Microscopic Anatomy of the Lining of Hemal Spaces in the Penaeid Shrimp, Sicyonia ingentis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Maintenance of Shrimp
2.2. Tissue Collection and Processing
2.3. Measurements
3. Results
3.1. Distribution of Hemal Spaces
3.2. Fibrillin-like Lining of Major Arteries
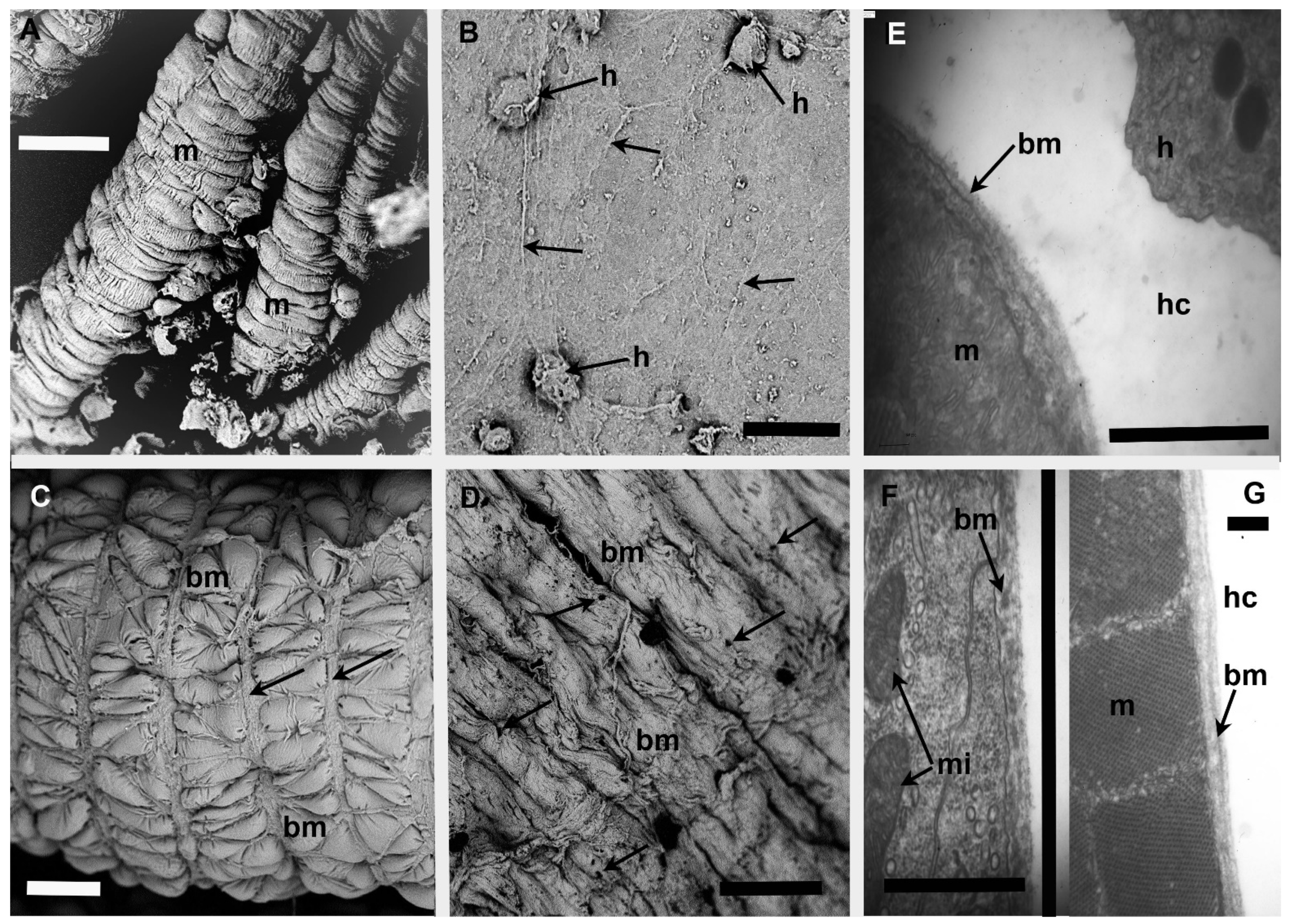
3.3. Tissues Lined by BM
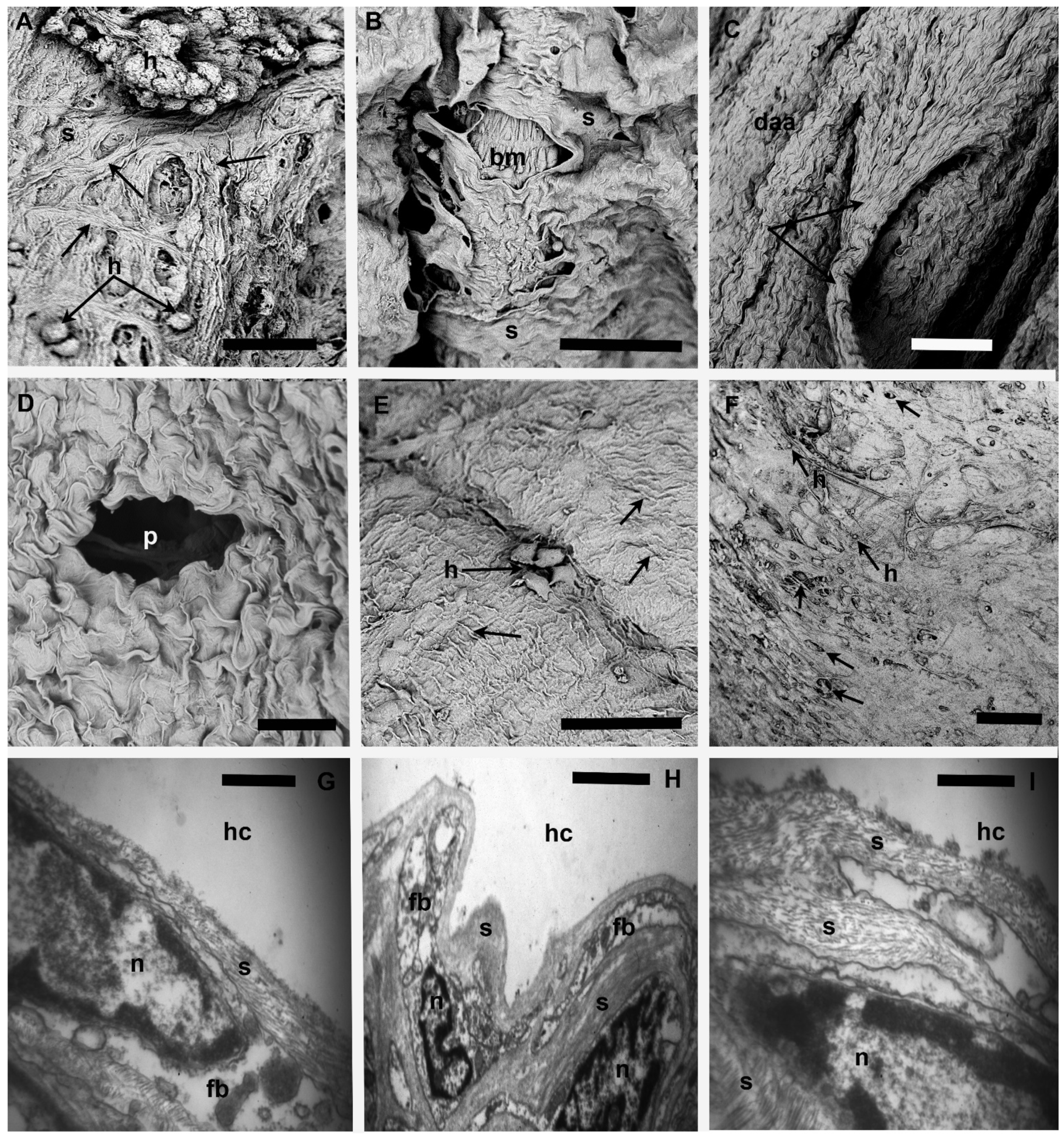
3.4. Tissues Lined by Connective Tissue Sheaths
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McGaw, I.J. The decapod crustacean circulatory system: A case that is neither open nor closed. Microsc. Microanal. 2005, 11, 18–36. [Google Scholar] [CrossRef]
- Reiber, C.L.; McGaw, I.J. A review of the “open” and “closed” circulatory systems: New terminology for complex invertebrate circulatory systems in light of current findings. Int. J. Zool. 2009, 2009, e301284. [Google Scholar] [CrossRef]
- McMahon, B.R. Control of cardiovascular function and its evolution in Crustacea. J. Exp. Biol. 2001, 204, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Salt, G. The Cellular Defense Reactions of Insects; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar] [CrossRef][Green Version]
- Rizki, T.M.; Rizki, R.M. The Cellular Defense System of Drosophila Melanogaster. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Springer: Boston, MA, USA, 1984; Volume 2, pp. 579–604. [Google Scholar] [CrossRef]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems—Not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Fong, K.M.; Holgate, S.T.; Holloway, J.W. The role of Toll-like receptors and related receptors of the innate immune system in asthma. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 23–28. [Google Scholar] [CrossRef]
- Davids, B.J.; Yoshino, T.P. Integrin-like RGD-dependent binding mechanism involved in the spreading response of circulating molluscan phagocytes. Dev. Comp. Immunol. 1998, 22, 39–53. [Google Scholar] [CrossRef]
- Holmblad, T.; Söderhäll, K. Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture 1999, 172, 111–123. [Google Scholar] [CrossRef]
- Plows, L.D.; Cook, R.T.; Davies, A.J.; Walker, A.J. Integrin engagement modulates the phosphorylation of focal adhesion kinase, phagocytosis, and cell spreading in molluscan defense cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 779–786. [Google Scholar] [CrossRef][Green Version]
