Naturally Occurring Rock Type Influences the Settlement of Fucus spiralis L. zygotes
Abstract
1. Introduction
2. Materials and Methods
2.1. Rock Types
2.2. Fucus Spiralis
2.3. Preparation of Settling Plates
2.4. Preparation of Zygote Solution
2.5. Settlement of Zygotes on Plates
2.6. Counting Methodology
2.7. Statistical Analyses
3. Results
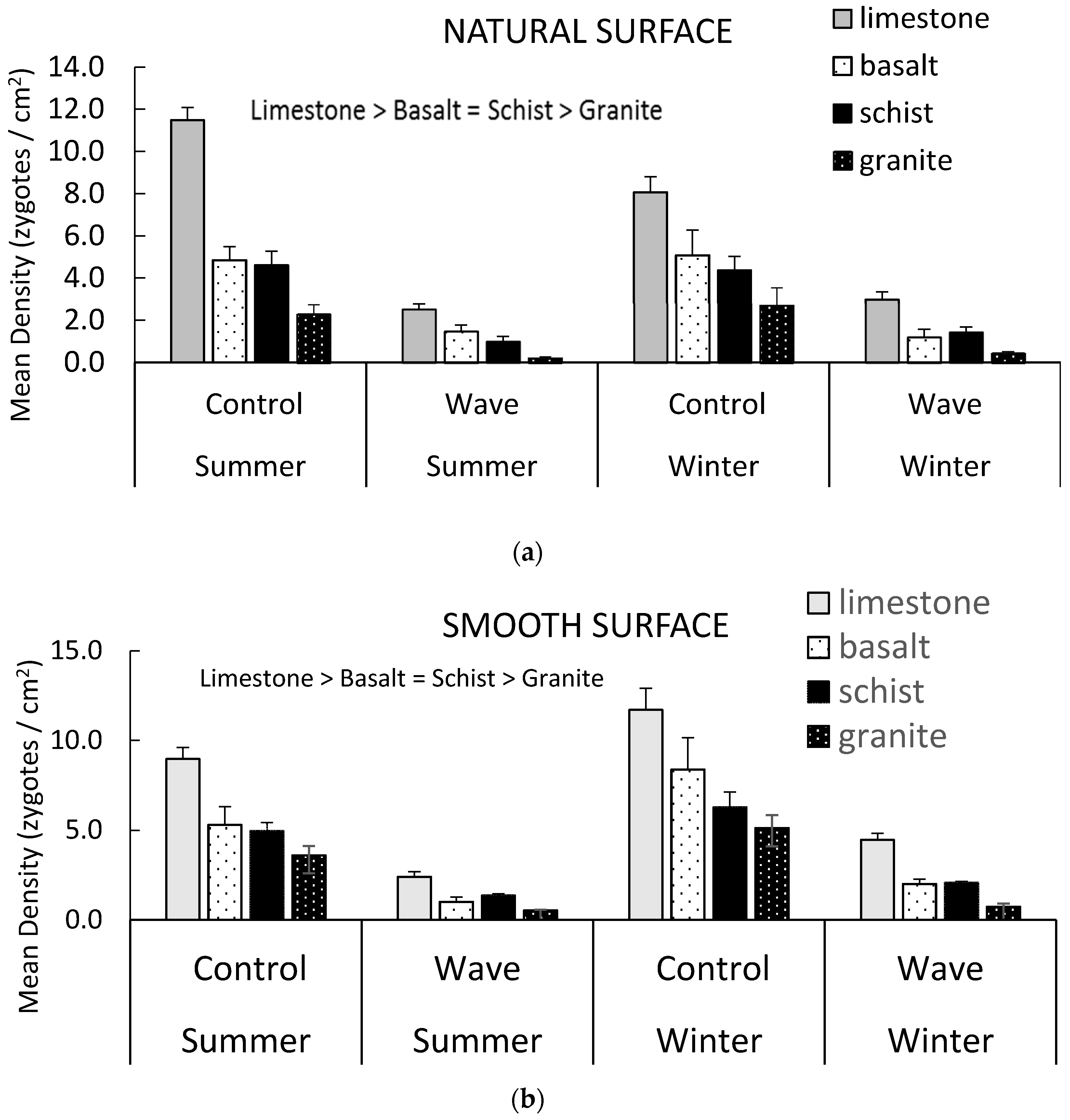
3.1. Initial Attachment
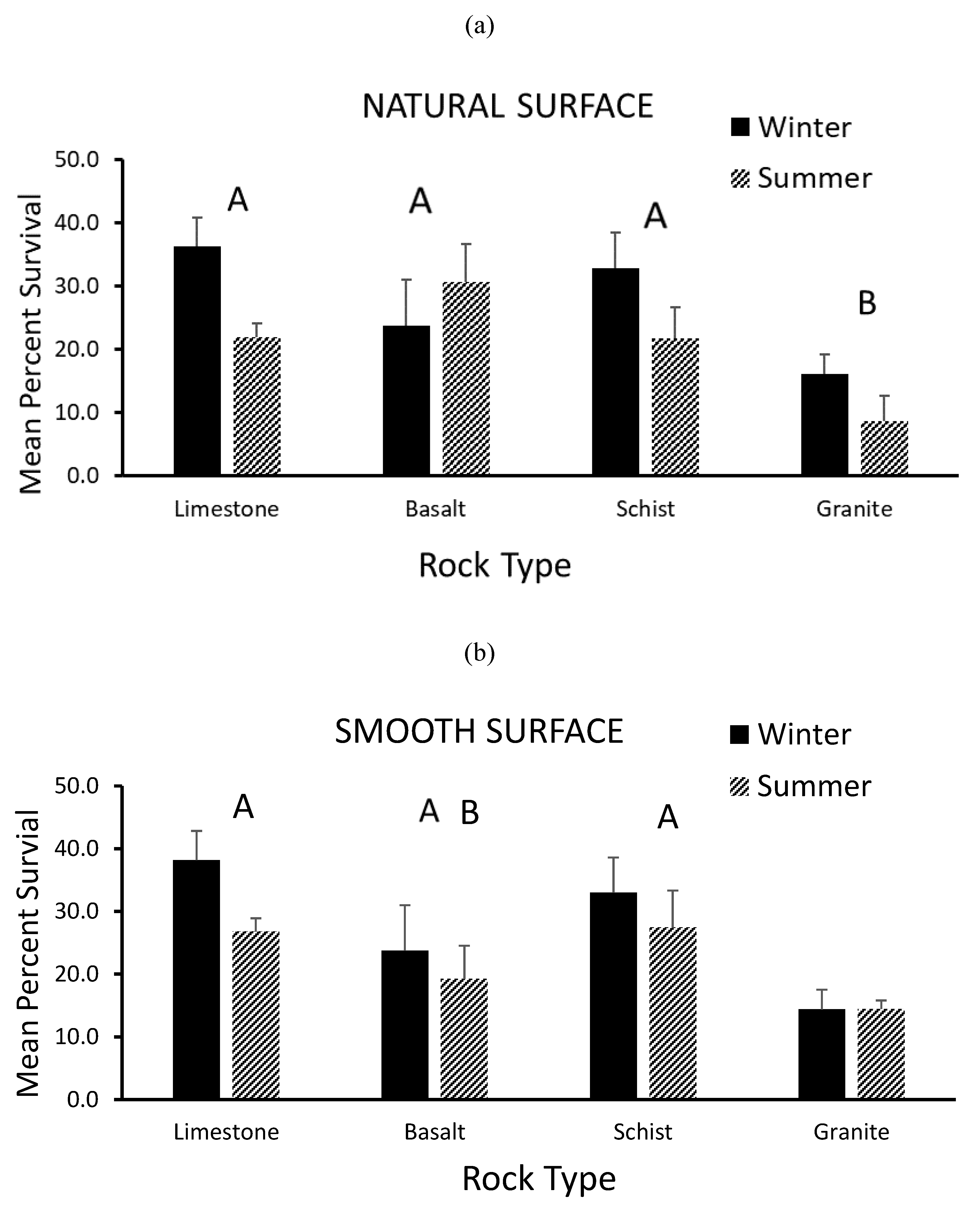
3.2. Survivorship
4. Discussion
4.1. Surface Roughness
4.2. Chemical Interactions between Adhesives and Substrata
4.3. Free Energy of Rock Surfaces
4.4. Other Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, J.R. Water movements and their role in rocky shore ecology. Sarsia 1968, 34, 13–36. [Google Scholar] [CrossRef]
- Crisp, D.J. Factors influencing the settlement of marine invertebrate larvae. In Chemoreception in Marine Organisms; Grant, P.T., Mackie, A., Eds.; Academic Press: London, UK, 1974; pp. 177–265. [Google Scholar]
- Lobban, C.S.; Harrison, P.; Duncan, A.J. The Physiological Ecology of Seaweeds; Cambridge University Press: New York, NY, USA, 1985. [Google Scholar]
- Mullineux, L.S.; Butnam, C.A. Initial contact, exploration, and attachment of barnacle (Balanus amphitrite) cyprids settling in flow. Mar. Biol. 1991, 110, 93–103. [Google Scholar] [CrossRef]
- Smith, C.M. Diversity in intertidal habitats: An assessment of the marine algae of select islands in the Hawaiian Archipelago. Pac. Sci. 1992, 46, 466–479. [Google Scholar]
- Gaines, S.; Brown, S.; Roughgarden, J. Spatial variation in larval concentrations as a cause of spatial variation in settlement of the barnacle Balanus glandula. Oecologia 1985, 67, 267–272. [Google Scholar] [CrossRef]
- Raimondi, P. Rock type affects settlement, recruitment, and zonation of the barnacle Chthamalus anispoma Pilsbury. J. Exp. Mar. Biol. Ecol. 1988, 123, 253–267. [Google Scholar] [CrossRef]
- Menge, B.A. Relative importance of recruitment and other causes of variation in rocky intertidal community structure. J. Exp. Mar. Biol. Ecol. 1991, 146, 69–100. [Google Scholar] [CrossRef]
- Valdivia, H.; Aguilera, M.A.; Navarrete, S.A.; Broitman, B.R. Disentangling the effects of propagule supply and environmental filtering on the spatial structure of a rocky shore metacommunity. Mar. Ecol. Prog. Ser. 2015, 538, 67–79. [Google Scholar] [CrossRef]
- Pornerat, C.M.; Reiner, E.R. The influence of surface angle and light on the attachment of barnacles and other sedentary organisms. Biol. Bull. 1942, 82, 14–25. [Google Scholar]
