The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling
Abstract
1. Introduction
2. Materials and Methods
2.1. UVC and Color
2.2. UVC and Reflectance
2.3. UVC and Exposure Intervals
2.4. Immersion
2.5. Statistical Analysis
3. Results
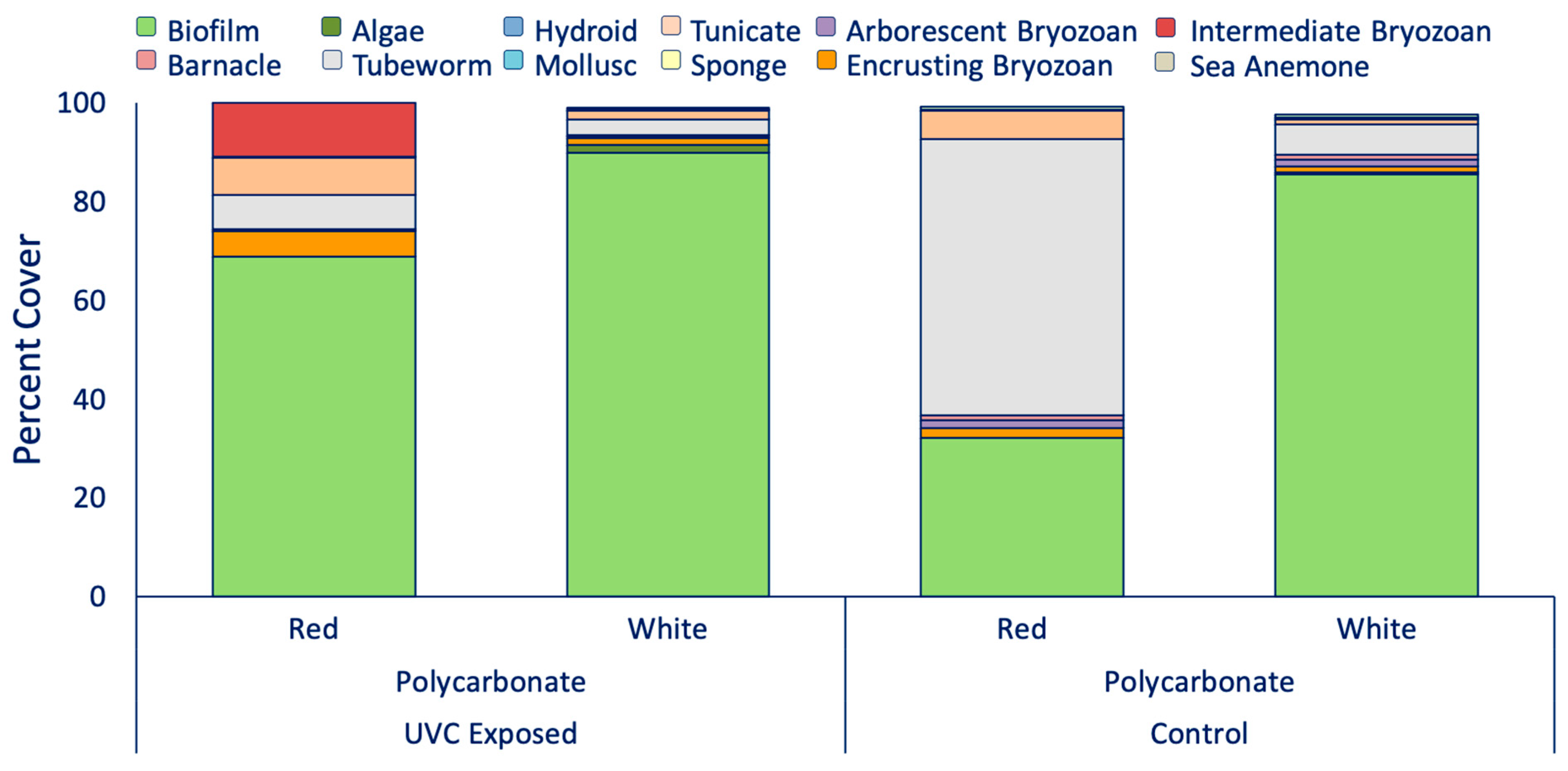
3.1. UVC and Color
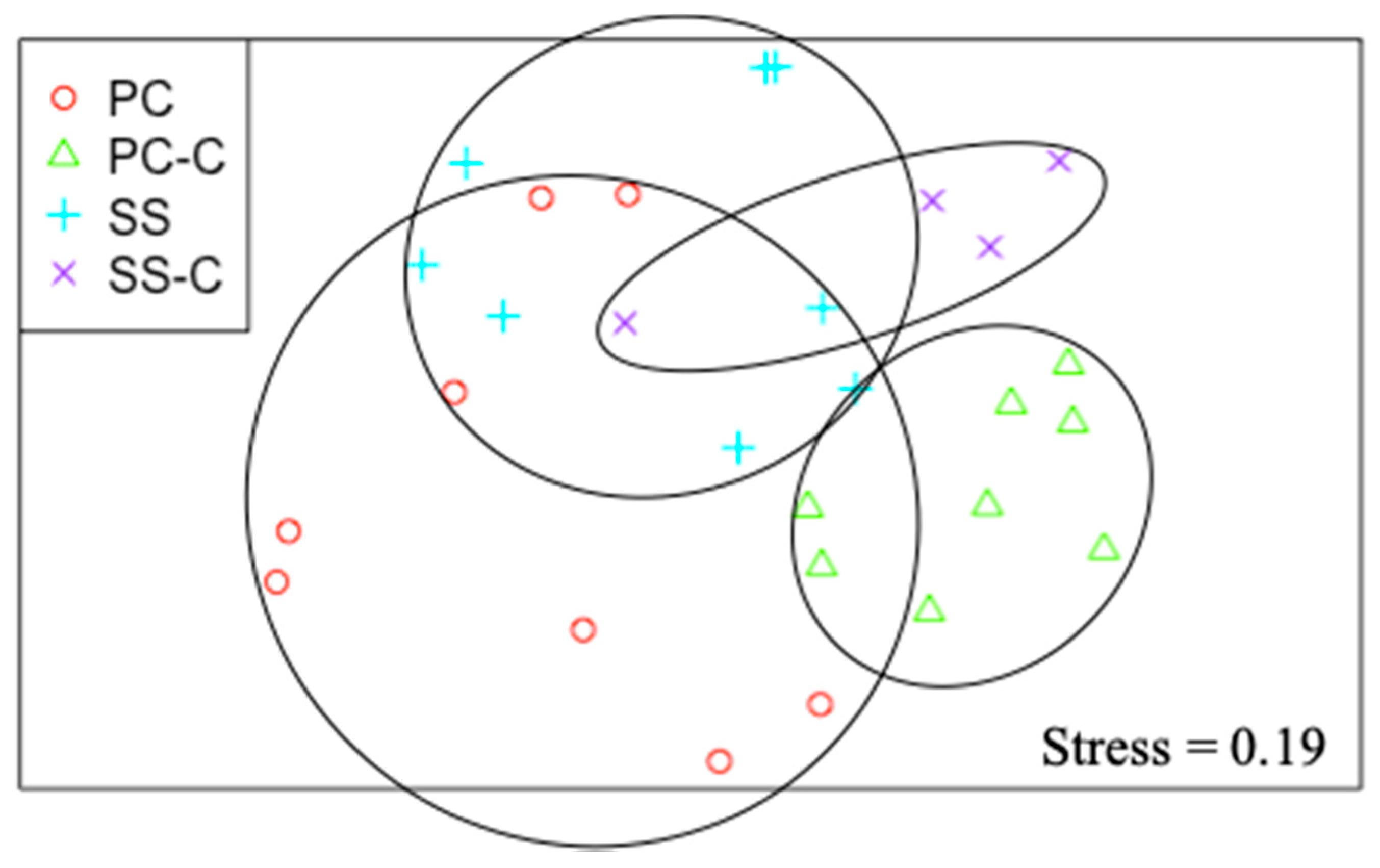
3.2. UVC and Reflectance
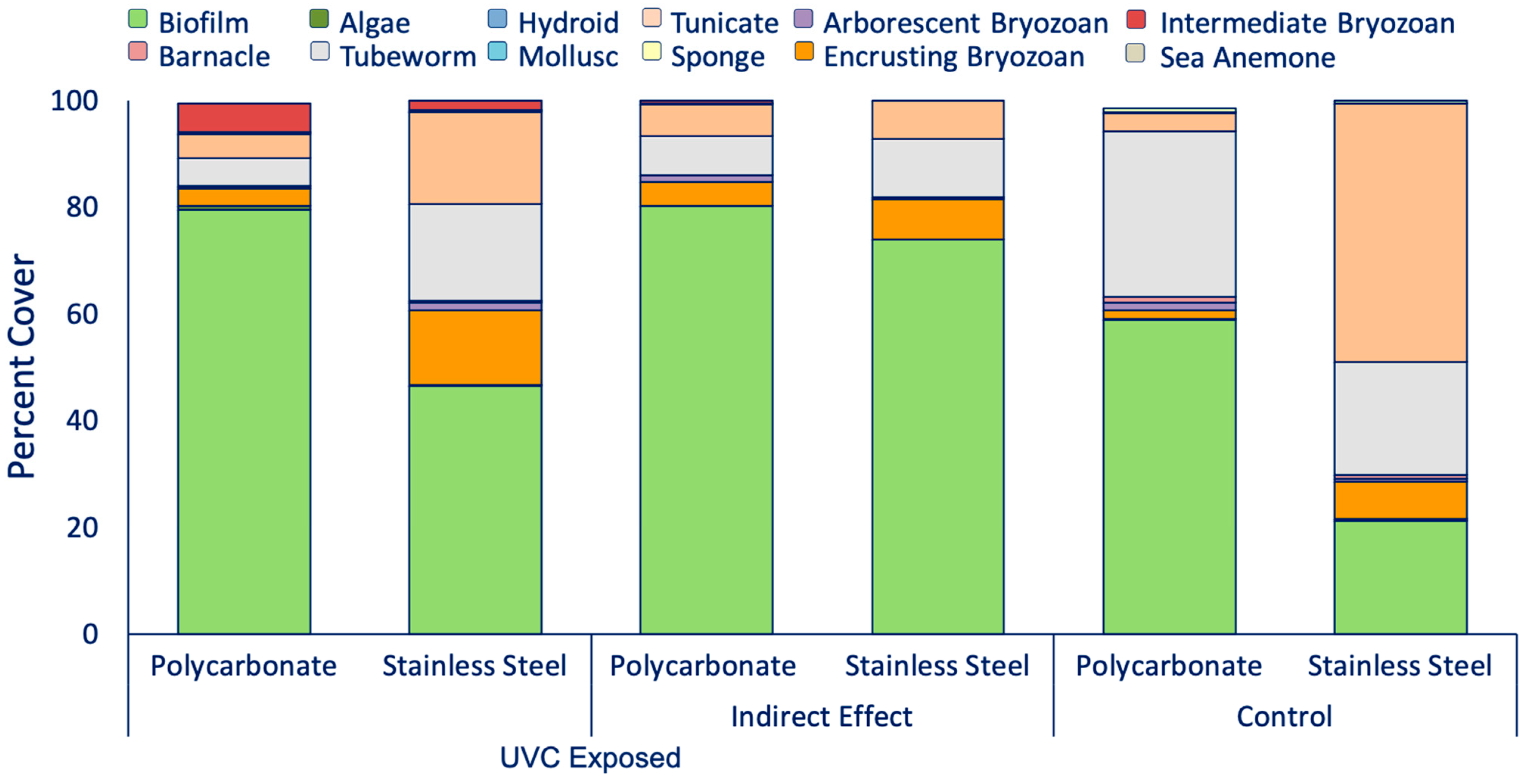
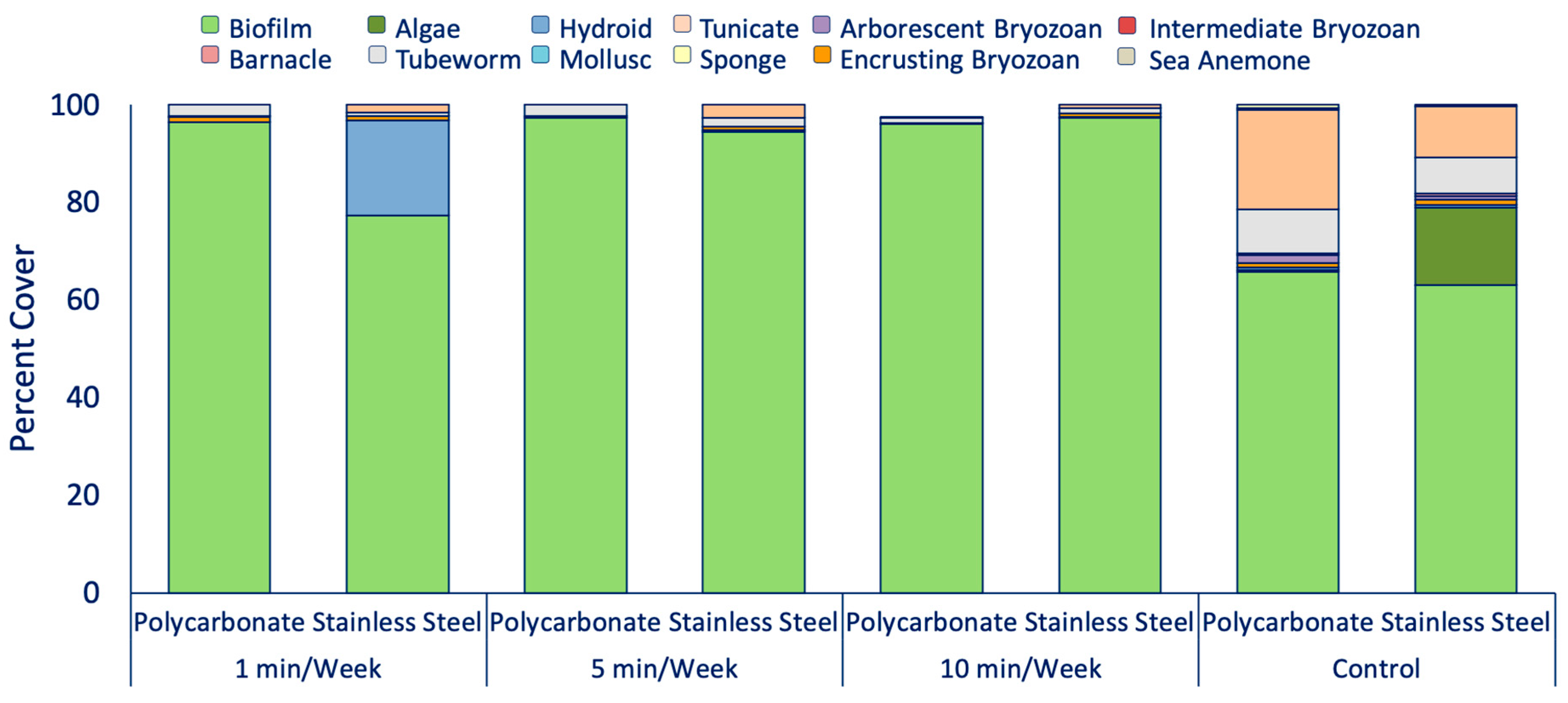
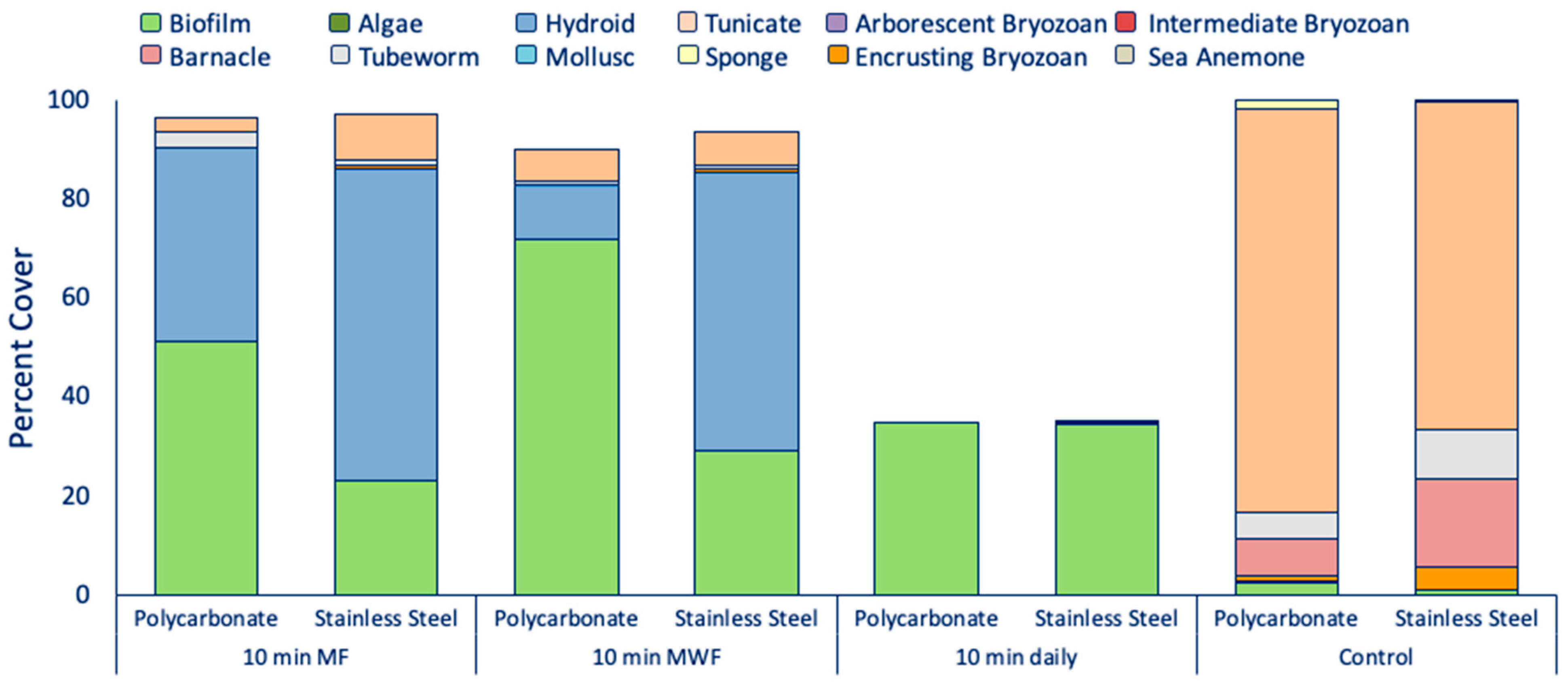
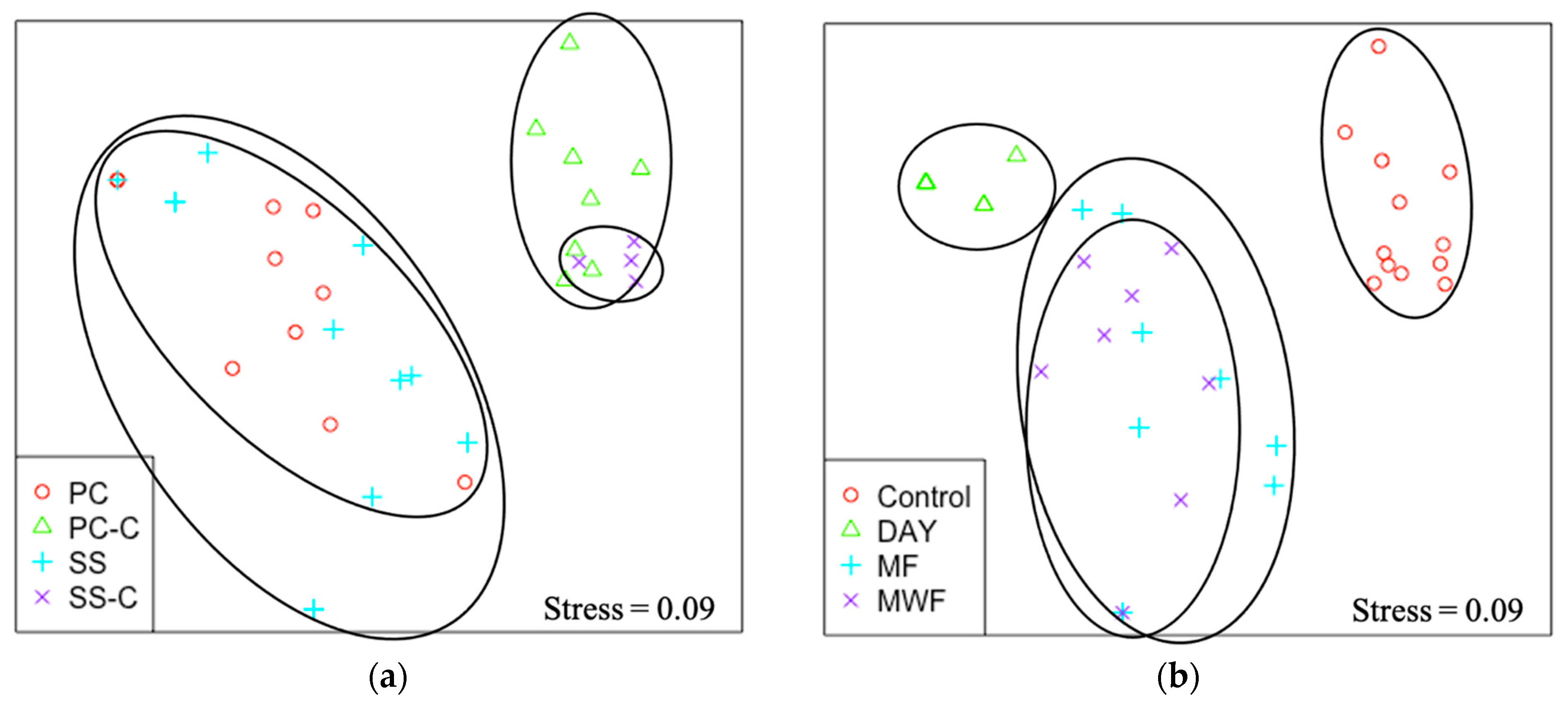
3.3. Determination of UVC Exposure Intervals
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callow, M.E.; Callow, J.E. Marine Biofouling: A Sticky Problem. Biologist (London) 2002, 49, 10–14. [Google Scholar]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine Invasive Alien Species: A Threat to Global Biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Coutts, A.D.M.; Taylor, M.D. A Preliminary Investigation of Biosecurity Risks Associated with Biofouling on Merchant Vessels in New Zealand. N. Z. J. Mar. Freshw. Res. 2004, 38, 215–229. [Google Scholar] [CrossRef]
- Campbell, M.L.; Hewitt, C.L. Assessing the Port to Port Risk of Vessel Movements Vectoring Non-Indigenous Marine Species within and across Domestic Australian Borders. Biofouling 2011, 27, 631–644. [Google Scholar] [CrossRef]