- Kawasaki, M.; Delamare-Deboutteville, J.; Dang, C.; Barnes, A.C. Hemiuroid trematode sporocysts are undetected by hemocytes of their intermediate host, the ark cockle Anadara trapezia: Potential role of surface carbohydrates in successful parasitism. Fish Shellfish Immunol. 2013, 35, 1937–1947. [Google Scholar] [CrossRef]
- Chen, J.H.; Bayne, C.J. Bivalve mollusc hemocyte behaviors: Characterization of hemocyte aggregation and adhesion and their inhibition in the California Mussel (Mytilus californianus). Biol. Bull. 1995, 188, 255–266. [Google Scholar] [CrossRef]
- Oka, M. Studies on Penaeus orientalis Kishinouye—VIII Structure of the newly found lymphoid organ. Bull. Jpn. Soc. Sci. Fisher 1969, 35, 245–250. [Google Scholar] [CrossRef]
- Van de Braak, C.B.T.; Botterblom, M.H.A.; Liu, W.; Taverne, N.; van der Knaap, W.P.W.; Rombout, J.H.W.M. The role of the haematopoietic tissue in haemocyte production and maturation in the black tiger shrimp (Penaeus monodon). Fish Shellfish Immunol. 2002, 12, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.G.; Hose, J.E.; Kim, J.J. Structure of hematopoietic nodules in the ridgeback prawn, Sicyonia ingentis: Light and electron microscopic observations. J. Morphol. 1987, 192, 204. [Google Scholar] [CrossRef]
- Martin, G.G.; Hose, J.E.; Corzine, C.J. Morphological comparison of major arteries in the ridgeback prawn, Sicyonia ingentis. J. Morphol. 1989, 200, 175–183. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Schittny, J.C. Molecular architecture of basement membranes. FASEB J. 1990, 4, 1577–1590. [Google Scholar] [CrossRef]
- Asem, E.K.; Feng, S.; Stingley-Salazar, S.; Turek, J.; Peter, A.; Robinson, P. Basal lamina of avian ovarian follicle: Influence on morphology of granulosa cells in-vitro. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 125, 189–201. [Google Scholar] [CrossRef]
- LeBleu, V.S.; MacDonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef]
- Pedersen, K.J. Invited Review: Structure and composition of basement membranes and other basal matrix systems in selected invertebrates. Acta Zool. 1991, 72, 181–201. [Google Scholar] [CrossRef]
- Timpl, R.; Dziadek, M. Structure, development, and molecular pathology of basement membranes. Int. Rev. Exp. Pathol. 1986, 29, 1–112. [Google Scholar] [PubMed]
- Timpl, R. Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 1989, 180, 487–502. [Google Scholar] [CrossRef]