- Barnes, H.; Crisp, D.J.; Powell, H.T. Observations on the orientation of some species of barnacles. J. Anim. Ecol. 1951, 20, 227–241. [Google Scholar] [CrossRef]
- Vaselli, S.; Bertocci, I.; Maggi, E.; Benedetti-Cecchi, L. Assessing the consequences of sea level rise: Effects of changes in the slope of the substratum on sessile assemblages of rocky shorelines. Mar. Ecol. Prog. Ser. 2008, 368, 9–22. [Google Scholar] [CrossRef]
- Firth, L.B.; White, F.J.; Schofield, M.; Hanley, M.E.; Burrows, M.T.; Thompson, R.C.; Skov, M.W.; Evans, A.J.; Moore, P.J.; Hawkins, S.J. Facing the future: The importance of substratum features for ecological engineering of artificial habitats in the rocky intertidal. Mar. Fresh. Res. 2014, 67, 131–143. [Google Scholar] [CrossRef]
- Paine, R.T. Disaster, catastrophe, and local persistence of the sea palm Postelsia palmaeformis. Science 1979, 17, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, E.F.; Calvin, J.; Hedgpeth, J.W.; Phillips, D.W. Between Pacific Tides, 5th ed.; Stanford Univ. Press: Palo Alto, CA, USA, 1985. [Google Scholar]
- Vadas, R.L.; Wright, W.A.; Miller, S.L. Recruitment of Ascophyllum nodosum: Wave action as a source of mortality. Mar. Ecol. Prog. Ser. 1990, 61, 263–272. [Google Scholar] [CrossRef]
- Petraitis, P.S.; Methratta, E.T.; Rhile, E.C.; Vidargas, N.A.; Dudgeon, S.R. Experimental confirmation of multiple community states in a marine ecosystem. Oecologia 2009, 161, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Menge, D.N.L. Dynamics of coastal meta-ecosystems: The intermittent upwelling hypothesis and a test in rocky intertidal regions. Ecol. Monogr. 2013, 83, 283–310. [Google Scholar] [CrossRef]
- Ibanez-Erquiaga, B.; Pacheco, A.S.; Rivadeneira, M.M.; Tejada, C.L. Biogeographical zonation of rocky intertidal communities along the coast of Peru (3.5–13.5° S Southeast Pacific). PLoS ONE 2018, 13, e0208244. [Google Scholar] [CrossRef] [PubMed]
- Fenberg, P.B.; Menge, B.A.; Raimondi, P.T.; Rivadeneira, M.M. Biogeographic structure of the northeastern Pacific rocky intertidal: The role of upwelling and dispersal drive patterns. Ecography 2015, 38, 83–95. [Google Scholar] [CrossRef]
- Grabowska, M.; Grzelak, K.; Kuklińsk, P. Rock encrusting assemblages: Structure and distribution along the Baltic Sea. J. Sea Res. 2015, 103, 24–31. [Google Scholar] [CrossRef]
