Distribution and Sources of Organic Carbon in Surface Intertidal Sediments of the Rudong Coast, Jiangsu Province, China
Abstract
:1. Introduction
2. Materials and Methods
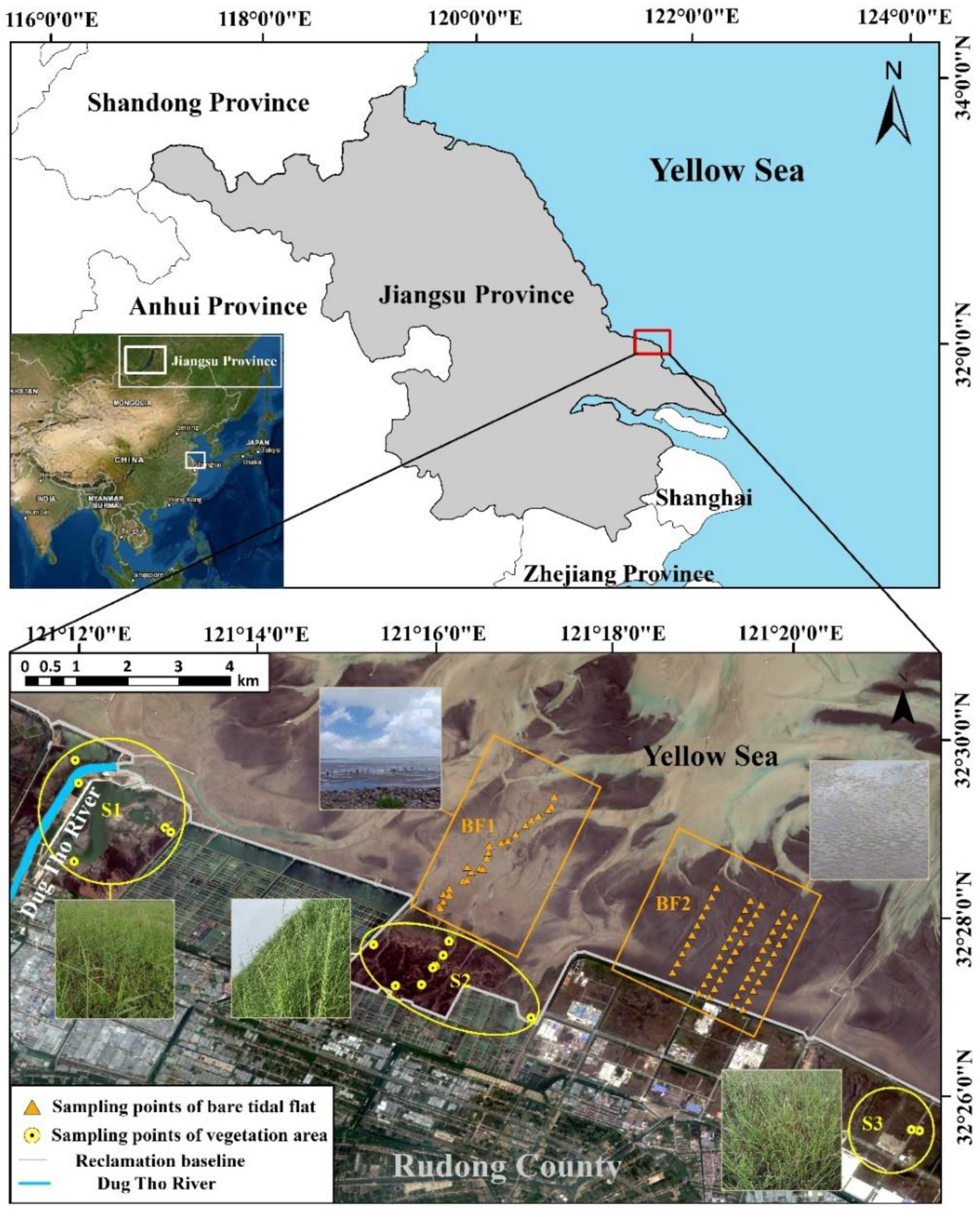
2.1. Study Area
2.2. Sample Collection and Experimental Methods
2.2.1. Sample Collection
2.2.2. Sample Measurement
2.2.3. Organic Carbon Source Analysis
3. Results
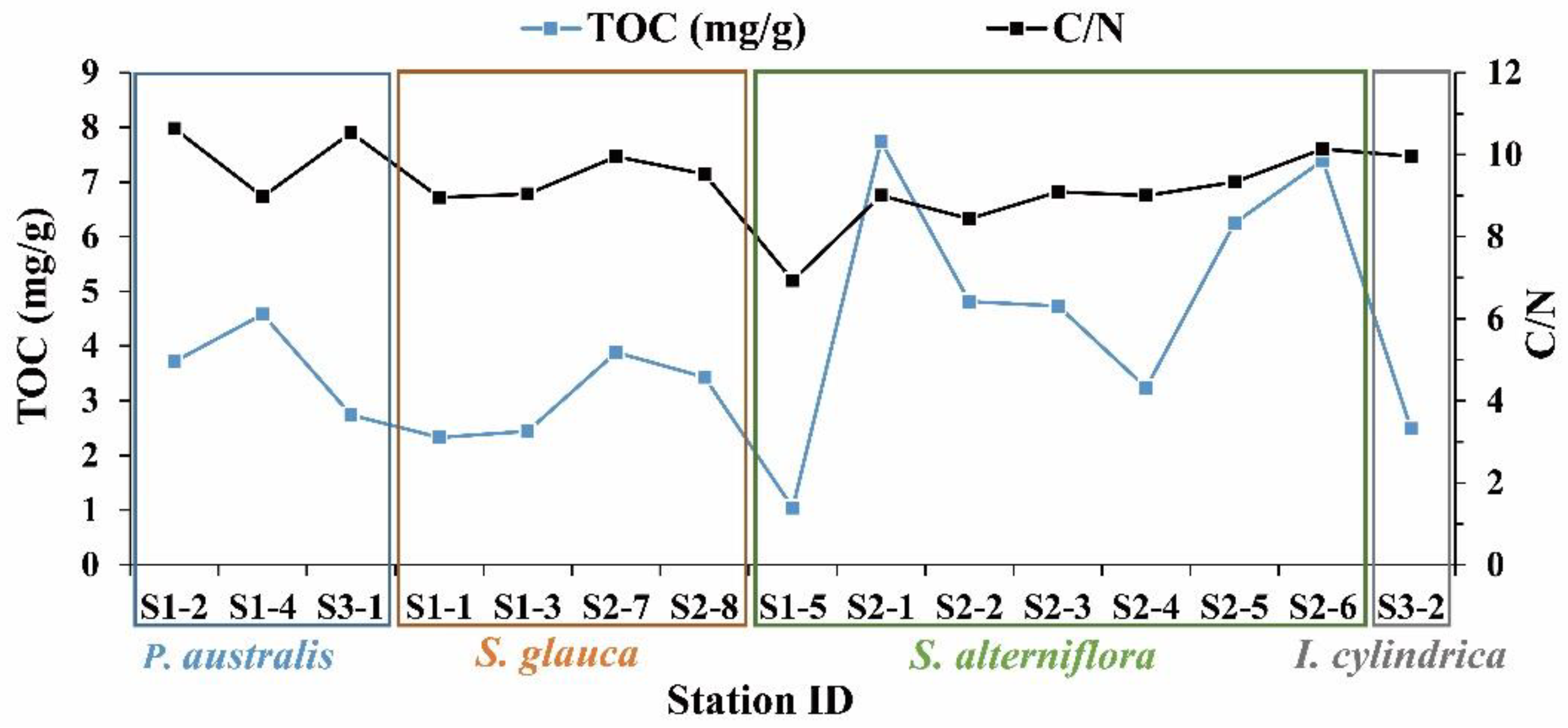
3.1. Distribution of TOC and C/N in Surface Sediments
3.1.1. Vegetated Saltmarsh Areas
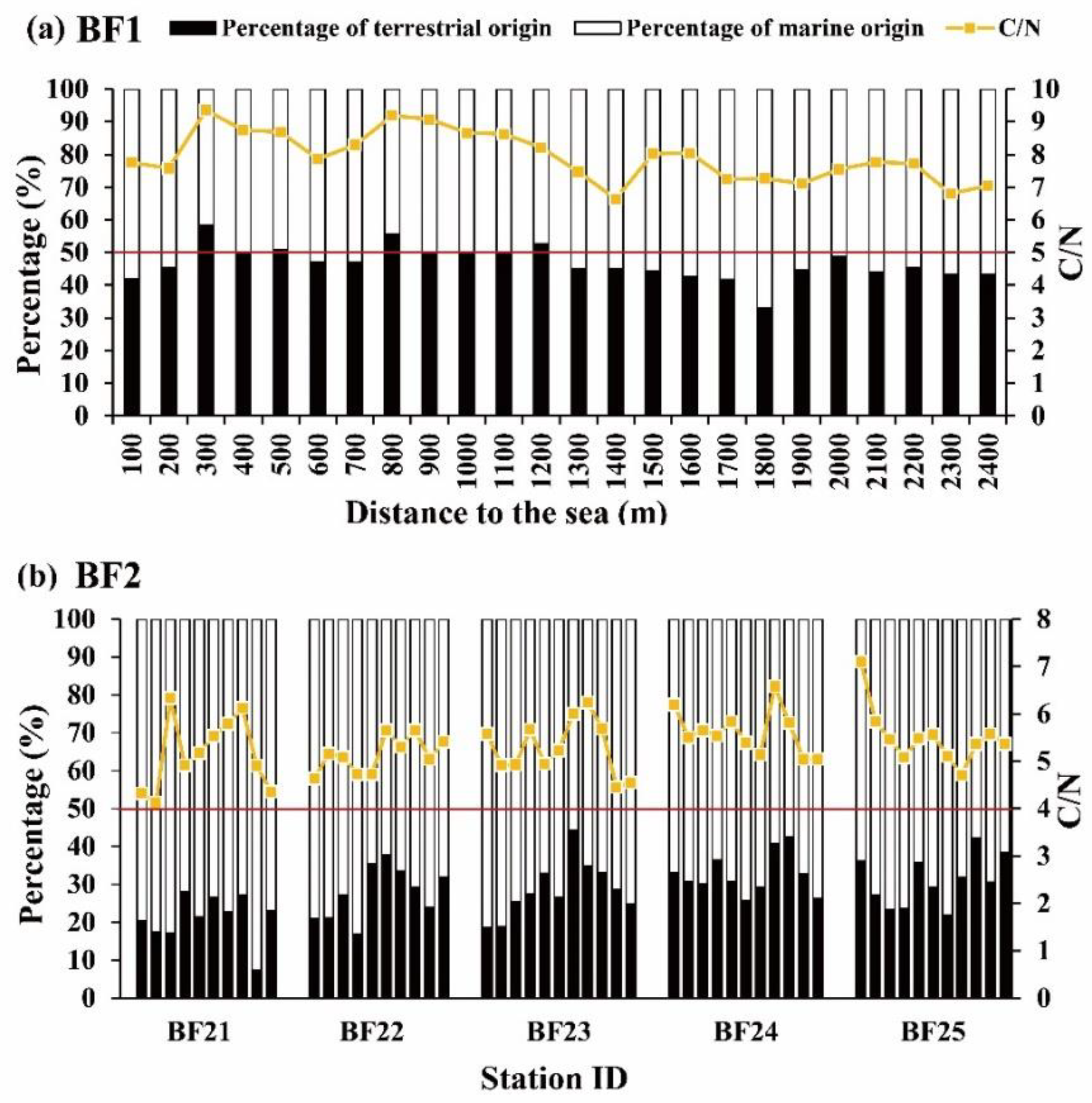
3.1.2. Bare Tidal Flat Sediments
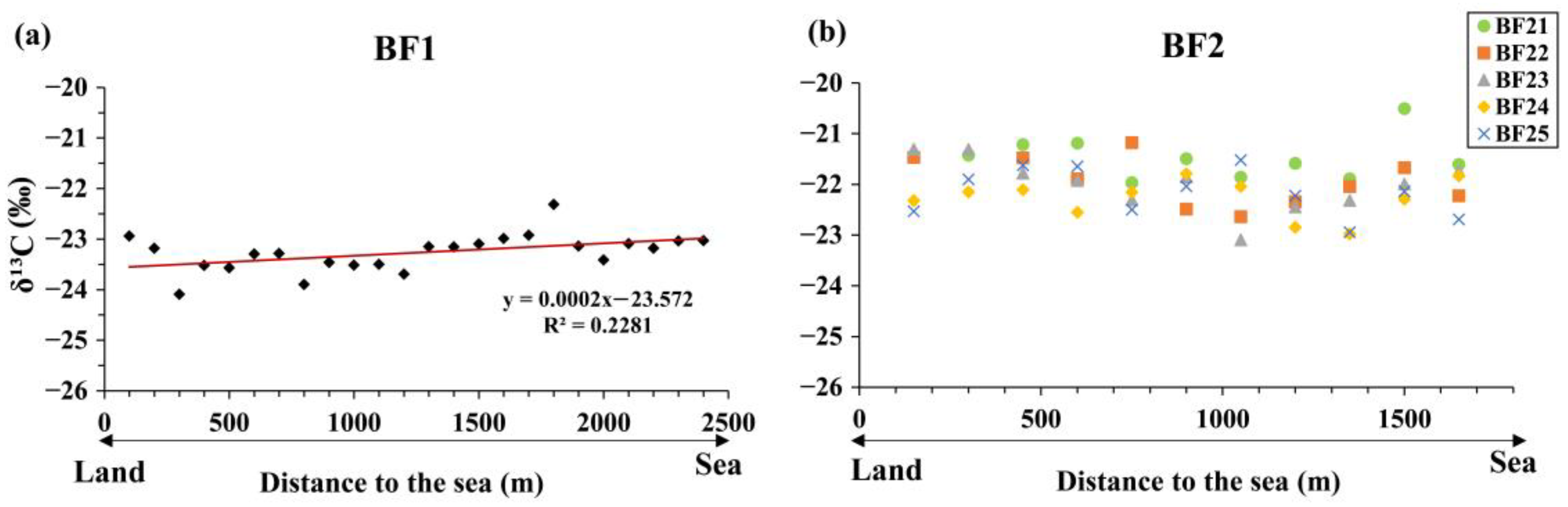
3.2. Stable Carbon Isotopes in Tidal Flats
3.2.1. δ13C of Vegetated Saltmarsh Sediments
3.2.2. δ13C of Bare Tidal Flat Sediments
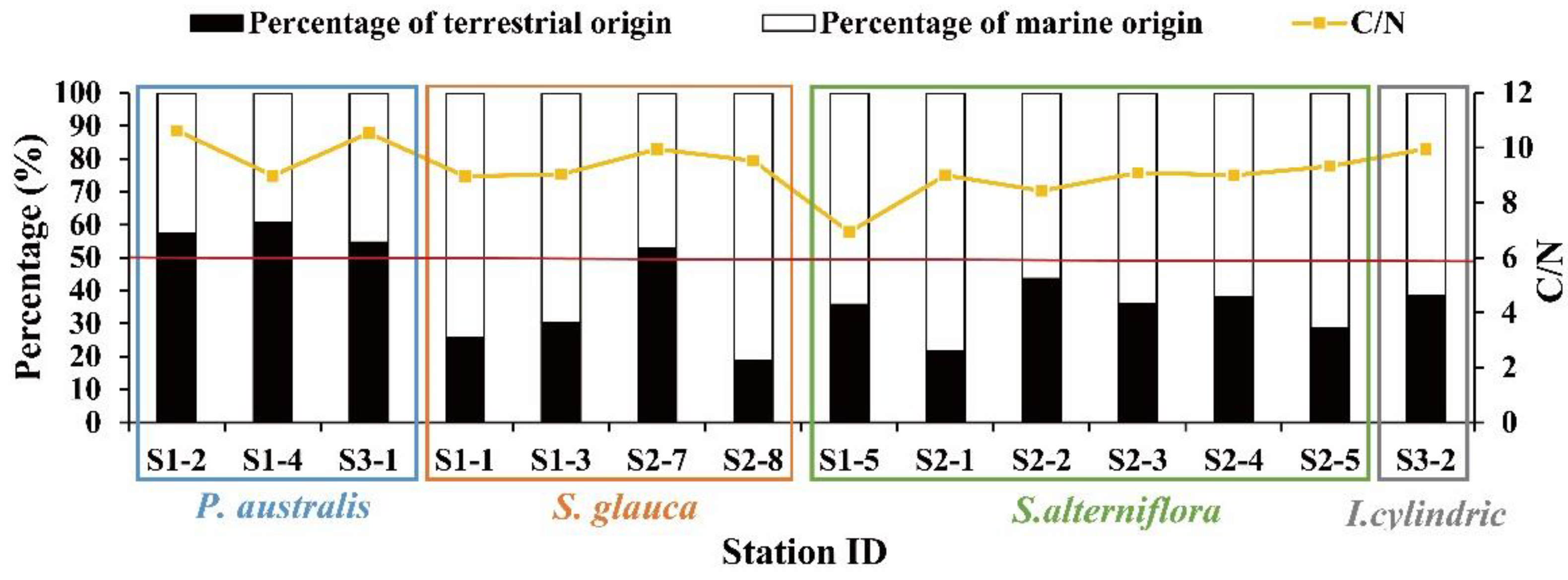
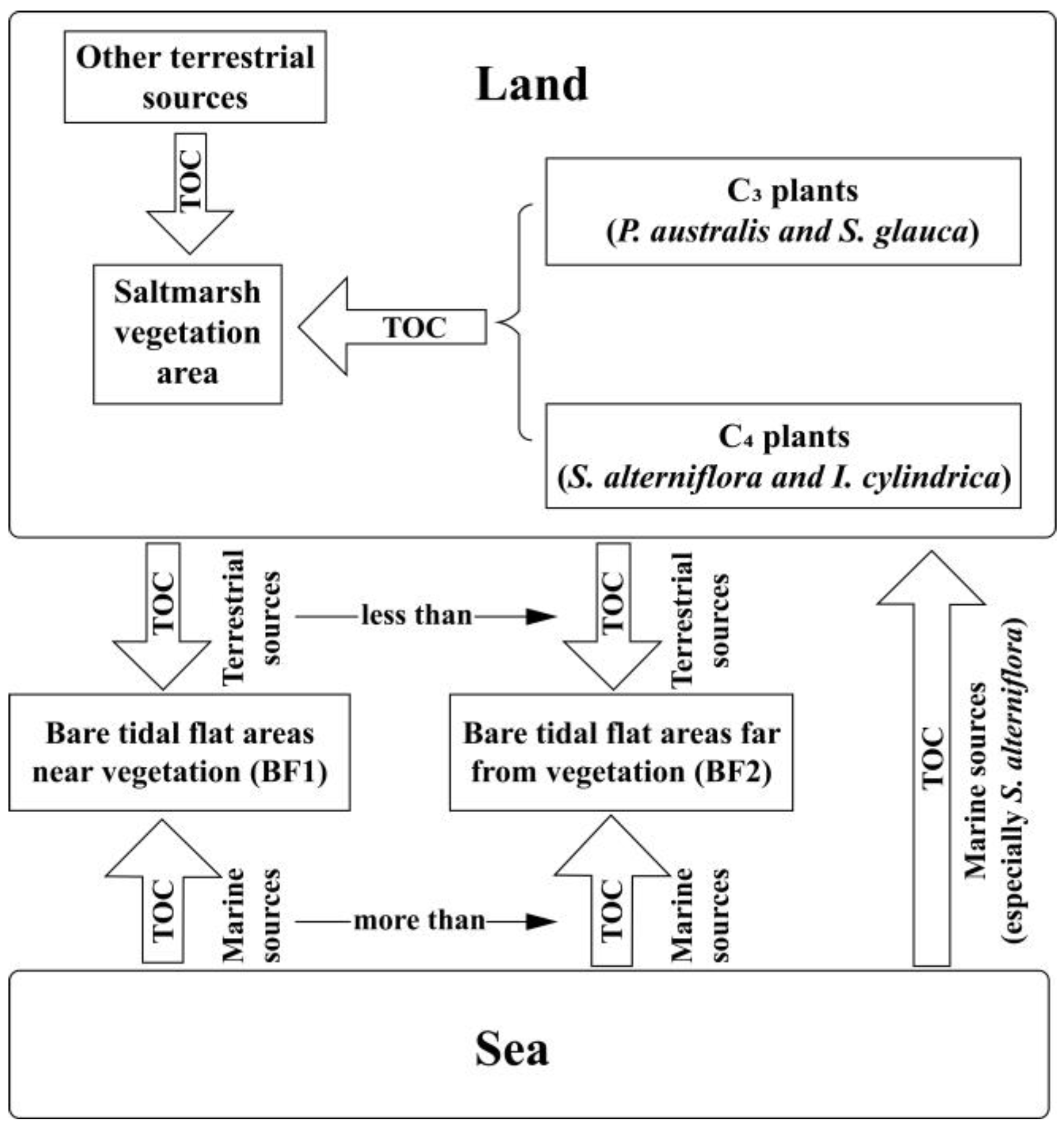
3.3. Organic Carbon Sources
3.3.1. Vegetated Saltmarsh Sediments
3.3.2. Bare Tidal Flat Sediments
3.4. Correlation Analysis Results
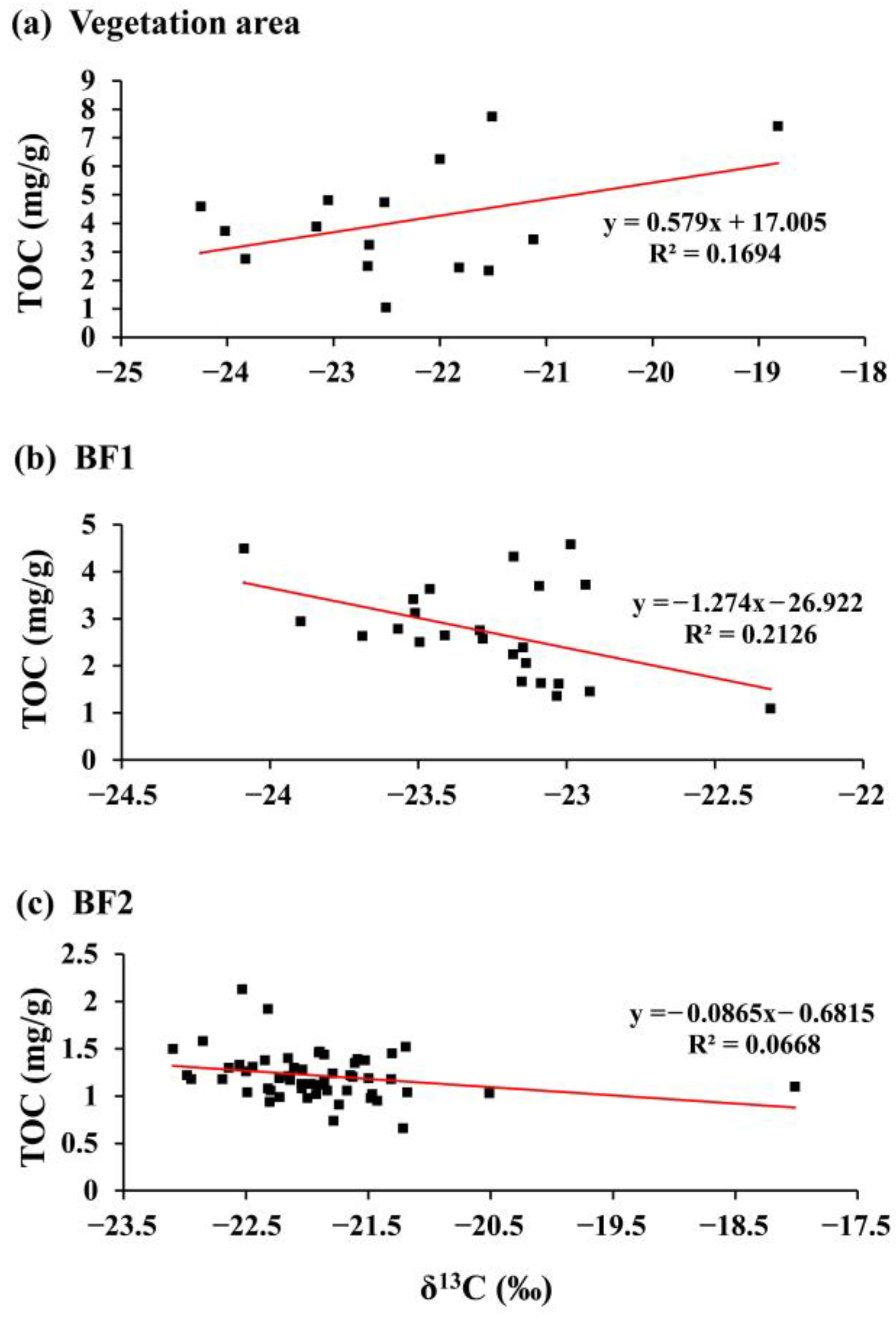
3.4.1. Surface Sediment δ13C Values and TOC
3.4.2. Surface Sediment 𝛿13C Values and C/N Ratios
4. Discussion
4.1. Effect of Coastal Vegetation on Organic Carbon Content
4.2. Surface Sediment 𝛿13C Values in Relation to TOC
4.3. Sources and Influencing Factors of Organic Carbon in Surface Sediments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdes, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy in Binding Carbon: A Rapid Response Assessment; United Nations Environment Programme, GRID-Arenal: Arendal, Norway, 2009. [Google Scholar]