- Swain, G. Redefining antifouling coatings. J. Prot. Coat. Linings 1999, 16, 26–35. [Google Scholar]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine Paints: The Particular Case of Antifouling Paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Srinivasan, M.; Swain, G.W. Managing the Use of Copper-Based Antifouling Paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Swain, G.W.; Kovach, B.; Touzot, A.; Casse, F.; Kavanagh, C.J. Measuring the Performance of Today’s Antifouling Coatings. J. Ship Prod. 2007, 23, 164–170. [Google Scholar] [CrossRef]
- Tribou, M.; Swain, G. The Use of Proactive In-Water Grooming to Improve the Performance of Ship Hull Antifouling Coatings. Biofouling 2010, 26, 47–56. [Google Scholar] [CrossRef]
- Swain, G.; Melissa, T.; Harrison, G.; Kelli, H. In-Water Grooming of Fouling Control Coatings: From Research to Reality; PortPIC: Hamburg, Germany, 2020; pp. 29–37. [Google Scholar]
- Tribou, M.; Swain, G. The Effects of Grooming on a Copper Ablative Coating: A Six Year Study. Biofouling 2017, 33, 494–504. [Google Scholar] [CrossRef]
- Hearin, J.; Hunsucker, K.Z.; Swain, G.; Stephens, A.; Gardner, H.; Lieberman, K.; Harper, M. Analysis of Long-Term Mechanical Grooming on Large-Scale Test Panels Coated with an Antifouling and a Fouling-Release Coating. Biofouling 2015, 31, 625–638. [Google Scholar] [CrossRef]
- Woods, C.M.C.; Floerl, O.; Jones, L. Biosecurity Risks Associated with In-Water and Shore-Based Marine Vessel Hull Cleaning Operations. Mar. Pollut. Bull. 2012, 64, 1392–1401. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Kolch, A.; Gebel, J.; Oguma, K.; Sommer, R. The Photochemical Basis of UV Disinfection. In Proceedings of the European Conference on UV Radiation-Effects and Technologies, Karlsruhe, Germany, 22–24 September 2004; pp. 22–24. [Google Scholar]
- Bak, J.; Ladefoged, S.D.; Tvede, M.; Begovic, T.; Gregersen, A. Dose Requirements for UVC Disinfection of Catheter Biofilms. Biofouling 2009, 25, 289–296. [Google Scholar] [CrossRef]
- Salters, B.; Piola, R. UVC Light for Antifouling. Mar. Technol. Soc. J. 2017, 51, 59–70. [Google Scholar] [CrossRef]
- Gleason, D.F.; Edmunds, P.J.; Gates, R.D. Ultraviolet Radiation Effects on the Behavior and Recruitment of Larvae from the Reef Coral Porites Astreoides. Mar. Biol. 2006, 148, 503–512. [Google Scholar] [CrossRef]