- Malm, T.; Kautsky, L.; Claesson, T. The density and survival vesiculous L. (Fucales, Phaeophyta) on different bedrock types on a Baltic Sea moaraine coast. Botanica Mar. 2003, 46, 256–262. [Google Scholar] [CrossRef]
- Guidetti, P.; Bianchi, C.N.; Chiantore, M.; Schiaparelli, S.; Morri, C.; Cattaneo-Vietti, R. Living on the rocks: Substrate mineralogy and the structure of subtidal rocky substrate communities in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2004, 274, 57–68. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Ostalé-Valriberas, E.; Farfán, G.M.; Espinosa, F. Substratum type affects recruitment and development of marine assemblages over artificial substrata: A case study in the Albaron Sea. Est. Coast. Shelf Sci. 2018, 204, 56–65. [Google Scholar] [CrossRef]
- Liversage, K.; Janetzki, N.; Benkendorff, K. Association of benthic fauna with different rock types, and evidence of changing effects during succession. Mar. Ecol. Prog. Ser. 2014, 505, 131–143. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Bavestrello, G.; Benatti, U.; Calcinai, B.; Cattaneo-Vietti, R.; Cortesogno, L.; Gaggero, L.; Giovine, M.; Puce, S.; et al. Organism-quartz interactions in structuring benthic communities. Towards a marine bio-mineralogy? Ecol. Lett. 1999, 2, 1–3. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bianchi, C.N.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Morri, C.; Puce, S.; Sara, M. Bio-mineralogy as a structuring factor for marine epibenthic communities. Mar. Ecol. Prog. Ser. 2000, 193, 241–249. [Google Scholar] [CrossRef]
- Moore, H.B.; Kitching, J.A. The biology of Chthamalus stellatus (Poli). J. Mar. Biol. Assoc. UK 1939, 23, 521–541. [Google Scholar] [CrossRef][Green Version]
- Crisp, D.J.; Barnes, H. The orientation and distribution of barnacles and settlement with particular reference to surface contour. J. Anim. Ecol. 1954, 23, 142–162. [Google Scholar] [CrossRef]
- Crisp, D.J.; Ryland, J.S. Influence of filming and surface texture on the settlement of marine organisms. Nature 1960, 185, 119. [Google Scholar] [CrossRef]
- Harlin, M.M.; Lindbergh, J. Selection of substrata by seaweeds: Optimal surface relief. Mar. Biol. 1977, 40, 33–40. [Google Scholar] [CrossRef]
- Wethey, D.S. Ranking of settlement cues by barnacle larvae: Influence of surface contour. Bull. Mar. Sci. 1986, 39, 393–400. [Google Scholar]
- Barkai, A.; Branch, G.B. The influence of predation and substrate complexity on recruitment to settlement plates: A test of the theory of alternative states. J. Exp. Mar. Biol. Ecol. 1988, 124, 215–237. [Google Scholar] [CrossRef]
