3. General Design of Experiments
4.4. Total and Differential Counts of Leukocytes in the Blood and in the BALF
4.5. Analysis of Markers of Inflammation, Oxidative Stress, and Fibrosis by Enzyme Immunosorbent Analysis (ELISA) Methods
4.6. Analysis of Markers of Inflammation, Oxidative Stress, and Fibrosis by Polymerase Chain Reaction (PCR) Methods
4.7. Determination of Wet-Dry (W/D) Lung Weight Ratio
4.8. Immunohistochemical Analyses of Fibrotic Changes in the Lung
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, H.; Goh, N.S.L.; Leong, T.L.; Hoy, R. Silica-associated lung disease: An old-world exposure in modern industries. Respirology 2019, 24, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F., Jr.; Thakur, S.A.; Holian, A. Silica binding and toxicity in alveolar macrophages. Free Radic. Biol. Med. 2008, 44, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre. Toxicol. 2016, 13, 51. [Google Scholar] [CrossRef]
- Adamcakova, J.; Mokra, D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021, 22, 4162. [Google Scholar] [CrossRef]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef]
- Thacker, E.L. Lung inflammatory responses. Vet. Res. 2006, 37, 469–486. [Google Scholar] [CrossRef]
- Esmaeil, N.; Gharagozloo, M.; Rezaei, A.; Grunig, G. Dust events, pulmonary diseases and immune system. Am. J. Clin. Exp. Immunol. 2014, 3, 20–29. [Google Scholar]
- Øvrevik, J.; Refsnes, M.; Namork, E.; Becher, R.; Sandnes, D.; Schwarze, P.E.; Låg, M. Mechanisms of silica-induced IL-8 release from A549 cells: Initial kinase-activation does not require EGFR activation or particle uptake. Toxicology 2006, 227, 105–116. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Dos Santos, G.; Kutuzov, M.A.; Ridge, K.M. The inflammasome in lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L627–L633. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-β-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- Cullinan, P.; Reid, P. Pneumoconiosis. Prim. Care Respir. J. 2013, 22, 249–252. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Jia, Q.; Bo, C.; Shao, H.; Zhang, Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol. Lett. 2019, 300, 59–66. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, G.; Jiang, H.; Zhang, C. High-dose N-acetylcysteine decreases silica-induced lung fibrosis in the rat. J. Int. Med. Res. 2013, 41, 1179–1186. [Google Scholar] [CrossRef]
- Huang, H.; Chen, M.; Liu, F.; Wu, H.; Wang, J.; Chen, J.; Liu, M.; Li, X. N-acetylcysteine tiherapeutically protects against pulmonary fibrosis in a mouse model of silicosis. Biosci. Rep. 2019, 39, BSR20190681. [Google Scholar] [CrossRef]
- Song, L.; Weng, D.; Dai, W.; Tang, W.; Chen, S.; Li, C.; Chen, Y.; Liu, F.; Chen, J. Th17 can regulate silica-induced lung inflammation through an IL-1beta-dependent mechanism. J. Cell. Mol. Med. 2014, 18, 1773–1784. [Google Scholar] [CrossRef]