- Hung, O.S.; Gosselin, L.A.; Thiyagarajan, V.; Wu, R.S.S.; Qian, P.Y. Do Effects of Ultraviolet Radiation on Microbial Films Have Indirect Effects on Larval Attachment of the Barnacle Balanus Amphitrite? J. Exp. Mar. Bio. Ecol. 2005, 323, 16–26. [Google Scholar] [CrossRef]
- Hung, O.S.; Thiyagarajan, V.; Wu, R.S.S.; Qian, P.Y. Effect of Ultraviolet Radiation on Biofilms and Subsequent Larval Settlement of Hydroides Elegans. Mar. Ecol. Prog. Ser. 2005, 304, 155–166. [Google Scholar] [CrossRef]
- Chiang, W.L.; Au, D.W.T.; Yu, P.K.N.; Wu, R.S.S. UV-B Damages Eyes of Barnacle Larvae and Impairs Their Photoresponses and Settlement Success. Environ. Sci. Technol. 2003, 37, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Hunsucker, K.Z.; Braga, C.; Gardner, H.; Jongerius, M.; Hietbrink, R.; Salters, B.; Swain, G. Using Ultraviolet Light for Improved Antifouling Performance on Ship Hull Coatings. Biofouling 2019, 35, 658–668. [Google Scholar] [CrossRef]
- Braga, C.; Hunsucker, K.; Gardner, H.; Swain, G. A Novel Design to Investigate the Impacts of UV Exposure on Marine Biofouling. Appl. Ocean Res. 2020, 101, 102226. [Google Scholar] [CrossRef]
- Swain, G.; Herpe, S.; Ralston, E.; Tribou, M. Short-Term Testing of Antifouling Surfaces: The Importance of Colour. Biofouling 2006, 22, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.; Beard, M.; Miller, M.W. Coral Larvae Settle at a Higher Frequency on Red Surfaces. Coral Reefs 2011, 30, 667–676. [Google Scholar] [CrossRef]