- Fidler, A.L.; Darris, C.E.; Chetyrkin, S.V.; Pedchenko, V.K.; Boudko, S.P.; Brown, K.L.; Jerome, W.G.; Hudson, J.K.; Roka, A.; Hudson, B.G. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife 2017, 6, e24176. [Google Scholar] [CrossRef] [PubMed]
- Haraida, S.; Nerlich, A.G.; Wiest, I.; Schleicher, E.; Löhrs, U. Distribution of basement membrane components in normal adipose tissue and in benign and malignant tumors of lipomatous origin. Mod. Pathol. 1996, 9, 137–144. [Google Scholar]
- Sanes, J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003, 278, 12601–12604. [Google Scholar] [CrossRef]
- Bruggink, A.H.; van Oosterhout, M.; de Jonge, N.; Cleutjens, J.P.M.; van Wichen, D.F.; van Kuik, J.; Tilanus, M.G.J.; Gmelig-Meyling, F.H.J.; van den Tweel, J.G.; de Weger, R.A. Type IV collagen degradation in the myocardial basement membrane after unloading of the failing heart by a left ventricular assist device. Lab. Invest. 2007, 87, 1125–1137. [Google Scholar] [CrossRef]
- Yang, H.; Borg, T.K.; Wang, Z.; Ma, Z.; Gao, B.Z. Role of the basement membrane in regulation of cardiac electrical properties. Ann. Biomed. Eng. 2014, 42, 1148–1157. [Google Scholar] [CrossRef]
- Grzelkowska-Kowalczyk, K. The Importance of Extracellular Matrix in Skeletal Muscle Development and Function. In Composition and Function of the Extracellular Matrix in the Human Body; IntechOpen: London, UK, 2016; pp. 3–24. [Google Scholar] [CrossRef]
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.B.; Prifti, E.; Bouillot, J.L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clèment, K. Increased basement membrane components in adipose tissue during obesity: Links with TGFβ and metabolic phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Yamada, K.M. Basement membranes in development and disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef] [PubMed]
- Bussiere, C.T.; Wright, G.M.; DeMont, M.E. The mechanical function and structure of aortic microfibrils in the lobster Homarus americanus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 417–428. [Google Scholar] [CrossRef]
- Davison, I.G.; Wright, G.M.; DeMont, M.E. The structure and physical properties of invertebrate and primitive vertebrate arteries. J. Exp. Biol. 1995, 198, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- McConnell, C.J.; Wright, G.M.; DeMont, M.E. The modulus of elasticity of lobster aorta microfibrils. Experientia 1996, 52, 918–921. [Google Scholar] [CrossRef]