- Sugimoto, N.; Suzukawa, M.; Nagase, H.; Koizumi, Y.; Ro, S.; Kobayashi, K.; Yoshihara, H.; Kojima, Y.; Kamiyama-Hara, A.; Hebisawa, A.; et al. IL-9 Blockade Suppresses Silica-induced Lung Inflammation and Fibrosis in Mice. Am. J. Respir. Cell Mol. Biol. 2019, 60, 232–243. [Google Scholar] [CrossRef]
- Rabolli, V.; Lo Re, S.; Uwambayinema, F.; Yakoub, Y.; Lison, D.; Huaux, F. Lung fibrosis induced by crystalline silica particles is uncoupled from lung inflammation in NMRI mice. Toxicol. Lett. 2011, 203, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, R.R.; Elkashef, W.F.; Said, E. Tadalafil reduces airway hyperactivity and protects against lung and respiratory airways dysfunction in a rat model of silicosis. Int. Immunopharmacol. 2016, 40, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.P.T.; Lima, J.G.M.E.; Farias-Filho, F.A.; Jannini de Sá, Y.A.P.; de Arantes, A.C.S.; Guimarães, F.V.; Carvalho, V.F.; Hogaboam, C.; Wallace, J.; Martins, M.A.; et al. Intranasal Flunisolide Suppresses Pathological Alterations Caused by Silica Particles in the Lungs of Mice. Front. Endocrinol. 2020, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova, J.; Mokra, D. Herbal compounds in the treatment of pulmonary silicosis. Physiol. Res. 2021, 70, S275–S287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, Q.; Xu, C.; Zhao, J.; Li, S.; Wang, Y.; Tian, L. Sodium tanshinone IIA sulfonate attenuates silica-induced pulmonary fibrosis in rats via activation of the Nrf2 and thioredoxin system. Environ. Toxicol. Pharmacol. 2020, 80, 103461. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Cao, Z.; Gu, J.; Pei, L.; Jia, M.; Su, M. Kaempferol Modulates Autophagy and Alleviates Silica-Induced Pulmonary Fibrosis. DNA. Cell. Biol. 2019, 38, 1418–1426. [Google Scholar] [CrossRef]
- Li, N.; Feng, F.; Wu, K.; Zhang, H.; Zhang, W.; Wang, W. Inhibitory effects of astragaloside IV on silica-induced pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed. Pharmacother. 2019, 119, 109387. [Google Scholar] [CrossRef]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878–1892. [Google Scholar] [CrossRef]
- Peng, H.; Wang, R.; Deng, H.; Wang, Y.; Tang, J.; Cao, F.; Wang, J. Protective effects of oleanolic acid on oxidative stress and the expression of cytokines and collagen by the AKT/NF κB pathway in silicotic rats. Mol. Med. Rep. 2017, 15, 3121–3128. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Fang, J.; Zhang, J.; Chen, Y.; Yeo, A.J.; Lavin, M.F.; Yu, G.; Shao, H. Hesperetin attenuates silica-induced lung injury by reducing oxidative damage and inflammatory response. Exp. Ther. Med. 2021, 21, 297. [Google Scholar] [CrossRef]
- Yang, T.; Wang, J.; Pang, Y.; Dang, X.; Ren, H.; Liu, Y.; Chen, M.; Shang, D. Emodin suppresses silica-induced lung fibrosis by promoting Sirt1 signaling via direct contact. Mol. Med. Rep. 2016, 14, 4643–4649. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Romano, A.; Martel, F. The Role of EGCG in Breast Cancer Prevention and Therapy. Mini Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Unno, K.; Pervin, M.; Taguchi, K.; Konishi, T.; Nakamura, Y. Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules 2020, 25, 1484. [Google Scholar] [CrossRef]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food. 2019, 22, 753–770. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol. Pharm. Bull. 2008, 31, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009, 22, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 849–859. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wei, L.; Sun, W.L.; Wang, L.; Yang, Z.L.; Liu, Y.; Zheng, K.; Wang, Y.; Zhang, W.J. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int. J. Mol. Med. 2014, 34, 92–102. [Google Scholar] [CrossRef]
- Hamdy, M.A.; El-Maraghy, S.A.; Kortam, M.A. Modulatory effects of curcumin and green tea extract against experimentally induced pulmonary fibrosis: A comparison with N-acetyl cysteine. J. Biochem. Mol. Toxicol. 2012, 26, 461–468. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, B.K.; Oh, Y.M.; Lee, Y.S.; Lee, D.S.; Kim, H.K.; Kim, J.Y.; Shim, T.S.; Lee, S.D. Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and endothelin-l expression. Lung 2006, 184, 287–295. [Google Scholar] [CrossRef]
- Yao, J.-J.; Ma, Q.-Q.; Shen, W.-W.; Li, L.-C.; Hu, D. Nano-enabled delivery of EGCG ameliorates silica-induced pulmonary fibrosis in rats. Toxicology 2022, 469, 153114. [Google Scholar] [CrossRef]
- Huaux, F. New developments in the understanding of immunology in silicosis. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 168–173. [Google Scholar] [CrossRef]
- Davis, G.S.; Holmes, C.E.; Pfeiffer, L.M.; Hemenway, D.R. Lymphocytes, lymphokines, and silicosis. J. Environ. Pathol. Toxicol. Oncol. 2001, 20 (Suppl. S1), 53–65. [Google Scholar] [CrossRef]
- Reasor, M.J.; Antonini, J.M. Pulmonary responses to single versus multiple intratracheal instillations of silica in rats. J. Toxicol. Environ. Health-Part A 2001, 62, 9–21. [Google Scholar] [CrossRef]
- Nakano-Narusawa, Y.; Yokohira, M.; Yamakawa, K.; Saoo, K.; Imaida, K.; Matsuda, Y. Single Intratracheal Quartz Instillation Induced Chronic Inflammation and Tumourigenesis in Rat Lungs. Sci. Rep. 2020, 10, 6647. [Google Scholar] [CrossRef]
- Adamson, I.Y.R.; Bowden, D.H. Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am. J. Pathol. 1984, 117, 37–43. [Google Scholar]
- El-Kashef, D.H. Nicorandil ameliorates pulmonary inflammation and fibrosis in a rat model of silicosis. Int. Immunopharmacol. 2018, 64, 289–297. [Google Scholar] [CrossRef]
- Marques Da Silva, V.; Benjdir, M.; Montagne, P.; Pairon, J.C.; Lanone, S.; Andujar, P. Pulmonary Toxicity of Silica Linked to Its Micro- or Nanometric Particle Size and Crystal Structure: A Review. Nanomaterials 2022, 12, 2392. [Google Scholar] [CrossRef]
- Øvrevik, J.; Låg, M.; Holme, J.A.; Schwarze, P.E.; Refsnes, M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology 2009, 259, 46–53. [Google Scholar] [CrossRef]
- Porter, D.W.; Ye, J.; Ma, J.; Barger, M.; Robinson, V.A.; Ramsey, D.; McLaurin, J.; Khan, A.; Landsittel, D.; Teass, A.; et al. Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhal. Toxicol. 2002, 14, 349–367. [Google Scholar] [CrossRef]