- Chabot, R.; Bourget, E. Influence of substratum heterogeneity and settled barnacle density on the settlement of Cypris larvae. Mar. Biol. 1988, 97, 45–56. [Google Scholar] [CrossRef]
- Le Tourneux, F.; Bourget, E. Importance of physical and biological settlement cues used at different spatial scales by the larvae of Semibalanus balanoides. Mar. Biol. 1988, 97, 57–66. [Google Scholar] [CrossRef]
- Wells, J.; Moll, E.J.; Bolton, J.J. Substrate as a determinant of marine intertidal algal communities at Smitswinkle Bay, False Cay, Cape. Mar. Bot. 1989, 32, 499–502. [Google Scholar] [CrossRef]
- Anderson, M.J.; Underwood, A.J. Effects of substratum on the recruitment and development of an intertidal estuarine fouling assemblage. J. Exp. Mar. Biol. Ecol. 1994, 184, 217–236. [Google Scholar] [CrossRef]
- Gersun, L.; Anderson, R.J.; Hart, J.R.; Maneveldt, G.W.; Bolton, J.J. Sublittoral seaweed communities on natural and artificial substrata in a high-latitude coral community in South Africa. Afr. J. Mar. Sci. 2016, 38, 303–316. [Google Scholar] [CrossRef]
- Chase, A.L.; Dijkstra, J.A.; Harris, L.G. The influence of substrate material on ascidian larval settlement. Mar. Poll. Bull. 2016, 106, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.M.; Maassen, S.; Rong, R.G.Z.; Benke, P.I.; Lim, C.-S.; Marzinelli, E.M.; Janczewski, D.; Teo, S.L.-M.; Vancso, G.J. Effect of variations in micropatterns and surface modulus on marine fouling of engineering polymers. Appl. Mater. Interfaces 2017, 9, 17508–17516. [Google Scholar] [CrossRef]
- Kerrison, P.; Stanley, M.; Mitchell, E.; Cunningham, L.; Hughes, A. A life-stage conflict of interest in kelp: Higher meiospore settlement where sporophyte attachment is weak. Algal Res. 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Erramilli, S.; Genzer, J. Influences of surface topography attributes on settlement and adhesion of natural and synthetic species. Soft Matter 2019, 15, 4045–4067. [Google Scholar] [CrossRef]
- Hardy, F.G.; Moss, B.L. The effects of the substratum on the morphology of the rhizoids of Fucus germlings: Est. Coast. Mar. Sci. 1979, 9, 577–584. [Google Scholar] [CrossRef]
- Fletcher, R.L.; Callow, M. The settlement, attachment, and establishment of marine algal spores. Brit. Phycol. J. 1992, 27, 303–329. [Google Scholar] [CrossRef]