- Howard, J.; Sutton-Grier, A.; Herr, D.; Kleypas, J.; Landis, E.; Mcleod, E.; Pidgeon, E.; Simpson, S. Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 2017, 15, 42–50. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Fennessy, M.S. Carbon storage in US wetlands. Nat. Commun. 2016, 7, 13835. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.C.; Boone Kauffman, J.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Tang, L.; Gao, Y.; Li, B.; Wang, Q.; Wang, C.; Zhao, B. Spartina alterniflora with high tolerance to salt stress changes vegetation pattern by outcompeting native species. Ecosphere 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Allison, S.K. Ecological Restoration and Environmental Change: Renewing Damaged Ecosystems; Routledge: London, UK, 2012. [Google Scholar]
- Li, C.; Tao, Y.; Zhao, M.; Yu, K.; Xu, L.; Fang, S. Soil characteristics and their potential thresholds associated with Scirpus mariqueter distribution on a reclaimed wetland coast. J. Coastal Conserv. 2018, 22, 1107–1116. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, M.; Mora, G.; Graniero, L.E.; Surge, D. Carbon and nitrogen tracers of land use effects on net ecosystem metabolism in mangrove estuaries, southwest Florida. Estuar. Coast. Shelf Sci. 2016, 181, 14–26. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Chen, X.; Yin, S.; Cheng, X.; An, S. Consequences of short-term C4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China. Ecol. Eng. 2013, 61, 50–57. [Google Scholar] [CrossRef]
- Hu, L.; Shi, X.; Bai, Y.; Qiao, S.; Li, L.; Yu, Y.; Yang, G.; Ma, D.; Guo, Z. Recent organic carbon sequestration in the shelf sediments of the Bohai Sea and Yellow Sea, China. J. Mar. Syst. 2016, 155, 50–58. [Google Scholar] [CrossRef]