- Bowden, D.H.; Hedgecock, C.; Adamson, I.Y. Silica-induced pulmonary fibrosis involves the reaction of particles with interstitial rather than alveolar macrophages. J. Pathol. 1989, 158, 73–80. [Google Scholar] [CrossRef]
- Lapp, N.L.; Castranova, V. How silicosis and coal workers’ pneumoconiosis develop-a cellular assessment. Occup. Med. 1993, 8, 35–56. [Google Scholar]
- Kumar, R.K. Quantitative immunohistologic assessment of lymphocyte populations in the pulmonary inflammatory response to intratracheal silica. Am. J. Pathol. 1989, 135, 605–614. [Google Scholar]
- Zhu, Z.; Yang, G.; Wang, Y.; Yang, J.; Gao, A.; Niu, P.; Tian, L. Suppression of thioredoxin system contributes to silica-induced oxidative stress and pulmonary fibrogenesis in rats. Toxicol. Lett. 2013, 222, 289–294. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Fan, J.; Hou, Z.; Wang, T.; Wu, B.; Ni, C. Annexin A5 promotes macrophage activation and contributes to pulmonary fibrosis induced by silica particles. Toxicol. Ind. Health 2016, 32, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Sato, T.; Shigemori, S.; Shimosato, T.; Shinkai, M.; Kaneko, T. Regulatory role of heme oxygenase-1 in silica-induced lung injury. Respir. Res. 2018, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeno, M.; Honma, K.; Yamauchi, H.; Saito, Y.; Sasaki, T.; Morikubo, H.; Nagashima, Y.; Takagi, S.; Yamanaka, K.; et al. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am. J. Respir. Crit. Care Med. 2006, 174, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Chen, M.; Jiang, Y.; Chen, M.; Zhou, T.; Wang, Y.; Hou, Z.; Ren, L. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int. J. Nanomed. 2014, 9, 2073–2087. [Google Scholar] [CrossRef]
- Lin, C.; Zhao, X.; Sun, D.; Zhang, L.; Fang, W.; Zhu, T.; Wang, Q.; Liu, B.; Wei, S.; Chen, G.; et al. Transcriptional activation of follistatin by Nrf2 protects pulmonary epithelial cells against silica nanoparticle-induced oxidative stress. Sci. Rep. 2016, 6, 21133. [Google Scholar] [CrossRef]
- Liu, W.; Hu, T.; Zhou, L.; Wu, D.; Huang, X.; Ren, X.; Lv, Y.; Hong, W.; Huang, G.; Lin, Z.; et al. Nrf2 protects against oxidative stress induced by SiO2 nanoparticles. Nanomedicine 2017, 12, 2303–2318. [Google Scholar] [CrossRef]
- Lee, K.I.; Su, C.C.; Fang, K.M.; Wu, C.C.; Wu, C.T.; Chen, Y.W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci. Rep. 2020, 10, 9928. [Google Scholar] [CrossRef]
- Pang, X.; Shao, L.; Nie, X.; Yan, H.; Li, C.; Yeo, A.J.; Lavin, M.F.; Xia, Q.; Shao, H.; Yu, G.; et al. Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int. Immunopharmacol. 2021, 91, 107277. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X. NF-kappaB, a pivotal transcription factor in silica-induced diseases. Mol. Cell. Biochem. 2002, 234, 169–176. [Google Scholar] [CrossRef]
