Cirsilineol Treatment Attenuates PM2.5-Induced Lung Injury in Mice
Abstract
1. Introduction
2. Results
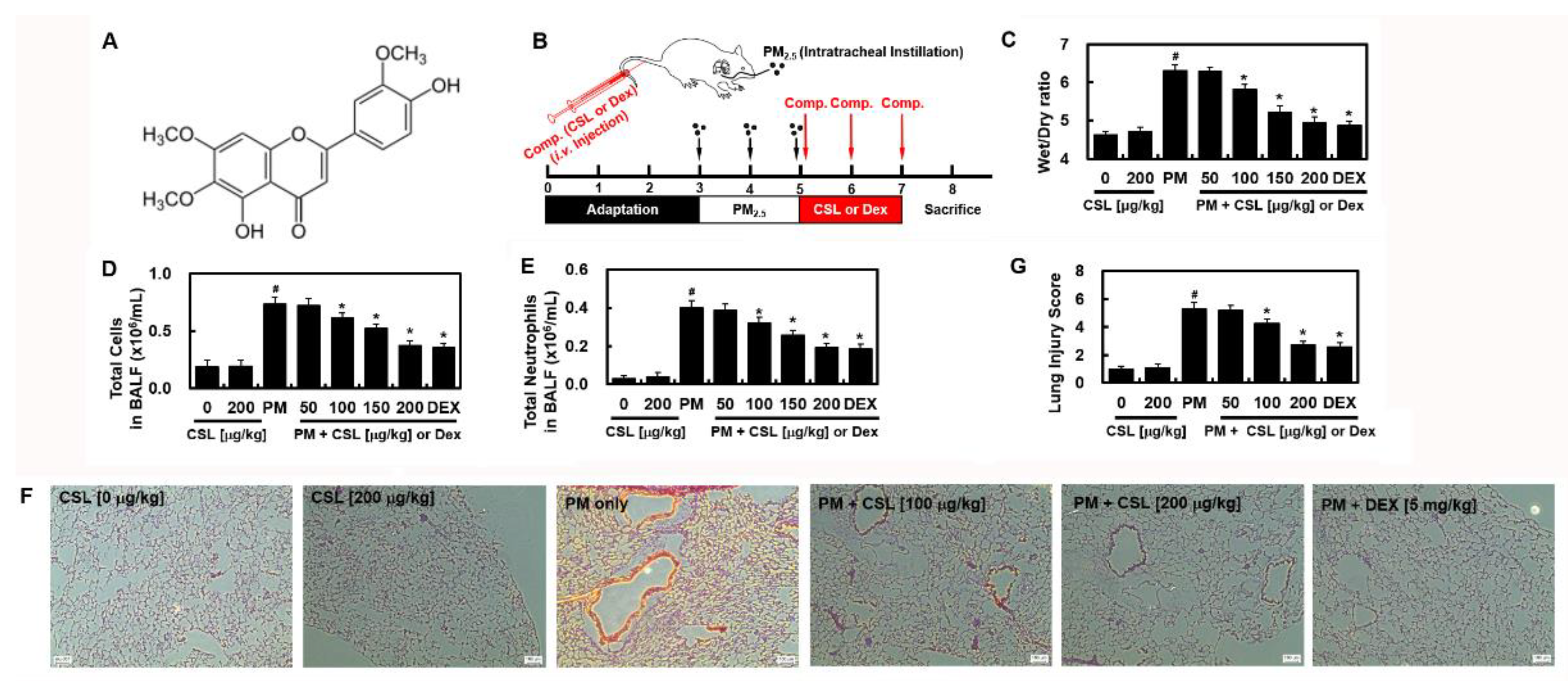
2.1. CSL Protects against PM2.5-Induced Lung Toxicity
2.2. CSL Prevents PM2.5-Induced Autophagy Dysfunction
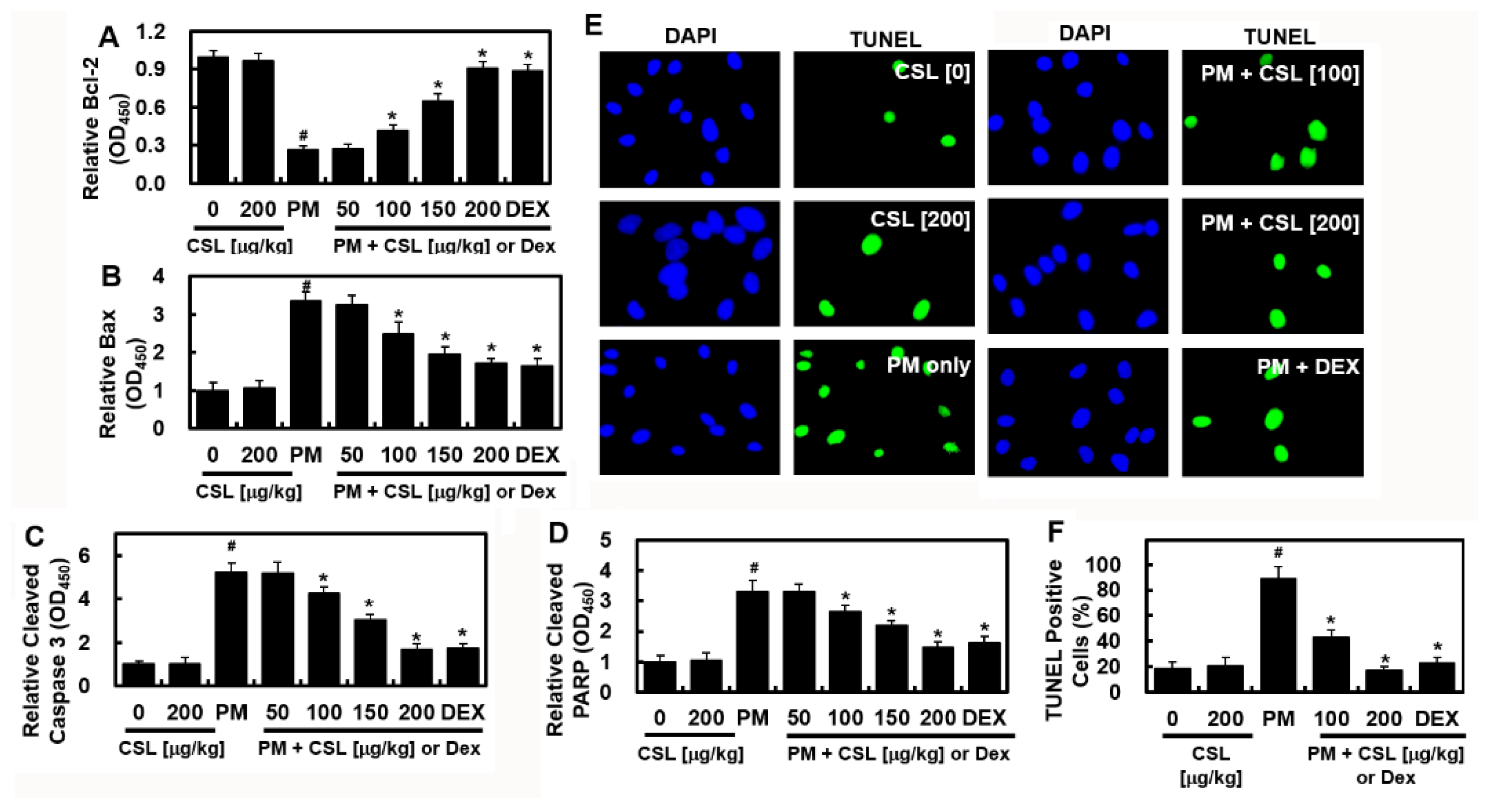
2.3. CSL Inhibits PM2.5-Induced Apoptosis in Mice Lung Tissues
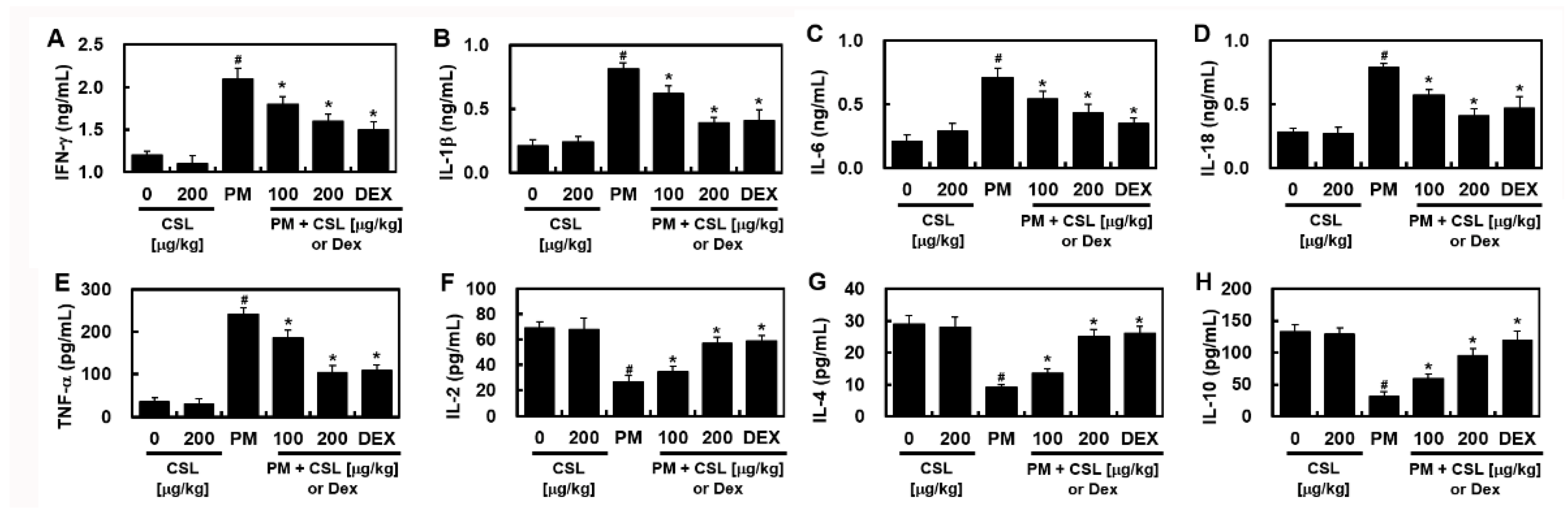
2.4. CSL Protected PM2.5-Induced Pulmonary Inflammatory Responses in Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
4.3. Lung Wet/Dry (W/D) Weight Ratios
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Cell Counts in BALF Samples
4.7. Western Blotting
4.8. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Chen, Y.; Yu, Z.; Ding, H.; Ma, Z. The influence of PM2.5 on lung injury and cytokines in mice. Exp. Ther. Med. 2019, 18, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Ji, X.; Li, G.; Sang, N. Ambient PM2.5 causes lung injuries and coupled energy metabolic disorder. Ecotoxicol. Environ. Saf. 2019, 170, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, T.; Wang, F.; Ji, T.; Guo, Z. Seasonal variation of polybrominated diphenyl ethers in PM2.5 aerosols over the East China Sea. Chemosphere 2015, 119, 675–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, G.; Sang, N. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: A study based on myocardial toxicity. Environ. Pollut. 2016, 216, 380–390. [Google Scholar] [CrossRef]
- Gong, H., Jr.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Geller, M.D.; Sioutas, C. Respiratory responses to exposures with fine particulates and nitrogen dioxide in the elderly with and without COPD. Inhal. Toxicol. 2005, 17, 123–132. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, G.; Li, Y.; Tan, M.; Wang, W.; Chen, J.; Hwu, Y.; Hsu, P.C.; Je, J.H.; Margaritondo, G.; et al. Synchrotron microradiography study on acute lung injury of mouse caused by PM(2.5) aerosols. Eur. J. Radiol. 2006, 58, 266–272. [Google Scholar] [CrossRef]