- Kang, X.; Song, J.; Yuan, H.; Li, X.; Li, N.; Duan, L. The sources and composition of organic matter in sediments of the Jiaozhou Bay: Implications for environmental changes on a centennial time scale. Acta Oceanol. Sin. 2017, 36, 68–78. [Google Scholar] [CrossRef]
- Lo, V.B.; Bouma, T.J.; van Belzen, J.; Van Colen, C.; Airoldi, L. Interactive effects of vegetation and sediment properties on erosion of salt marshes in the Northern Adriatic Sea. Mar. Environ. Res. 2017, 131, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Nie, M.; Li, B.; Wu, J. The transfer and allocation of newly fixed C by invasive Spartina alterniflora and native Phragmites australis to soil microbiota. Soil Biol. Biochem. 2017, 113, 231–239. [Google Scholar] [CrossRef]
- Zhou, C.; An, S.; Deng, Z.; Yin, D.; Zhi, Y.; Sun, Z.; Zhao, H.; Zhou, L.; Fang, C.; Qian, C. Sulfur storage changed by exotic Spartina alterniflora in coastal saltmarshes of China. Ecol. Eng. 2009, 35, 536–543. [Google Scholar] [CrossRef]
- Bromberg Gedan, K.; Silliman, B.R.; Bertness, M.D. Centuries of Human-Driven Change in Salt Marsh Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, D.P.; McBride, C.; Özkundakci, D.; Schallenberg, M.; Verburg, P.; Winton, M.D.; Kelly, D.; Hendy, C.; Ye, W. Effects of climate change on New Zealand Lakes. In Climatic Change and Global Warming of Inland Waters: Impacts and Mitigation for Ecosystems and Societies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 337–366. [Google Scholar]