- Peeters, P.M.; Eurlings, I.M.; Perkins, T.N.; Wouters, E.F.; Schins, R.P.; Borm, P.J.; Drommer, W.; Reynaert, N.L.; Albrecht, C. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part. Fibre Toxicol. 2014, 11, 58. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Wang, Y.; Wang, J.; Zhou, F.; Wang, H.; Xie, W.; Kong, H. NLRP3 inflammasome inhibition attenuates silica-induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells. Exp. Cell. Res. 2018, 362, 489–497. [Google Scholar] [CrossRef]
- Jiang, Q.T.; Han, L.; Liu, X.; Zhu, B.L. Upregulation of Slc26a4 in the Early Development of Silicosis via GEO Database Analysis in vivo and in vitro. Biomed. Environ. Sci. 2019, 32, 938–943. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Li, T.; Jin, F.; Li, Y.; Xu, H.; Yang, F. Transcriptomic analysis identifies upregulation of secreted phosphoprotein 1 in silicotic rats. Exp. Ther. Med. 2021, 21, 579. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Yucesoy, B.; Vallyathan, V.; Landsittel, D.P.; Simeonova, P.; Luster, M.I. Cytokine polymorphisms in silicosis and other pneumoconioses. Mol. Cell. Biochem. 2002, 234, 219–224. [Google Scholar] [CrossRef]
- Karkale, S.; Khurana, A.; Saifi, M.A.; Godugu, C.; Talla, V. Oropharyngeal administration of silica in Swiss mice: A robust and reproducible model of occupational pulmonary fibrosis. Pulm. Pharmacol. Ther. 2018, 51, 32–40. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Grunig, G.; Marsh, L.M.; Esmaeil, N.; Jackson, K.; Gordon, T.; Reibman, J.; Kwapiszewska, G.; Park, S.H. Perspective: Ambient air pollution: Inflammatory response and effects on the lung’s vasculature. Pulm. Circ. 2014, 4, 25–35. [Google Scholar] [CrossRef]
- Tamagawa, E.; Bai, N.; Morimoto, K.; Gray, C.; Mui, T.; Yatera, K.; Zhang, X.; Xing, L.; Li, Y.; Laher, I.; et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L79–L85. [Google Scholar] [CrossRef]
- Nurkiewicz, T.R.; Porter, D.W.; Hubbs, A.F.; Stone, S.; Moseley, A.M.; Cumpston, J.L.; Goodwill, A.G.; Frisbee, S.J.; Perrotta, P.L.; Brock, R.W.; et al. Pulmonary particulate matter and systemic microvascular dysfunction. Res. Rep. Health Eff. Inst. 2011, 164, 3–48. [Google Scholar]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox. Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (-)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Yokohira, M.; Hashimoto, N.; Yamakawa, K.; Kishi, S.; Ninomiya, F.; Kanie, S.; Saoo, K.; Imaida, K. Rat strain differences in levels and effects of chronic inflammation due to intratracheal instillation of quartz on lung tumorigenesis induced by DHPN. Exp. Toxicol. Pathol. 2014, 66, 391–401. [Google Scholar] [CrossRef]
- Gharaee-Kermani, M.; Hatano, K.; Nozaki, Y.; Phan, S.H. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 2005, 166, 1593–1606. [Google Scholar] [CrossRef]
- Ray, J.L.; Holian, A. Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica. Inhal. Toxicol. 2019, 31, 285–297. [Google Scholar] [CrossRef]
- Gharaee-Kermani, M.; Ullenbruch, M.; Phan, S.H. Animal models of pulmonary fibrosis. Methods. Mol. Med. 2005, 117, 251–259. [Google Scholar] [CrossRef]