- Wang, J.S.; Tseng, C.Y.; Chao, M.W. Diesel Exhaust Particles Contribute to Endothelia Apoptosis via Autophagy Pathway. Toxicol. Sci. 2017, 156, 72–83. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review. Sci. Total Environ. 2017, 586, 610–622. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Wu, Y.F.; Wang, P.L.; Wu, Y.P.; Li, Z.Y.; Zhao, Y.; Zhou, J.S.; Zhu, C.; Cao, C.; Mao, Y.Y.; et al. Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy 2016, 12, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.P.; Pinheiro, N.M.; Mernak, M.I.; Righetti, R.F.; Martins, M.A.; Lago, J.H.; Lopes, F.D.; Tiberio, I.F.; Prado, C.M. Evidences of Herbal Medicine-Derived Natural Products Effects in Inflammatory Lung Diseases. Mediat. Inflamm. 2016, 2016, 2348968. [Google Scholar] [CrossRef]
- Lu, A.P.; Jia, H.W.; Xiao, C.; Lu, Q.P. Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 2004, 10, 1854–1856. [Google Scholar] [CrossRef]
- Yin, Y.; Gong, F.Y.; Wu, X.X.; Sun, Y.; Li, Y.H.; Chen, T.; Xu, Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008, 120, 1–6. [Google Scholar] [CrossRef]
- Sheng, X.; Sun, Y.; Yin, Y.; Chen, T.; Xu, Q. Cirsilineol inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway. J. Pharm. Pharmacol. 2008, 60, 1523–1529. [Google Scholar] [CrossRef]
- Bai, N.S.; He, K.; Zhou, Z.; Lai, C.S.; Zhang, L.; Quan, Z.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010, 122, 831–835. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, A.L.N.; Viana, L.; Silva, M.G.; Lavor, E.M.; Oliveira, R.G.; Alencar, E.B.; Lima, R.S.; Mendes, R.L.; Rolim, L.A.; et al. beta-Cyclodextrin complex improves the bioavailability and antitumor potential of cirsiliol, a flavone isolated from Leonotis nepetifolia (Lamiaceae). Heliyon 2019, 5, e01692. [Google Scholar] [CrossRef]