- Callow, M.E.; Fletcher, R.L. The influence of low surface energy materials on bioadhesion—A review. J. Int. Biodeterior. Biodegrad. 1994, 34, 333–348. [Google Scholar] [CrossRef]
- Pradhan, S.; Kumar, S.; Mohanty, S.; Nayak, S.K. Environmentally Benign Fouling-Resistant Marine Coatings: A Review. Polym.-Plast. Technol. Eng. 2019, 58, 498–518. [Google Scholar] [CrossRef]
- Leonardi, A.K.; Ober, C.K. Polymer-based marine antifouling and fouling release surfaces: Strategies for synthesis and modification. Ann. Rev. Chem. Biomol. Engin. 2019, 10, 241–264. [Google Scholar] [CrossRef]
- Chapman, A.R.O. Functional ecology of fucoid algae: Twenty-three years of progress. Phycologia 1995, 34, 1–32. [Google Scholar] [CrossRef]
- Norton, T.A.; Fetter, R. The settlement of Sargassum muticum propagules in stationary and flowing water. J. Mar. Biol. Assoc. UK 1981, 61, 929–940. [Google Scholar] [CrossRef]
- Le, H.N.; Hughes, A.D.; Kerrison, P.D. Early development and substrate twice selection for the cultivation of Sargassum muticum (Yendo) Fensholt under laboratory conditions. J. Appl. Phycol. 2018, 30, 2475–2483. [Google Scholar] [CrossRef]
- Taylor, D.I.; Schiel, D.R. Wave-related mortality in zygotes of habit-forming algae from different exposures in southern New Zealand: The importance of ‘stickability’. J. Exper. Mar. Biol. Ecol. 2003, 290, 229–245. [Google Scholar] [CrossRef]
- Dimartino, S.; Mather, A.V.; Nowell-Usticke, J.S.; Fischer, B.; Nock, V. Investigation of the adhesive from Hormosira banksia germlings and its performance over different material surfaces and topographies. Inter. J. Adhes. Adhes. 2017, 75, 114–123. [Google Scholar] [CrossRef]
- Hussey, A.M., II; Berry, H.N., IV. Bedrock Geology of the Bath 1:100,000 Map Sheet, Coastal Maine; Map 02–152; Maine Geological Survey: Augusta, ME, USA, 2002. [Google Scholar]
- Vreeland, V.; Grotkopp, S.; Espinosa, S.; Quiroz, D.; Laetsch, W.M.; West, J. The pattern of cell wall adhesive formation by Fucus zygotes. Hydrobiologia 1993, 260/261, 485–491. [Google Scholar] [CrossRef]
- Niemeck, R.A.; Mathieson, A.C. An ecological study of Fucus spiralis (L.). J. Exp. Mar. Biol. Ecol. 1976, 24, 33–48. [Google Scholar] [CrossRef]
- Thelin, I. Effects, en culture, de deux petroles bruts et d’un petrolier sur les zygotes et les plantules, de Fucus serratus Linnaeus (Fucales, Phaeophyceae). Bot. Mar. 1981, 24, 515–519. [Google Scholar] [CrossRef]