- Matsumura, K.; Qian, P.-Y. Larval Vision Contributes to Gregarious Settlement in Barnacles: Adult Red Fluorescence as a Possible Visual Signal. J. Exp. Biol. 2014, 217, 743–750. [Google Scholar] [CrossRef]
- Satheesh, S.; Wesley, S.G. Influence of Substratum Colour on the Recruitment of Macrofouling Communities. J. Mar. Biol. Assoc. U. K. 2010, 90, 941–946. [Google Scholar] [CrossRef]
- Taki, Y.; Ogasawara, Y.; Ido, Y.; Yokoyama, N. Color Factors Influencing Larval Settlement of Barnacles, Balanus Amphitrite Subspp. Nippon Suisan Gakkaishi 1980, 46, 133–138. [Google Scholar] [CrossRef][Green Version]
- Robson, M.A.; Williams, D.; Wolff, K.; Thomason, J.C. The Effect of Surface Colour on the Adhesion Strength of Elminius Modestus Darwin on a Commercial Non-Biocidal Antifouling Coating at Two Locations in the UK. Biofouling 2009, 25, 215–227. [Google Scholar] [CrossRef]
- Zwinkels, J.C.; Noël, M.; Dodd, C.X. Procedures and Standards for Accurate Spectrophotometric Measurements of Specular Reflectance. Appl. Opt. 1994, 33, 7933–7944. [Google Scholar] [CrossRef]
- Citek, K. Anti-Reflective Coatings Reflect Ultraviolet Radiation. Optometry 2008, 79, 143–148. [Google Scholar] [CrossRef]