- Capon, S.J.; Bunn, S.E. Assessing climate change risks and prioritising adaptation options using a water ecosystem services-based approach. Water Ecosyst. Serv. 2015, 17–25. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Herman, P.M.J. Organic matter processing in tidal estuaries. Mar. Chem. 2007, 106, 127–147. [Google Scholar] [CrossRef]
- Hu, L.; Guo, Z.; Feng, J.; Yang, Z.; Fang, M. Distributions and sources of bulk organic matter and aliphatic hydrocarbons in surface sediments of the Bohai Sea, China. Mar. Chem. 2009, 113, 197–211. [Google Scholar] [CrossRef]
- Burdige, D.J. Preservation of Organic Matter in Marine Sediments: Controls, Mechanisms, and an Imbalance in Sediment Organic Carbon Budgets? Chem. Rev. 2007, 107, 467–485. [Google Scholar] [CrossRef]
- Ma, L.; Yang, L.; Xia, L.; Shen, M.; Yin, S.; Li, Y. Long-Term Effects of Inorganic and Organic Amendments on Organic Carbon in a Paddy Soil of the Taihu Lake Region, China. Pedosphere 2011, 21, 186–196. [Google Scholar] [CrossRef]
- Smith, C.B.; Cohen, M.C.L.; Pessenda, L.C.R.; França, M.C.; Guimarães, J.T.F. Holocenic proxies of sedimentary organic matter and the evolution of Lake Arari-Amazon Region. Catena 2012, 90, 26–38. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, K.; Yang, Y.; Zhang, D. Distribution characteristics and sources of sedimentary organic matter in the pearl river estuary and adjacent coastal waters, southern China. J. Earth Sci. 2013, 24, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Minoura, K.; Hoshino, K.; Nakamura, T.; Wada, E. Late Pleistocene-Holocene paleoproductivity circulation in the Japan Sea: Sea-level control on δ13C and δ15N records of sediment organic material. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 135, 41–50. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Sonninen, E.; Korhola, A.; Weckström, K. Identifying recent sources of organic matter enrichment and eutrophication trends at coastal sites using stable nitrogen and carbon isotope ratios in sediment cores. J. Paleolimnol. 2013, 50, 191–206. [Google Scholar] [CrossRef]
- Oczkowski, A.; Markham, E.; Hanson, A.; Wigand, C. Carbon stable isotopes as indicators of coastal eutrophication. Ecol. Appl. 2014, 24, 457–466. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Processes 2001, 15, 1301–1346. [Google Scholar] [CrossRef]
- Rong, Q.; Liu, J.; Cai, Y.; Lu, Z.; Zhao, Z.; Yue, W.; Xia, J. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix chinensis Lour. in the Laizhou Bay coastal wetland, China. Ecol. Eng. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Emeis, K.; Wang, Y.; Richard, P. Distribution and sources of organic matter in surface sediments of Bohai Sea near the Yellow River Estuary, China. Estuar. Coast. Shelf Sci. 2015, 165, 128–136. [Google Scholar] [CrossRef]
- Hedges, J.I.; Keil, R.G.; Benner, R. What happens to terrestrial organic matter in the ocean? Org. Geochem. 1997, 27, 195–212. [Google Scholar] [CrossRef]
- Deegan, L.A.; Garritt, R.H. Evidence for spatial variability in estuarine food webs. Mar. Ecol. Prog. Ser. 1997, 147, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ding, W.; Luo, J.; Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil Biol. Biochem. 2010, 42, 1712–1720. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora Enhanced Ecosystem Carbon and Nitrogen Stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, R.; Xiong, J.; Guo, H.; Zhao, B. Contribution of invasive species Spartina alterniflora to soil organic carbon pool in coastal wetland: Stable isotope approach. Chin. J. Plant Ecol. 2015, 39, 941–949. [Google Scholar]