- Vadas, R.L.; Johnson, S.; Norton, T.A. Recruitment and mortality of early post-settlement stages of benthic algae. Brit. Phycol. J. 1992, 27, 331–351. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables; W. H. Freeman and Company: San Francisco, CA, USA, 1969. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Potin, P.; Leblanc, C. Phenolic-based adhesives of marine brown algae. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 105–124. [Google Scholar]
- Britton, R.; Ben-Yehuda, M.; Davidovich, M.; Balazs, Y.; Potin, P.; Delage, L.; Colin, C.; Bianco-Peled, H. Structure of algal-born phenolic polymeric adhesives. Macromol. Biosci. 2006, 6, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Bourget, E.; DeGuise, J.; Daigle, G. Scale of substratum heterogeneity structural complexity, and the early establishment of a marine epibenthic community. J. Exp. Mar. Biol. Ecol. 1994, 181, 31–51. [Google Scholar] [CrossRef]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.G. Engineered Antifouling Microtopographies—Effect of Feature Size, Geometry, and Roughness on Settlement of Zoospores of the Green Alga Ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.P.; Sturgess, C.J.; Davies, M.S. The effect of rock-type on the settlement of Balanus balanoides (L.) cyprids. Biofouling 1997, 11, 137–147. [Google Scholar] [CrossRef]
- McGuinness, K.A.; Underwood, A.J. Habitat structure of communities on intertidal boulders. J. Exp. Mar. Biol. Ecol. 1986, 104, 97–123. [Google Scholar] [CrossRef]
- Barnes, H. Surface roughness and the settlement of Balanus balanoides. Arch. Soc. Zool.-Bot. Fenn. Vanamo 1956, 10, 164–168. [Google Scholar]
- Caffey, H.M. No effect of naturally occurring rock types on settlement or survival of the intertidal barnacle Tessoropora rosea. J. Exp. Mar. Biol. Ecol. 1982, 63, 119–132. [Google Scholar] [CrossRef]
- Caffey, H.M. Spatial and temporal variation in settlement and recruitment of intertidal barnacles. Ecol. Monogr. 1994, 55, 313–332. [Google Scholar] [CrossRef]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Rittschof, D.; Holm, E.; Schmidt, A.R. Factors influencing initial larval settlement temporal, spatial and surface molecular components. J. Exp. Mar. Biol. Ecol. 1991, 150, 203–211. [Google Scholar] [CrossRef]