- Piha-Gossack, A.; Sossin, W.; Reinhardt, D.P. The evolution of extracellular fibrillins and their functional domains. PLoS ONE. 2012, 7, e33560. [Google Scholar] [CrossRef]
- Kielty, C.M.; Baldock, C.; Lee, D.; Rock, M.J.; Ashworth, J.L.; Shuttleworth, C.A. Fibrillin: From microfibril assembly to biomechanical function. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 207–217. [Google Scholar] [CrossRef]
- Ramirez, F.; Sakai, L.Y.; Dietz, H.C.; Rifkin, D.B. Fibrillin microfibrils: Multipurpose extracellular networks in organismal physiology. Physiol. Genom. 2004, 19, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.A.; Robertson, I.B.; Handford, P.A. Dissecting the fibrillin microfibril: Structural insights into organization and function. Structure 2012, 20, 215–225. [Google Scholar] [CrossRef]
- Chan, K.S.; Cavey, M.L.; Wilkens, J.L. Microscopic anatomy of the thin-walled vessels leaving the heart of the lobster Homarus americanus: Anterior lateral arteries. Invertebr. Biol. 2006, 125, 70–82. [Google Scholar] [CrossRef]
- Factor, J.R.; Naar, M. The digestive system of the lobster, Homarus americanus: I. Connective tissue of the digestive gland. J. Morphol. 1985, 184, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.P.; Liu, Y.M. The connective tissue sheath of the nerve as effective diffusion barrier. J. Cell. Comp. Physiol. 1949, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Krnjević, K. The connective tissue of the frog sciatic nerve. Q. J. Exp. Physiol. Cogn. Med. Sci. Transl. Integr. 1954, 39, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Twarog, B.M.; Roeder, K.D. Properties of the connective tissue sheath of the cockroach abdominal nerve cord. Biol. Bull. 1956, 111, 278–286. [Google Scholar] [CrossRef]
- Ashhurst, D.E.; Chapman, J.A. The tissue sheath of the nervous system of Locusta migratoria: An electron microscope study. J. Cell Sci. 1961, 3, 463–467. [Google Scholar] [CrossRef]
- Anderson, S.L. Multiple spawning and molt synchrony in a free spawning shrimp (Sicyonia ingentis: Penaeoidea). Biol. Bull. 1985, 168, 377–394. [Google Scholar] [CrossRef]
- Humason, G.L. Animal Tissue Techniques, 4th ed.; WH Freeman and Co.: San Francisco, CA, USA, 1979; p. 641. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Talbot, P. The ovary of the lobster, Homarus americanus. I. Architecture of the mature ovary. J. Ultrastruct. Res. 1981, 76, 235–248. [Google Scholar] [CrossRef]