- Lakatos, H.F.; Burgess, H.A.; Thatcher, T.H.; Redonnet, M.R.; Hernady, E.; Williams, J.P.; Sime, P.J. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp. Lung. Res. 2006, 32, 181–199. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem. Biol. Interact. 2009, 180, 271–280. [Google Scholar] [CrossRef]
- Mikolka, P.; Kopincova, J.; Mikusiakova, L.T.; Kosutova, P.; Calkovska, A.; Mokra, D. Antiinflammatory Effect of N-Acetylcysteine Combined with Exogenous Surfactant in Meconium-Induced Lung Injury. Adv. Exp. Med. Biol. 2016, 934, 63–75. [Google Scholar] [CrossRef]
| Control14d | Model14d | EGCG14d | Control28d | Model28d | EGCG28d | |
|---|---|---|---|---|---|---|
| Total count of leukocytes in the blood (×103/µL) | ||||||
| 3.7 ± 1.2 | 5.4 ± 1.2 * | 6.4 ± 1.7 | 3.7 ± 1.1 | 5.0 ± 1.2 | 6.5 ± 0.8 & | |
| Absolute counts of leukocyte types in the blood (×103/µL) | ||||||
| Neu | 0.64 ± 0.17 | 0.94 ± 0.38 | 2.20 ± 0.65 ++ | 0.52 ± 0.17 | 0.76 ± 0.29 | 2.02 ± 0.60 &&& |
| Lym | 2.90 ± 1.10 | 4.26 ± 0.84 * | 4.50 ± 1.50 | 3.00 ± 0.93 | 3.96 ± 1.00 | 4.10 ± 0.87 |
| Mon | 0.08 ± 0.05 | 0.12 ± 0.06 | 0.20 ± 0.05 | 0.09 ± 0.04 | 0.16 ± 0.06 # | 0.26 ± 0.05 && |
| Eos | 0.05 ± 0.06 | 0.05 ± 0.03 | 0.07 ± 0.03 | 0.05 ± 0.04 | 0.07 ± 0.05 | 0.06 ± 0.03 |
| Relative counts of leukocyte types in the blood (%) | ||||||
| Neu | 18.4 ± 4.9 | 17.0 ± 4.2 | 32.3 ± 9.3 + | 14.6 ± 3.7 | 15.4 ± 4.6 | 31.6 ± 9.2 &&& |
| Lym | 78.3 ± 4.7 | 79.8 ± 5.0 | 63.5 ± 10.5 ++ | 81.7 ± 3.8 | 79.9 ± 5.6 | 63.5 ± 9.6 && |
| Mon | 2.0 ± 1.0 | 2.3 ± 0.8 | 3.0 ± 1.0 | 2.4 ± 1.1 | 3.2 ± 1.0 | 4.0 ± 0.5 |
| Eos | 1.3 ± 1.3 | 0.9 ± 0.5 | 1.1 ± 0.6 | 1.3 ± 1.2 | 1.4 ± 0.7 | 0.8 ± 0.4 & |
| Control14d | Model14d | EGCG14d | Control28d | Model28d | EGCG28d | |
|---|---|---|---|---|---|---|
| Total count of leukocytes in the BALF (×103/mL) | ||||||
| 70.9 ± 52.1 | 97.5 ± 41.0 | 95.0 ± 35.8 | 121.3 ± 44.9 | 135.7 ± 68.5 | 96.3 ± 36.6 | |
| Absolute counts of leukocyte types in the BALF (×103/mL) | ||||||
| Mac | 97.7 ± 41.5 | 86.1 ± 44.5 | 89.1 ± 35.1 | 142.1 ± 101.5 | 108.3 ± 61.0 | 90.6 ± 38.5 |
| Neu | 2.3 ± 1.7 | 12.4 ± 4.7 *** | 3.7 ± 2.0 +++ | 3.8 ± 1.5 | 20.8 ± 10.3 ### | 9.3 ± 3.1 & |
| Lym | 1.0 ± 0.6 | 2.1 ± 1.7 | 1.5 ± 1.2 | 1.0 ± 0.7 | 4.7 ± 1.9 ## | 1.8 ± 1.2 & |
| Eos | 0.3 ± 0.3 | 0.7 ± 0.3 * | 0.7 ± 0.3 | 0.2 ± 0.2 | 1.7 ± 1.5 # | 0.8 ± 0.8 |
| Relative counts of leukocyte types in the BALF (%) | ||||||
| Mac | 96.7 ± 1.1 | 82.6 ± 6.5 *** | 92.9 ± 4.4 ++ | 96.1 ± 1.1 | 77.0 ± 8.9 ### | 87.2 ± 5.8 & |
| Neu | 2.1 ± 0.9 | 14.7 ± 6.9 *** | 4.6 ± 3.5 ++ | 3.1 ± 1.2 | 17.4 ± 6.9 ### | 10.2 ± 5.2 & |
| Lym | 0.9 ± 0.3 | 1.9 ± 1.1 * | 1.7 ± 1.3 | 0.7 ± 0.2 | 3.9 ± 1.8 ### | 1.8 ± 1.2 & |
| Eos | 0.2 ± 0.3 | 0.8 ± 0.2 ** | 0.8 ± 0.4 | 0.2 ± 0.2 | 1.8 ± 1.4 ### | 0.8 ± 0.6 |
| Markers | After 14 Days | After 28 Days |
|---|---|---|
| Inflammation | ||
| Total blood leukocytes | ↑ NS | ↑ p < 0.05 |
| Absolute blood neutrophils | ↑ p < 0.01 | ↑ p < 0.001 |
| Absolute blood lymphocytes | ↑ NS | ↑ NS |
| Absolute blood monocytes | ↑ NS | ↑ p < 0.01 |
| Absolute blood eosinophils | ↑ NS | ↓ NS |