- Jaycock, M.J.; Parfitt, G.D. Chemistry of Interfaces; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Adamson, A.W. Physical Chemistry of Surfaces; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Holm, E.R.; Cannon, G.; Roberts, D.; Schmidt, A.R.; Sutherland, J.P.; Rittschof, D. The influence of initial surface chemistry on development of the fouling community at Beaufort North Carolina. J. Exp. Mar. Biol. Ecol. 1997, 215, 189–203. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Pogorzelski, S.J.; Pospiech, A.; Boniewicz-Szmyt, B. Monitoring of marine biofilm formation dynamics at submerged solid surfaced with miltitechnique sensors. Front. Mar. Sci. 2018, 10, 363. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between microbial biofilms and marine fouling algae: A mini review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef]
- Hou, Y.; Xiaoping, J.; Li, J.; Xianghang, L. Adhesion between asphalt and recycled concrete aggregate and its impact on properties of asphalt mixture. Materials 2018, 11, 2528. [Google Scholar] [CrossRef]
- Wright, J.; Reed, R.H. Effects of osmotic stress on gamete size, rhizoid initiation and germling growth in fucoid algae. Brit. Phycol. J. 1990, 25, 149–155. [Google Scholar] [CrossRef]
- Maki, J.S.; Rittschof, D.; Schmidt, A.R.; Snyder, A.G.; Mitchell, R. Factors controlling attachment of bryozoan larvae: A comparison of bacterial films and unfilmed surfaces. Biol. Bull. 1989, 177, 295–302. [Google Scholar] [CrossRef]
- Becker, K. Attachment strength and colonization patterns of two macrofouling species on substrata with different surface tension (in situ studies). Mar. Biol. 1993, 117, 301–309. [Google Scholar] [CrossRef]
- Allen, T.F.H. Scale in microscopic algal ecology: A neglected dimension. Phycologia 1977, 16, 253–257. [Google Scholar] [CrossRef]
- Amsler, C.D.; Reed, D.C.; Neushul, M. The microclimate inhabited by macroalgal propagules. Brit. Phycol. J. 1992, 27, 253–270. [Google Scholar] [CrossRef]
- Maki, J.S.; Rittschof, D.; Mitchell, R. Inhibition of larval barnacle attachment to bacterial films: An investigation of physical properties. Microb. Ecol. 1992, 23, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pershing, A.J.; Alexander, M.A.; Brady, D.C.; Brickman, D.; Curchitser, E.N.; Diamond, A.W.; McClenachan, L.M.; Mills, K.E.; Nichols, O.C.; Pendleton, D.E.; et al. Climate impacts of the Gulf of Maine ecosystem: A review of observed and expected changes in 2050 from rising temperatures. Elem. Sci. Anth. 2021, 9, 76. [Google Scholar] [CrossRef]
| Natural Surface | |||||
| Source | df | SS | MS | F | p |
| Season | 1 | 0.0001 | 0.0001 | 0.01 | 0.92 |
| Rock type | 3 | 2.3207 | 0.7735 | 53.9 | 0.0001 |
| Wave | 1 | 3.4149 | 3.4149 | 237.9 | 0.0001 |
| Interaction (season x rock) | 3 | 0.0374 | 0.0125 | 0.86 | 0.46 |