- Odselius, R.; Elofsson, R. The basement membrane of the insect and crustacean compound eye: Definition, fine structure, and comparative morphology. Cell Tissue Res. 1981, 216, 205–214. [Google Scholar] [CrossRef]
- Nakao, T. The fine structure and innervation of gill lamellae in Anodonta. Cell Tissue Res. 1975, 157, 239–254. [Google Scholar] [CrossRef]
- Miner, J.H. Glomerular filtration: The charge debate charges ahead. Kidney Int. 2008, 74, 259–261. [Google Scholar] [CrossRef]
- Ashhurst, D.E. Integument, Respiration, and Circulation. In Comprehensive Insect Physiology Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 3, pp. 249–287. [Google Scholar]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chem. 2007, 103, 1199–1207. [Google Scholar] [CrossRef]
- Yoshinaka, R.; Mizuta, S.; Itoh, Y.; Sato, M. Two genetically distinct types of collagen in kuruma prawn Penaeus japonicus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 451–456. [Google Scholar] [CrossRef]
- Sivakumar, P.; Suguna, L.; Chandrakasan, G. Molecular species of collagen in the intramuscular connective tissues of the marine crab, Scylla serrata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 555–562. [Google Scholar] [CrossRef]
- Mirre, C.; Le Parco, Y.; Knibiehler, B. Collagen IV is present in the developing CNS during Drosophila neurogenesis. J. Neurosci. Res. 1992, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the ‘omics’ era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Perdomo-Morales, R.; Montero-Alejo, V.; Perera, E. The clotting system in decapod crustaceans: History, current knowledge and what we need to know beyond the models. Fish Shellfish Immunol. 2019, 84, 204–212. [Google Scholar] [CrossRef]
- Zucker, M.B.; Borrelli, J. Platelet clumping produced by connective tissue suspensions and by collagen. Proc. Soc. Exp. Biol. Med. 1962, 109, 779–787. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; Anderson, K.R.; Topper, J.N. The critical role of mechanical forces in blood vessel development, physiology and pathology. J. Vasc. Surg. 1999, 29, 1104–1151. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. Digestion and Nutrition. In The Principles of Insect Physiology; Springer: Dordrecht, The Netherlands, 1972; pp. 476–552. [Google Scholar] [CrossRef]
- Ball, E.E.; de Couet, H.G.; Horn, P.L.; Quinn, J.M.A. Haemocytes secrete basement membrane components in embryonic locusts. Development 1987, 99, 255–259. [Google Scholar] [CrossRef]