- ASTM International. A480/A480M-20a Standard Specification for General Requirements for Flat-Rolled Stainless and Heat-Resisting Steel Plate, Sheet, and Strip; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM International. 6990-05 Standard Practice for Evaluating Biofouling Resistance and Physical Performance of Marine Coating Systems; Paint-Tests for Formulated Products and Applied Coatings; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Kim, D.-K.; Kang, D.-H. Effect of Surface Characteristics on the Bactericidal Efficacy of UVC LEDs. Food Control. 2020, 108, 106869. [Google Scholar] [CrossRef]
- Perepelizin, P.V.; Boltovskoy, D. Effects of 254 Nm UV Irradiation on the Mobility and Survival of Larvae of the Invasive Fouling Mussel Limnoperna Fortunei. Biofouling 2014, 30, 197–202. [Google Scholar] [CrossRef]
- Van Dijk, J.; de Louw, M.D.E.; Kalis, L.P.A.; Morgan, E.R. Ultraviolet Light Increases Mortality of Nematode Larvae and Can Explain Patterns of Larval Availability at Pasture. Int. J. Parasitol. 2009, 39, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Tjandraatmadja, G.F.; Burn, L.S.; Jollands, M.J. The effects of ultraviolet radiation on polycarbonate grazing. In Durability of Building Materials and Components 8; Lacasseand, M.A., Vanier, D.J., Eds.; National Research Council, Institute for Research in Construction: Ottawa, ON, Canada, 1999; pp. 884–898. [Google Scholar]