| Interaction (season x wave) | 1 | 0.0261 | 0.0261 | 1.82 | 0.18 |
| Interaction (rock x wave) | 3 | 0.0131 | 0.0044 | 0.30 | 0.82 |
| Interaction (season x rock) | |||||
| x wave | 3 | 0.0433 | 0.0144 | 1.01 | 0.39 |
| Error | 64 | 0.9185 | 0.0144 | ||
| Total | 79 | 6.7742 | 0.0858 | ||
| Smooth Surface | |||||
| Source | df | SS | MS | F | p |
| Season | 1 | 0.328 | 0.328 | 23.29 | 0.0001 |
| Rock type | 3 | 1.433 | 0.478 | 33.91 | 0.0001 |
| Wave | 1 | 4.065 | 4.065 | 288.68 | 0.0001 |
| Interaction (season x rock) | 3 | 0.025 | 0.008 | 0.6 | 0.62 |
| Interaction (season x wave) | 1 | 0.0004 | 0.0005 | 0.03 | 0.85 |
| Interaction (rock x wave) | 3 | 0.044 | 0.014 | 1.05 | 0.37 |
| Interaction (season x rock) | |||||
| x wave | 3 | 0.016 | 0.005 | 0.38 | 0.77 |
| Error | 64 | 0.901 | 0.014 | ||
| Total | 79 | 6.812 | 0.086 |
| Natural Surface | |||||
| Source | df | SS | MS | F | p |
| Season | 1 | 0.0908 | 0.0908 | 4.11 | 0.051 |
| Rock type | 3 | 0.3953 | 0.1318 | 5.96 | 0.002 |
| Interaction (season x type) | 3 | 0.1133 | 0.0377 | 1.71 | 0.181 |
| Error | 32 | 0.7076 | 0.0221 | ||
| Total | 39 | 1.3071 | |||
| Smooth Surface | |||||
| Source | df | SS | MS | F | p |
| Season | 1 | 0.0218 | 0.218 | 1.66 | 0.207 |
| Rock type | 3 | 0.2698 | 0.089 | 6.83 | 0.001 |
| Interaction (season x type) | 3 | 0.0258 | 0.0086 | 0.65 | 0.587 |
| Error | 32 | 0.4216 | 0.0132 | ||
| Total | 39 | 0.7391 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrose, W.G., Jr.; Renaud, P.E.; Adler, D.C.; Vadas, R.L. Naturally Occurring Rock Type Influences the Settlement of Fucus spiralis L. zygotes. J. Mar. Sci. Eng. 2021, 9, 927. https://doi.org/10.3390/jmse9090927
Ambrose WG Jr., Renaud PE, Adler DC, Vadas RL. Naturally Occurring Rock Type Influences the Settlement of Fucus spiralis L. zygotes. Journal of Marine Science and Engineering. 2021; 9(9):927. https://doi.org/10.3390/jmse9090927
Chicago/Turabian StyleAmbrose, William G., Jr., Paul E. Renaud, David C. Adler, and Robert L. Vadas. 2021. "Naturally Occurring Rock Type Influences the Settlement of Fucus spiralis L. zygotes" Journal of Marine Science and Engineering 9, no. 9: 927. https://doi.org/10.3390/jmse9090927
APA StyleAmbrose, W. G., Jr., Renaud, P. E., Adler, D. C., & Vadas, R. L. (2021). Naturally Occurring Rock Type Influences the Settlement of Fucus spiralis L. zygotes. Journal of Marine Science and Engineering, 9(9), 927. https://doi.org/10.3390/jmse9090927