| Total BALF leukocytes | ↓ NS | ↓ NS |
| Absolute BALF macrophages | ↑ NS | ↓ NS |
| Absolute BALF neutrophils | ↓ p < 0.001 | ↓ p < 0.05 |
| Absolute BALF lymphocytes | ↓ NS | ↓ p < 0.05 |
| Absolute BALF eosinophils | - | ↓ NS |
| W/D ratio | - | ↓ NS |
| NLRP3 | ↓ p < 0.05 | - |
| NF-κB | ↓ NS | ↑ NS |
| TNFα | ↓ p < 0.001 | ↓ p < 0.05 |
| IL-1β | ↓ NS | ↑ NS |
| IL-6 | - | ↓ p < 0.01 |
| CXCL1 | - | ↓ p < 0.01 |
| SLC26A4 | ↓ p < 0.05 | ↓ NS |
| Oxidative stress | ||
| 3-nitrotyrosine | ↓ NS | ↓ NS |
| TAC | ↓ NS | ↓ NS |
| SOD | ↓ p < 0.01 | ↓ p < 0.01 |
| Catalase | - | ↑ p < 0.05 |
| Nrf2 | - | ↑ NS |
| HO-1 | - | - |
| NQO1 | ↑ p < 0.01 | ↓ p < 0.05 |
| Apoptosis | ||
| Bax/Bcl2 | - | ↓ p < 0.05 |
| Fibrosis | ||
| Hydroxyproline | ↓ NS | ↓ NS |
| TGF-β1 | ↓ NS | ↓ NS |
| Collagen—bronchioles (IHI) | ↓ p < 0.01 | ↓ p < 0.01 |
| Collagen—vessels (IHI) | ↓ p < 0.01 | ↓ p < 0.01 |
| α-SMA—bronchioles (IHI) | ↓ p < 0.01 | ↓ p < 0.01 |
| α-SMA—vessels (IHI) | ↓ p < 0.01 | ↓ p < 0.05 |
| Primers | Forward | Reverse |
|---|---|---|
| Nrf2 | TCTGACTCCGGCATTTCACT | TGTTGGCTGTGCTTTAGGTC |
| NQO-1 | CATCATTTGGGCAAGTCC | ACAGCCGTGGCAGAACTA |
| Bcl-2 | GGGATGACTTCTCTCGTCGC | AGAGCGATGTTGTCCACCAG |
| Bax | AGGACGCATCCACCAAGAAG | GGGGGTCCCGAAGTAGGAAA |
| CXCL1 | GGCAGGGATTCACTTCAAGAACATC | AGTGTGGCTATGACTTCGGTTTGG |
| NF-κB | TCTGACTCCGGCATTTCACT | TGTTGGCTGTGCTTTAGGTC |
| SLC26A4 | GGGCAACCAAGAACGGGATTATAAG | TCTGGCTCTTCGACATCTTCATCAG |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamcakova, J.; Balentova, S.; Barosova, R.; Hanusrichterova, J.; Mikolka, P.; Prso, K.; Mokry, J.; Tatarkova, Z.; Kalenska, D.; Mokra, D. Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate on Markers of Inflammation and Fibrosis in a Rat Model of Pulmonary Silicosis. Int. J. Mol. Sci. 2023, 24, 1857. https://doi.org/10.3390/ijms24031857
Adamcakova J, Balentova S, Barosova R, Hanusrichterova J, Mikolka P, Prso K, Mokry J, Tatarkova Z, Kalenska D, Mokra D. Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate on Markers of Inflammation and Fibrosis in a Rat Model of Pulmonary Silicosis. International Journal of Molecular Sciences. 2023; 24(3):1857. https://doi.org/10.3390/ijms24031857
Chicago/Turabian StyleAdamcakova, Jana, Sona Balentova, Romana Barosova, Juliana Hanusrichterova, Pavol Mikolka, Kristian Prso, Juraj Mokry, Zuzana Tatarkova, Dagmar Kalenska, and Daniela Mokra. 2023. "Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate on Markers of Inflammation and Fibrosis in a Rat Model of Pulmonary Silicosis" International Journal of Molecular Sciences 24, no. 3: 1857. https://doi.org/10.3390/ijms24031857
APA StyleAdamcakova, J., Balentova, S., Barosova, R., Hanusrichterova, J., Mikolka, P., Prso, K., Mokry, J., Tatarkova, Z., Kalenska, D., & Mokra, D. (2023). Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate on Markers of Inflammation and Fibrosis in a Rat Model of Pulmonary Silicosis. International Journal of Molecular Sciences, 24(3), 1857. https://doi.org/10.3390/ijms24031857