- Yu, F.; Zong, Y.; Lloyd, J.M.; Huang, G.; Leng, M.; Kendrick, C.; Lamb, A.L.; Yim, W.W.S. Bulk organic δ13C and C/N as indicators for sediment sources in the Pearl River delta and estuary, southern China. Estuar. Coast. Shelf Sci. 2010, 87, 618–630. [Google Scholar] [CrossRef]
- Glaeser, L.C.; Vitt, D.H.; Ebbs, S. Responses of the wetland grass, Beckmannia syzigachne, to salinity and soil wetness: Consequences for wetland reclamation in the oil sands area of Alberta, Canada. Ecol. Eng. 2016, 86, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Gireeshkumar, T.R.; Deepulal, P.M.; Chandramohanakumar, N. Distribution and sources of sedimentary organic matter in a tropical estuary, south west coast of India (Cochin estuary): A baseline study. Mar. Pollut. Bull. 2013, 66, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Kanda, J. Seasonal variations and sources of sedimentary organic carbon in Tokyo Bay. Mar. Pollut. Bull. 2017, 114, 637–643. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Coffin, R.B.; Solorzano, L.; Cardenas, W.; Espinoza, J.; Twilley, R.R. Isotopic and Elemental Variations of Carbon and Nitrogen in a Mangrove Estuary. Estuar. Coast. Shelf Sci. 1996, 43, 781–800. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Batten, G. Stable soil organic matter: A comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 2011, 163, 197–208. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Zhang, Z.; Ren, J.; Cao, J. Seasonal variability of stable carbon and nitrogen isotope of suspended particulate matter in the Changjiang River. Oceanol. Limnol. Sin. 2002, 33, 546–552. [Google Scholar]