- Knibiehler, B.; Mirre, C.; Cecchini, J.P.; Le Parco, Y. Haemocytes accumulate collagen transcripts during Drosophila melanogaster metamorphosis. Roux’s Arch. Dev. Biol. 1987, 196, 243–247. [Google Scholar] [CrossRef]
- Uhrík, B.; Rýdlová, K.; Zacharová, D. The roles of haemocytes during degeneration and regeneration of crayfish muscle fibres. Cell Tissue Res. 1989, 255, 443–449. [Google Scholar] [CrossRef]
- Adachi, T.; Tomita, M.; Yoshizato, K. Synthesis of prolyl 4-hydroxylase alpha subunit and type IV collagen in hemocytic granular cells of silkworm, Bombyx mori: Involvement of type IV collagen in self-defense reaction and metamorphosis. Matrix Biol. 2005, 24, 136–154. [Google Scholar] [CrossRef]
- Franklin, B.M.; Maroudas, E.; Osborn, J.L. Sine-wave electrical stimulation initiates a voltage-gated potassium channel-dependent soft tissue response characterized by induction of hemocyte recruitment and collagen deposition. Physiol. Rep. 2016, 4, e12832. [Google Scholar] [CrossRef]
- Muñoz-Chápuli, R.; Carmona, R.; Guadix, J.A.; Macías, D.; Pérez-Pomares, J.M. The origin of the endothelial cells: An evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evol. Dev. 2005, 7, 351–358. [Google Scholar] [CrossRef]
- Hartenstein, V.; Mandal, L. The blood/vascular system in a phylogenetic perspective. BioEssays 2006, 28, 1203–1210. [Google Scholar] [CrossRef]
- Söderhäll, K.; Cerenius, L. Crustacean immunity. Annu. Rev. Fish Dis. 1992, 2, 3–23. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidebottom, R.B.; Bang, S.; Martin, G. Microscopic Anatomy of the Lining of Hemal Spaces in the Penaeid Shrimp, Sicyonia ingentis. J. Mar. Sci. Eng. 2021, 9, 862. https://doi.org/10.3390/jmse9080862
Sidebottom RB, Bang S, Martin G. Microscopic Anatomy of the Lining of Hemal Spaces in the Penaeid Shrimp, Sicyonia ingentis. Journal of Marine Science and Engineering. 2021; 9(8):862. https://doi.org/10.3390/jmse9080862
Chicago/Turabian StyleSidebottom, Rachel Brittany, Sabi Bang, and Gary Martin. 2021. "Microscopic Anatomy of the Lining of Hemal Spaces in the Penaeid Shrimp, Sicyonia ingentis" Journal of Marine Science and Engineering 9, no. 8: 862. https://doi.org/10.3390/jmse9080862
APA StyleSidebottom, R. B., Bang, S., & Martin, G. (2021). Microscopic Anatomy of the Lining of Hemal Spaces in the Penaeid Shrimp, Sicyonia ingentis. Journal of Marine Science and Engineering, 9(8), 862. https://doi.org/10.3390/jmse9080862