- Ryan, E.; Turkmen, S.; Benson, S. An Investigation into the Application and Practical Use of (UV) Ultraviolet Light Technology for Marine Antifouling. Ocean Eng. 2020, 216, 107690. [Google Scholar] [CrossRef]
- Braga, C.; Hunsucker, K.; Erdogan, C.; Gardner, H.; Swain, G. The Use of a UVC Lamp Incorporated with an ROV to Prevent Biofouling: A Proof-of-Concept Study. Mar. Technol. Soc. J. 2020, 54, 76–83. [Google Scholar] [CrossRef]
- Korkut, E.; Atlar, M. An Experimental Investigation of the Effect of Foul Release Coating Application on Performance, Noise and Cavitation Characteristics of Marine Propellers. Ocean Eng. 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
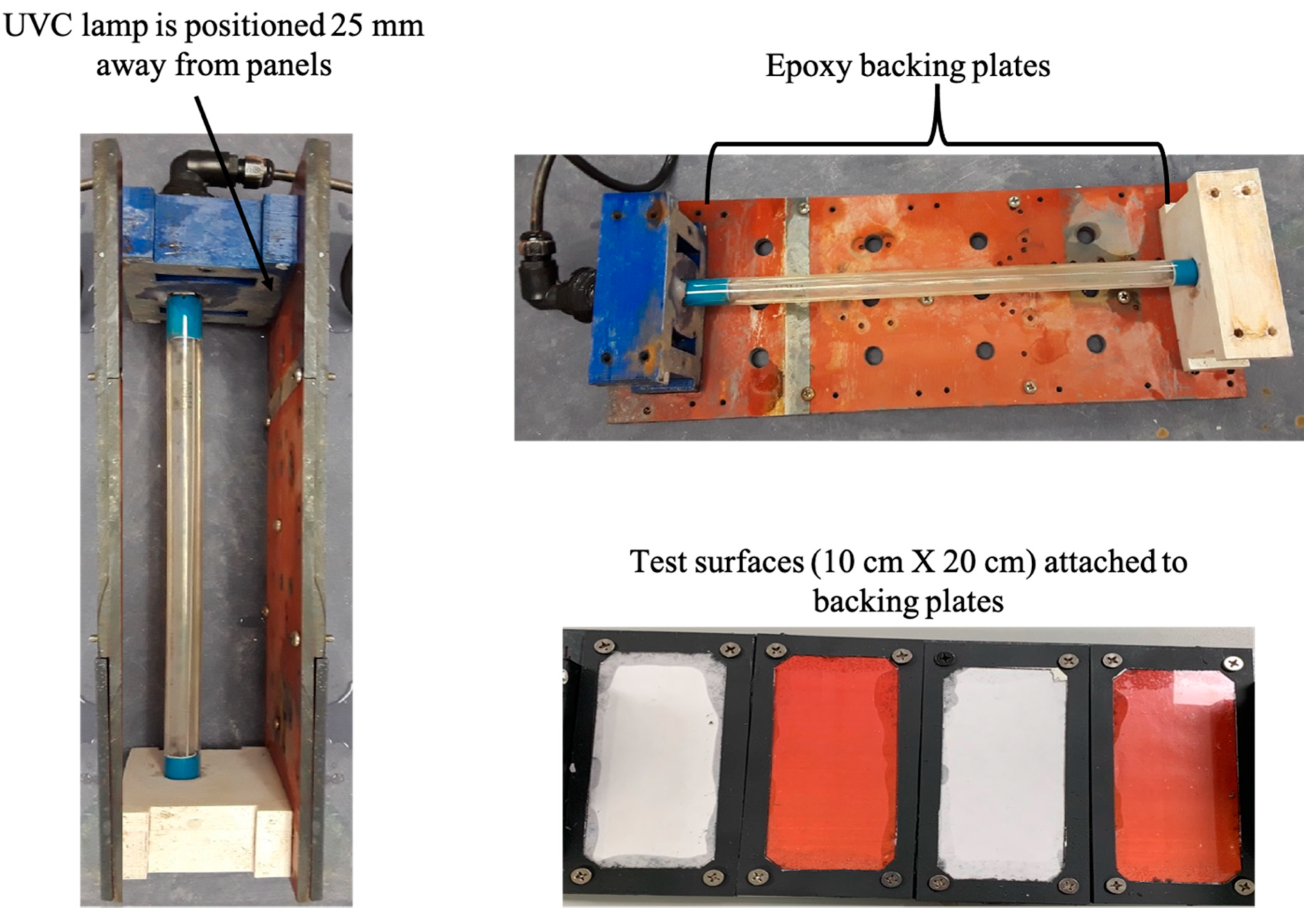

| Experiment | Panels | Time Frequency |
|---|---|---|
| UVC and Color | 4 Red and 4 White Polycarbonate (Box 1) | 1 min/day |
| UVC and Reflectance | 4 Red and White Polycarbonate (Box 1) | 1 min/day |
| 8 Stainless steel (Box 2) | ||
| 4 Polycarbonate and 4 Stainless steel (Box 3) | ||
| UVC and Exposure Intervals | 4 Polycarbonate and 4 Stainless steel | 1 min/week |