- Koziorowska, K.; Kuliński, K.; Pempkowiak, J. Sedimentary organic matter in two Spitsbergen fjords: Terrestrial and marine contributions based on carbon and nitrogen contents and stable isotopes composition. Cont. Shelf Res. 2016, 113, 38–46. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Olsen, C.R. Marine origin of Savannah River estuary sediments: Evidence from radioactive and stable isotope tracers. Estuar. Coast. Shelf Sci. 1992, 34, 95–107. [Google Scholar] [CrossRef]
- Mchenga, I.S.S.; Tsuchiya, M. Nutrient dynamics in mangrove crab burrow sediments subjected to anthropogenic input. J. Sea Res. 2007, 59, 103–113. [Google Scholar] [CrossRef]
- Owen, R.B.; Lee, R. Human impacts on organic matter sedimentation in a proximal shelf setting, Hong Kong. Cont. Shelf Res. 2003, 24, 583–602. [Google Scholar] [CrossRef]
- Gao, X.; Yang, Y.; Wang, C. Geochemistry of organic carbon and nitrogen in surface sediments of coastal Bohai Bay inferred from their ratios and stable isotopic signatures. Mar. Pollut. Bull. 2012, 64, 1148–1155. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | TOC (mg/g) | C/N |
|---|---|---|
| S1-1 | 2.33 | 8.96 |
| S1-2 | 3.72 | 10.63 |
| S1-3 | 2.44 | 9.04 |
| S1-4 | 4.58 | 8.98 |
| S1-5 | 1.04 | 6.93 |
| S2-1 | 7.74 | 9.00 |
| S2-2 | 4.81 | 8.44 |
| S2-3 | 4.73 | 9.10 |
| S2-4 | 3.24 | 9.00 |
| S2-5 | 6.25 | 9.33 |
| S2-6 | 7.4 | 10.14 |
| S2-7 | 3.88 | 9.95 |
| S2-8 | 3.43 | 9.53 |