- Li, Y.; Batibawa, J.W.; Du, Z.; Liang, S.; Duan, J.; Sun, Z. Acute exposure to PM2.5 triggers lung inflammatory response and apoptosis in rat. Ecotoxicol. Environ. Saf. 2021, 222, 112526. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lee, T.L.; Chen, Y.C.; Liang, C.J.; Wang, S.H.; Lue, J.H.; Tsai, J.S.; Lee, S.W.; Chen, S.H.; Yang, Y.F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-kappaB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Qiu, X.; Hu, X.; Shang, Y.; Pardo, M.; Fang, Y.; Wang, J.; Rudich, Y.; Zhu, T. Effects on IL-1beta signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ. Pollut 2018, 237, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, W.; Kim, E.; Ku, S.K.; Bae, J.S. Inhibitory effects of collismycin C and pyrisulfoxin A on particulate matter-induced pulmonary injury. Phytomedicine 2019, 62, 152939. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jeong, S.Y.; Gu, M.J.; Lim, J.S.; Park, E.K.; Baek, M.C.; Kim, J.S.; Hahn, D.; Bae, J.S. Inhibitory effects of compounds isolated from Dioscorea batatas Decne peel on particulate matter-induced pulmonary injury in mice. J. Toxicol. Environ. Health A 2019, 82, 727–740. [Google Scholar] [CrossRef]
- Xu, C.; Shi, Q.; Zhang, L.; Zhao, H. High molecular weight hyaluronan attenuates fine particulate matter-induced acute lung injury through inhibition of ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ. Toxicol. Pharm. 2018, 59, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, Q.; Zhang, X.; Wu, S.; Wang, S.; Chen, R.; Li, X. Role of astrocyte activation in fine particulate matter-enhancement of existing ischemic stroke in Sprague-Dawley male rats. J. Toxicol. Environ. Health A 2016, 79, 393–401. [Google Scholar] [CrossRef]
- Wang, N.; Mengersen, K.; Kimlin, M.; Zhou, M.; Tong, S.; Fang, L.; Wang, B.; Hu, W. Lung cancer and particulate pollution: A critical review of spatial and temporal analysis evidence. Environ. Res. 2018, 164, 585–596. [Google Scholar] [CrossRef]