| 4 Polycarbonate and 4 Stainless steel | 5 min/week | |
| 4 Polycarbonate and 4 Stainless steel | 10 min/week | |
| 4 Polycarbonate and 4 Stainless steel | 10 min/MF | |
| 4 Polycarbonate and 4 Stainless steel | 10 min/MWF | |
| 4 Polycarbonate and 4 Stainless steel | 10 min/day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, K.N.; Hunsucker, K.Z.; Gardner, H.; Hickman, K.; Swain, G. The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling. J. Mar. Sci. Eng. 2021, 9, 662. https://doi.org/10.3390/jmse9060662
Richard KN, Hunsucker KZ, Gardner H, Hickman K, Swain G. The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling. Journal of Marine Science and Engineering. 2021; 9(6):662. https://doi.org/10.3390/jmse9060662
Chicago/Turabian StyleRichard, Kailey N., Kelli Z. Hunsucker, Harrison Gardner, Kris Hickman, and Geoffrey Swain. 2021. "The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling" Journal of Marine Science and Engineering 9, no. 6: 662. https://doi.org/10.3390/jmse9060662
APA StyleRichard, K. N., Hunsucker, K. Z., Gardner, H., Hickman, K., & Swain, G. (2021). The Application of UVC Used in Synergy with Surface Material to Prevent Marine Biofouling. Journal of Marine Science and Engineering, 9(6), 662. https://doi.org/10.3390/jmse9060662