| S3-1 | 2.74 | 10.54 |
| S3-2 | 2.49 | 9.96 |
| Mean ± SD | 4.05 ± 1.84 | 9.3 ± 0.89 |
| Sample Name | Vegetation Type | δ13C of Surface Sediments (‰) | Mean ± SD (‰) |
|---|---|---|---|
| S1-1 | C3 plants | −21.54 | −22.82 ± 1.2 |
| S1-2 | −24.02 | ||
| S1-3 | −21.82 | ||
| S1-4 | −24.25 | ||
| S2-7 | −23.16 | ||
| S2-8 | −21.12 | ||
| S3-1 | −23.83 | ||
| S1-5 | C4 plants | −22.51 | −21.97 ± 1.27 |
| S2-1 | −21.51 | ||
| S2-2 | −23.05 | ||
| S2-3 | −22.52 | ||
| S2-4 | −22.67 | ||
| S2-5 | −22.00 | ||
| S2-6 | −18.82 | ||
| S3-2 | −22.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Ma, Q.; Lin, J.; Chen, Y.; Shu, Q. Distribution and Sources of Organic Carbon in Surface Intertidal Sediments of the Rudong Coast, Jiangsu Province, China. J. Mar. Sci. Eng. 2021, 9, 992. https://doi.org/10.3390/jmse9090992
Mao Y, Ma Q, Lin J, Chen Y, Shu Q. Distribution and Sources of Organic Carbon in Surface Intertidal Sediments of the Rudong Coast, Jiangsu Province, China. Journal of Marine Science and Engineering. 2021; 9(9):992. https://doi.org/10.3390/jmse9090992
Chicago/Turabian StyleMao, Yinan, Qingyun Ma, Jiaxin Lin, Ye Chen, and Qiang Shu. 2021. "Distribution and Sources of Organic Carbon in Surface Intertidal Sediments of the Rudong Coast, Jiangsu Province, China" Journal of Marine Science and Engineering 9, no. 9: 992. https://doi.org/10.3390/jmse9090992
APA StyleMao, Y., Ma, Q., Lin, J., Chen, Y., & Shu, Q. (2021). Distribution and Sources of Organic Carbon in Surface Intertidal Sediments of the Rudong Coast, Jiangsu Province, China. Journal of Marine Science and Engineering, 9(9), 992. https://doi.org/10.3390/jmse9090992