- Yan, X.D.; Wang, Q.M.; Tie, C.; Jin, H.T.; Han, Y.X.; Zhang, J.L.; Yu, X.M.; Hou, Q.; Zhang, P.P.; Wang, A.P.; et al. Polydatin protects the respiratory system from PM2.5 exposure. Sci. Rep. 2017, 7, 40030. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Fujishima, K.; Yatera, K.; Yamamoto, K. Usefulness of Intratracheal Instillation Studies for Estimating Nanoparticle-Induced Pulmonary Toxicity. Int. J. Mol. Sci. 2016, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.C.; Hsieh, W.Y.; Tsai, C.H.; Chen, C.Y.; Chang, H.F.; Lin, C.S. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Cloonan, S.M.; Haspel, J.A.; Choi, A.M.K. The emerging importance of autophagy in pulmonary diseases. Chest 2012, 142, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Wu, Y.F.; Lou, J.; Mao, Y.Y.; Shen, H.H.; Chen, Z.H. mTOR and autophagy in regulation of acute lung injury: A review and perspective. Microbes Infect. 2014, 16, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef] [PubMed]
- Woodward, N.C.; Levine, M.C.; Haghani, A.; Shirmohammadi, F.; Saffari, A.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflammation 2017, 14, 84. [Google Scholar] [CrossRef]
- Zeng, M.C.; Sang, W.H.; Chen, S.; Chen, R.; Zhang, H.L.; Xue, F.; Li, Z.M.; Liu, Y.; Gong, Y.S.; Zhang, H.Y.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef]
- Shao, X.; Lai, D.; Zhang, L.; Xu, H. Induction of Autophagy and Apoptosis via PI3K/AKT/TOR Pathways by Azadirachtin A in Spodoptera litura Cells. Sci. Rep. 2016, 6, 35482. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, Y.; Huang, Y.; Lu, Q.; Zheng, L.; Hu, D.; Feng, W.K.; Liu, Y.L.; Ji, K.T.; Zhang, H.Y.; et al. bFGF regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the PI3K/Akt/mTOR pathway. Sci. Rep. 2015, 5, 9287. [Google Scholar] [CrossRef]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6, 32. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Bergvall, C.; Westerholm, R. Determination of dibenzopyrenes in standard reference materials (SRM) 1649a, 1650, and 2975 using ultrasonically assisted extraction and LC-GC-MS. Anal. Bioanal. Chem. 2006, 384, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Choo, S.; Sim, H.; Bae, J.S. Inhibitory Activities of Ononin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 208–215. [Google Scholar] [CrossRef]
- Sim, H.; Noh, Y.; Choo, S.; Kim, N.; Lee, T.; Bae, J.S. Suppressive Activities of Fisetin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 568–574. [Google Scholar] [CrossRef]
- Wang, H.; Shen, X.; Tian, G.; Shi, X.; Huang, W.; Wu, Y.; Sun, L.; Peng, C.; Liu, S.; Huang, Y.; et al. AMPKalpha2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radic. Biol. Med. 2018, 121, 202–214. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, S.H.; Kim, N.; Lee, W.; Bae, J.-S. Renal Protective Effects of Sparstolonin B in a Mouse Model of Sepsis. Biotechnol. Bioprocess Eng. 2022, 27, 157–162. [Google Scholar] [CrossRef]
- Lee, I.C.; Bae, J.S. Hepatic Protective Effects of Jujuboside B through the Modulation of Inflammatory Pathways. Biotechnol. Bioprocess Eng. 2022, 27, 336–343. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Kim, G.O.; Bae, J.-S. Cirsilineol Treatment Attenuates PM2.5-Induced Lung Injury in Mice. Int. J. Mol. Sci. 2022, 23, 13948. https://doi.org/10.3390/ijms232213948
Kim C, Kim GO, Bae J-S. Cirsilineol Treatment Attenuates PM2.5-Induced Lung Injury in Mice. International Journal of Molecular Sciences. 2022; 23(22):13948. https://doi.org/10.3390/ijms232213948
Chicago/Turabian StyleKim, Chaeyeong, Go Oun Kim, and Jong-Sup Bae. 2022. "Cirsilineol Treatment Attenuates PM2.5-Induced Lung Injury in Mice" International Journal of Molecular Sciences 23, no. 22: 13948. https://doi.org/10.3390/ijms232213948
APA StyleKim, C., Kim, G. O., & Bae, J.-S. (2022). Cirsilineol Treatment Attenuates PM2.5-Induced Lung Injury in Mice. International Journal of Molecular Sciences, 23(22), 13948. https://doi.org/10.3390/ijms232213948







